Abstract
Low oxygen tension, i.e., hypoxia, is a pathophysiological component involved in many human disorders but is also a critically important phenomenon in normal development and differentiation. The ability of cells to survive under hypoxia or to adapt to it depends on a family of hypoxia-inducible transcription factors (HIFs) that induce the expression of a number of genes involved in hematopoiesis, angiogenesis, iron transport, glucose utilization, resistance to oxidative stress, cell proliferation, survival and apoptosis, and extracellular matrix homeostasis. We introduce here the recently identified molecular mechanisms responsible for the oxygen-dependent stability and activity of HIF, after which we focus on extracellular matrix genes as HIF targets. The vital role of the hypoxia response pathway in chondrogenesis and joint development is then discussed.
Keywords: Hypoxia, Hypoxia-inducible factor, Extracellular matrix, Cartilage, Joint
Introduction
An adequate supply of oxygen is essential for the function and survival of cells in the human body, and an abrupt decrease in oxygen availability caused by myocardial infarction or stroke is a major cause of mortality. Tissues may also suffer from chronic low oxygen tension in several pathological situations such as severe anemias and fibrotic diseases. Furthermore, hypoxia is clinically associated with tumor metastasis and poor prognosis. However, low oxygen tension also has an important role in normal development and differentiation and can be regarded as an essential regulatory signal during fetal development. The ability of cells to survive under hypoxia or to adapt to it depends on a family of hypoxia-inducible transcription factors (HIFs) that induce the expression of a number of genes involved in hematopoiesis, angiogenesis, iron transport, glucose utilization, resistance to oxidative stress and cell proliferation, survival and apoptosis. In addition, the expression of several genes involved in the synthesis of extracellular matrix is upregulated under hypoxic conditions (for reviews, see Schipani 2005; Bertout et al. 2008; Chandel and Simon 2008; Chowdury et al. 2008; Higgins et al. 2008; Kaelin and Ratcliffe 2008; Smith et al. 2008; Fraisl et al. 2009). In this review, we introduce recently identified molecular mechanisms responsible for the oxygen-dependent stability and activity of HIF, after which we focus on extracellular matrix genes as HIF targets and the role of the hypoxia response in chondrogenesis and joint development.
Oxygen-dependent regulation of stability and activity of HIF
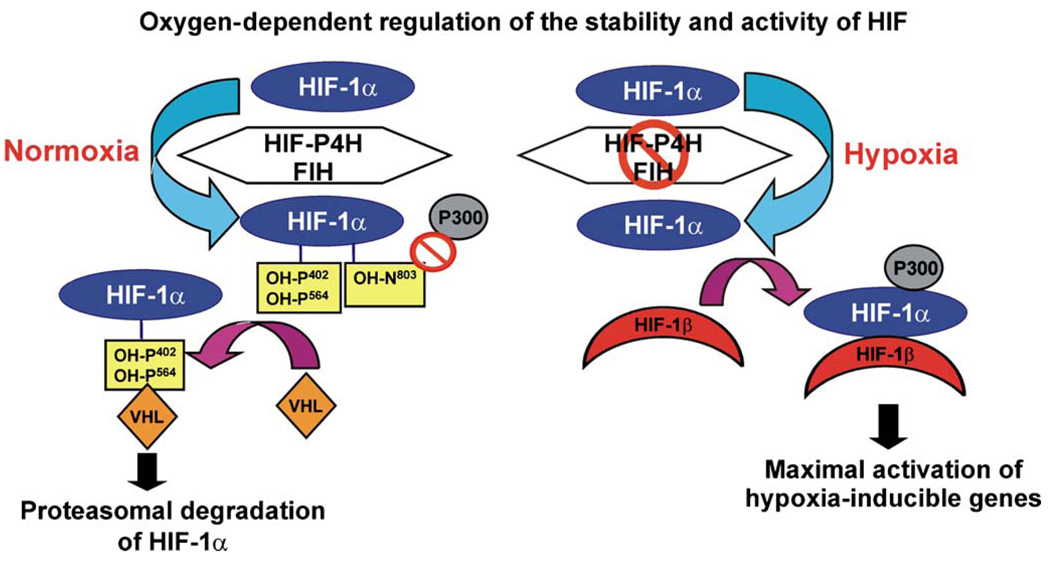
HIFs are the master regulators of hypoxia-responsive genes. They are αβ heterodimers in which the stability and activity of the α subunit is regulated in an oxygen-dependent manner. HIF-α has three isoforms in humans, of which HIF-1α and HIF-2α are the most extensively studied (Ratcliffe 2006). The proteolytic stability and transcriptional activity of the HIF-1α and HIF-2α subunits are regulated by two oxygen-dependent hydroxylation events. Under normoxic conditions, extremely low levels of the HIF-α subunits, if any, can be detected in cells. The HIF-α subunits contain an oxygen-dependent degradation domain in which proline residues in two -Leu-X-X-Leu-Ala-Pro- sequences are hydroxylated under normoxic conditions by a novel cytoplasmic and nuclear HIF prolyl 4-hydroxylase (HIF-P4H) family (Fig. 1; Bruick and McKnight 2001; Epstein et al. 2001; Ivan et al. 2001, 2002; Jaakkola et al. 2001; Yu et al. 2001). The 4-hydroxyproline residues formed by the HIF-P4Hs are required for the binding of HIF-α to the von Hippel-Lindau E3 ubiquitin ligase complex and its rapid subsequent proteasomal degradation in normoxia (Fig. 1). Under hypoxic conditions this oxygen-requiring hydroxylation is inhibited, and HIF-α escapes degradation and dimerizes with HIF-β (Fig. 1). The dimer is then translocated into the nucleus and becomes bound to the HIF-responsive elements in a number of hypoxia-regulated genes that facilitate adaptation to hypoxia and associated metabolic compromise (Fig. 1). The transcriptional activation capacity of HIF is regulated by yet another oxygen-dependent hydroxylation event. Hydroxylation of a critical asparagine residue in the C-terminal transactivation domain of HIF-α blocks its interaction with the transcriptional coactivator p300 (Fig. 1; Lando et al. 2002a). The asparaginyl hydroxylase catalyzing this modification is identical to a protein known as factor inhibiting HIF (Fig. 1; Hewitson et al. 2002; Lando et al. 2002b).
Fig. 1.
Regulation of the stability and activity of hypoxia-inducible transcription factor-1α (HIF-1α) by oxygen-dependent hydroxylation. Under normoxic conditions, HIF-1α is hydroxylated by HIF prolyl 4-hydroxylases (HIF-P4H) and factor inhibiting HIF (FIH). Hydroxylation of one or two specific prolines of the oxygen-dependent degradation domain by the HIF-P4Hs is required for binding of the von Hippel-Lindau E3 ubiquitin ligase complex (VHL) and for subsequent proteasomal degradation. Hydroxylation of a specific asparagine in the C-terminal transactivation domain by FIH blocks the binding of the transcriptional coactivator p300 (P300). Hypoxia inhibits the HIF-P4Hs and FIH, HIF-1α escapes degradation, forms a stable dimer with HIFβ, and binds p300. The active dimer binds to the HIF-responsive elements in a number of hypoxia-inducible genes and activates their transcription
Genes of collagens and their modifying enzymes as HIF targets
A hypoxia-responsive enhancer element was first identified in the gene for erythropoietin, a growth factor that stimulates erythrocyte production (Semenza et al. 1991). This led to the isolation, cloning, and identification of HIF as the major mediator of the hypoxia-inducible expression of erythropoietin (Semenza and Wang 1992; Wang et al. 1995). Since then, more than 40 HIF target genes have been identified (for a review, see Schofield and Ratcliffe 2004). The closely related HIF-1α and HIF-2α regulate common target genes but also show specificity in that HIF-1α appears to act more effectively on genes for glycolytic enzymes, for instance, and HIF-2α on the gene for erythropoietin (Hu et al. 2003; Warnecke et al. 2004; Wang et al. 2005; Elvidge et al. 2006; Gruber et al. 2007; Rankin et al. 2007).
Several genes involved in the synthesis, maintenance, and degradation of extracellular matrix are also regulated by HIF (Denko et al. 2003; Hofbauer et al. 2003; Wang et al. 2005; Chi et al. 2006; Elvidge et al. 2006; Erler et al. 2006; Pollard et al. 2008), and hypoxia is thus likely to regulate extracellular matrix homeostasis directly through the activity of HIF. Hypoxia has been shown to increase the rate of collagen synthesis in several in vivo and in vitro studies (Falanga et al. 1993; Durmowicz et al. 1994; Ostadal et al. 1995; Perhonen et al. 1997; Tamamori et al. 1997; Berg et al. 1998; Norman et al. 2000; Takahashi et al. 2000; Tajima et al. 2001; Horino et al. 2002). The exposure of rats to hypoxia, for example, has been shown to lead to an increase in the mRNA levels of proα1(I), proα1(III), and α2(IV) collagen chains in peripheral lung parenchyma (Berg et al. 1998) and of proα1(I), fibronectin, and tropoelastin in the pulmonary artery, where such an increase is correlated with enlarged vascular elastin and collagen fiber volumes in the adventitial layer (Durmowicz et al. 1994). The mRNA level of proα1(I) procollagen polypeptides has also been shown to be increased in fibroblasts originating from various tissues and cultured under hypoxic conditions (Falanga et al. 1993; Tamamori et al. 1997; Norman et al. 2000).
In certain other studies, increased deposition of type I and IV collagens has been detected under hypoxia with no increase in the corresponding collagen polypeptide mRNAs (Tajima et al. 2001; Horino et al. 2002). In these cases, increased mRNA and protein levels of collagen P4H-I, an essential enzyme required for the generation of stable triple-helical collagen molecules (Myllyharju and Kivirikko 2004; Myllyharju 2008), have been observed instead (Tajima et al. 2001, Horino et al. 2002). Three vertebrate collagen P4H isoenzymes, collagen P4Hs I–III, which differ in their catalytic α subunit, have been identified, collagen P4H-I being the major form in most cell types and tissues, with the exception of chondrocytes, osteoblasts, and capillary endothelial cells in which collagen P4H-II is the predominant form (Myllyharju and Kivirikko 2004; Holster et al. 2007; Myllyharju 2008). The type III collagen P4H is expressed in many adult and fetal human tissues, but at much lower levels than the other two isoenzymes (Kukkola et al. 2003). Increased mRNA levels of the α(I) and α(II) subunits of the vertebrate collagen P4Hs I and II have been reported in several additional studies (Takahashi et al. 2000; Hofbauer et al. 2003; Fähling et al. 2004; Elvidge et al. 2006; Pollard et al. 2008). A two-fold to three-fold increase in the mRNA abundance of the α(I) subunit has been observed in fetal rat lung fibroblasts cultured for 8 h under 0%–2% O2 (Takahashi et al. 2000), and the mRNA levels of the α(I) and α(II) subunits are increased five-fold and twelve-fold, respectively, with concomitant two–fold to 2.5-fold and three–fold to four-fold increases in the protein level, after a 24 h exposure to 1% O2 in a rat vascular smooth muscle cell line, whereas no induction has been detected in the mRNA levels of the α(III) subunit or proα1 (I) collagen (Hofbauer et al. 2003). More robust induction has been observed in a mouse juxtaglomerular cell line that responds to hypoxia rapidly, the increases in the α(I) and α(II) mRNAs being five-fold to eight-fold and 25–fold to 33-fold, respectively, after exposure to 0.5% O2 for 4.5 h (Hofbauer et al. 2003). Similar increases have also been recorded in a mouse hepatoma cell line and mouse embryonic fibroblasts in which the effect has been shown to be HIF-1-dependent (Hofbauer et al. 2003). In a MCF7 breast cancer cell line exposed to 1% O2 for 16 h, the α(I) and α(II) mRNAs are upregulated about five-fold and 7.5-fold, respectively, whereas no changes have been detected in the α(III) mRNA level (Elvidge et al. 2006; Pollard et al. 2008). Furthermore, increased synthesis of the α(I) protein under prolonged hypoxia has been shown to be regulated at the translational level by the interaction of an RNA-binding nucleolin at the untranslated region of the mRNA (Fähling et al. 2006).
Collagen polypeptides are also hydroxylated by lysyl hydroxylases (LHs), the hydroxylysine residues generated having at least two important functions (Kivirikko and Pihlajaniemi 1998; Myllyharju and Kivirikko 2004; Myllyharju 2005): they are essential for the stability of the intermolecular collagen crosslinks that provide the tensile strength and mechanical stability for collagens, and they serve as attachment sites for carbohydrates (Myllyharju 2005). The vertebrate LH family consists of three isoenzymes, each with specific roles in collagen synthesis (Heikkinen et al. 2000; Rautavuoma et al. 2002, 2004; Wang et al. 2002; van der Slot et al. 2003, 2004; Myllyharju and Kivirikko 2004; Myllyharju 2005; Ruotsalainen et al. 2006; Takaluoma et al. 2007a, b). The mRNA levels of LH1 and LH2 have also been shown, in several studies, to be increased by hypoxia. The LH1 and LH2 mRNA levels increase seven-fold and five-fold, respectively, in rat vascular smooth muscle cells exposed to 1% O2 for 24 h, and five-fold and two-fold in mouse juxtaglomerular cells cultured under 0.5% O2 for 4.5 h (Hofbauer et al. 2003). The LH2 mRNA level has been shown to be highly upregulated in hypoxic primary and transformed keratinocytes, but not in fibroblasts (Denko et al. 2003). The LH1 and LH2 mRNA levels increase about two-fold and 2.5-fold, respectively, in the MCF7 breast cancer cell line when exposed to 1% O2 for 16 h, whereas no changes have been detected in the LH3 mRNA level (Elvidge et al. 2006; Pollard et al. 2008).
Lysyl oxidase (LOX) catalyzes the crosslinking of collagen and elastin fibers (Lucero and Kagan 2006; Mäki 2009), and its activity is essential for the normal development and function of the cardiovascular and respiratory systems and for perinatal survival (Mäki et al. 2002, 2005; Hornstra et al. 2003). The LOX family contains, in addition to LOX, four LOX-like proteins, LOXL 1–4, which are also likely to be involved in extracellular matrix synthesis (Lucero and Kagan 2006; Payne et al. 2007; Mäki 2009). In addition, LOX and LOXL proteins have been demonstrated to influence chemotactic responses, proliferation, and shifts between normal and malignant phenotypes (Lucero and Kagan 2006; Payne et al. 2007). LOX has been identified both as a tumor suppressor that inhibits the function of ras and as a gene promoting metastasis (Lucero and Kagan 2006; Payne et al. 2007). The LOX gene is one of the most strongly hypoxia-induced genes (Denko et al. 2003; Wang et al. 2005; Elvidge et al. 2006) and has been shown to be essential for hypoxia-induced metastasis (Erler et al. 2006). Patients with highly LOX-expressing tumors have poor metastasis-free and overall survival rates, and the inhibition of the catalytic activity of Lox eliminates metastasis in mice with orthotopically grown breast cancer tumors (Erler et al. 2006). Recently, the hypoxia-induced LOX has been reported to play a critical function in the formation of the premetastatic niche where it crosslinks basement membrane collagen IV and recruits bone marrow cells to produce a matrix metalloproteinase (MMP) that enhances invasion and metastatic growth (Erler et al. 2009).
Hypoxia also induces gene products that are involved in the regulation of extracellular matrix turnover. In particular, it increases plasminogen activator inhibitor-I (Kietzmann et al. 1999; Koong et al. 2000), tissue-inhibitor of metalloproteinase-1 (Norman et al. 2000), and connective tissue growth factor (Higgins et al. 2004), all in a HIF-1α-dependent fashion. Most notably, the accumulation of metalloproteinases is often reduced under hypoxic conditions (Norman et al. 2000), although an increase of MMP13 has also been reported (Koong et al. 2000).
Fetal growth plate
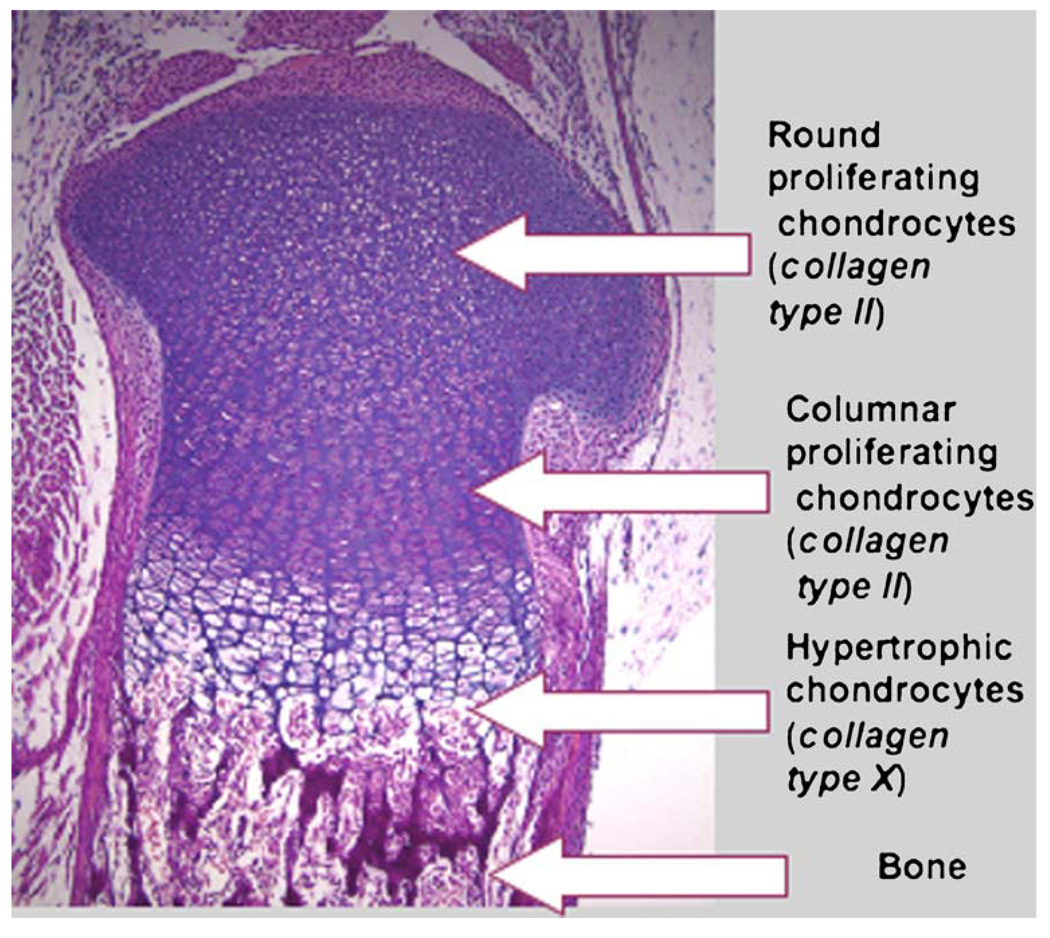
The fetal growth plate is a striking example of the critical and non-redundant role of HIF-1α in the survival and differentiation of hypoxic cells in vivo. Skeletal development depends on two mechanisms: intramembranous and endochondral ossification (Karsenty 2003). In intramembranous ossification, mesenchymal cells develop directly into osteoblasts and form the flat bones of the skull. The endochondral ossification that accounts for the development of most other bones involves a two-stage mechanism in which chondrocytes form a matrix template, the growth plate that is then replaced by bone. During endochondral bone development the growth plate chondrocytes undergo well-ordered and controlled phases of cell proliferation, maturation, and death (Fig. 2). The proliferative chondrocytes synthesize type II collagen and form a columnar layer (Fig. 2). They then stop proliferating and differentiate into post-mitotic hypertrophic cells (Fig. 2). Hypertrophic chondrocytes express predominantly type X collagen and mineralize their surrounding matrix (Fig. 2). Differentiation is followed by the death of the hypertrophic chondrocytes, by blood vessel invasion, and finally by replacement of the cartilaginous matrix with bone.
Fig. 2.
Hematoxylin and eosin staining of the proximal growth plate of mouse tibia at birth. ×10
The size of an organ is often stated to depend on two variables: cell number and cell size. This is only partly true for bone and cartilage, tissues in which the matrix is quantitatively as important as the cells. The cartilaginous matrix is formed by two components: proteoglycans and collagens. Proteoglycans are macromolecules that contain a core protein with multiple attached polysaccharide chains (Schwartz and Domowicz 2002). Because of their high content of charged polysaccharides, proteoglycans are highly hydrated. The collagens of the growth plate matrix consist of the fibrillar type II and XI collagens, the fibrilassociated type IX collagen that binds proteoglycans, and the sheet-forming type X collagen (Olsen 1996; Myllyharju and Kivirikko 2004). The type II and IX collagens are also found in the vitreous of the eye, whereas type II collagen is produced by the proliferating chondrocytes and upper hypertrophic chondrocytes in cartilage, and type X collagen is exclusively expressed by the hypertrophic chondrocytes (Fig. 2).
HIF-1α as a survival and differentiation factor in the fetal growth plate
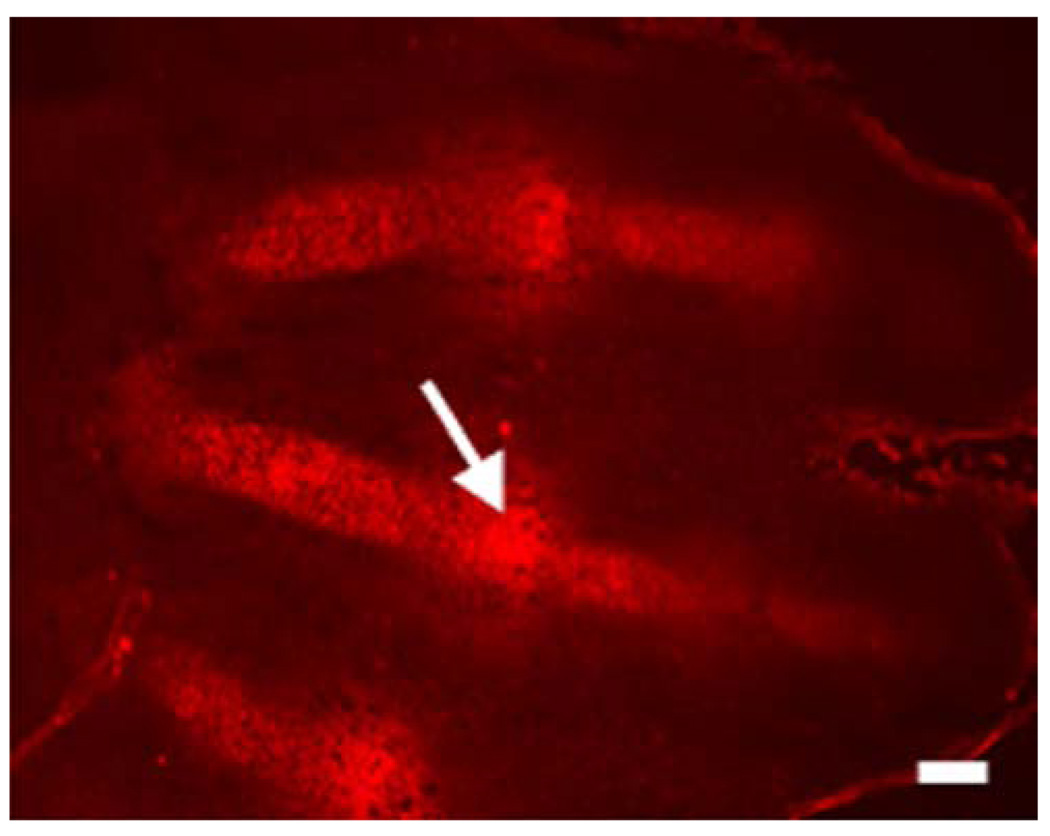
The fetal growth plate is unique among mesenchymal tissues, because it is avascular and requires an angiogenic switch for bone to replace it. Consistent with its avascularity, and differing from events observed in a postnatal setting (Shapiro et al. 1997), the fetal growth plate contains a hypoxic central region (Schipani et al. 2001). The presence and degree of hypoxia in mammalian fetal cartilage can be made evident by injecting EF5, a marker of bioreductive activity, into pregnant female mice at various gestational stages. EF5 reacts with cytoplasmic proteins in hypoxic cells, and these adducts can be detected with a specific antibody (Lord et al. 1993; Lee et al. 1996). The fetal chondrocytic growth plate has been shown to bind EF5, whereas no binding is detected in the surrounding soft tissues. The most hypoxic chondrocytes are located in the round proliferative layer near the joint space, in the center of the columnar proliferative layer, and in the upper portion of the hypertrophic zone (Fig. 3; Schipani et al. 2001). These findings document a gradient of oxygenation, from the proliferative to the hypertrophic zone and from the outer to the inner region of the fetal growth plate.
Fig. 3.
Histological section of the digital rays of an autopod of a mouse at embyonic day 13.5 (E13.5). Staining with the marker of hypoxia, EF5 (red), shows that the chondrocytes are hypoxic in the digital rays. The “interzones”, which will give rise to the perspective joints, are also highly hypoxic (white arrow). Bar 100 µm
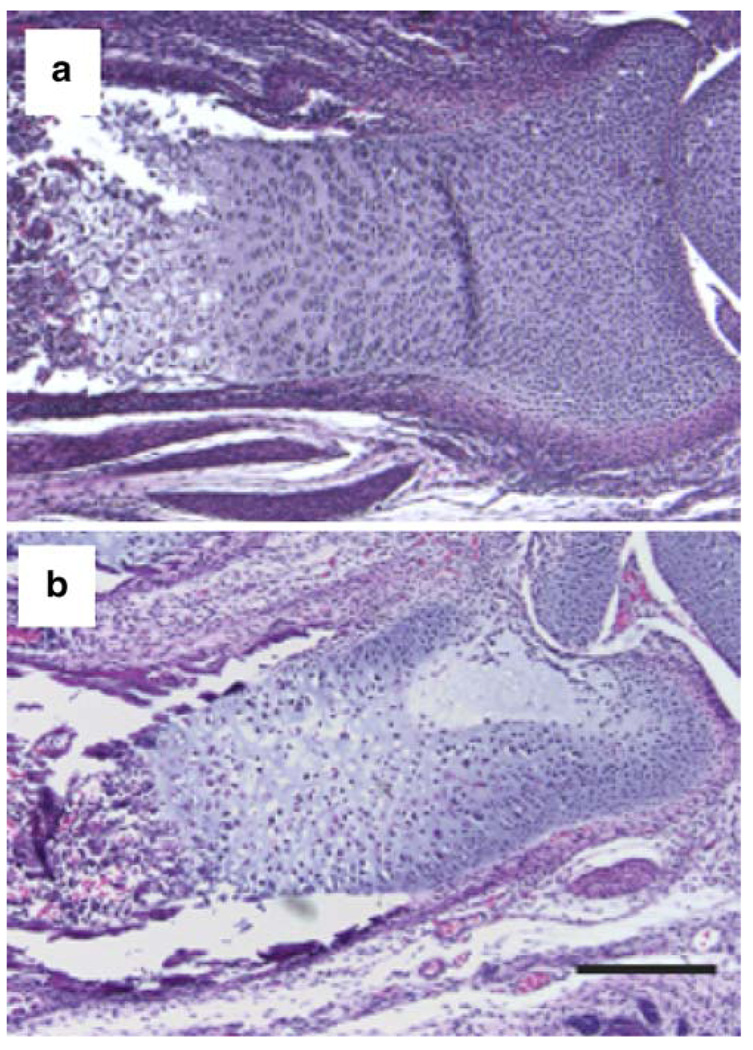
Analysis of genetically modified mice has demonstrated that HIF-1α is essential for endochondral bone development. With the aid of a Cre-loxP conditional knockout strategy in which the Cre-recombinase is driven by a fragment of the type II collagen promoter (Col2a1-Cre) and a floxed HIF-1α allele, Schipani et al. (2001) have been able to demonstrate the critical and non-redundant role of HIF-1α in endochondral bone development (Fig. 4). HIF-1α null chondrocytes (Col2a1-Cre;HIF-1αf/f) undergo massive cell death, particularly in the center of the developing growth plate, showing that HIF-1α is essential for the survival of hypoxic chondrocytes in vivo (Fig. 4; Schipani et al. 2001). The finding that the death of the cells at the center of the developing growth plate is not preceded by ectopic hypertrophy (Schipani et al. 2001) suggests that chondrocyte death secondary to the lack of HIF-1α is different at the molecular level from the chondrocyte apoptosis that precedes blood vessel invasion and the replacement of cartilage with bone.
Fig. 4.
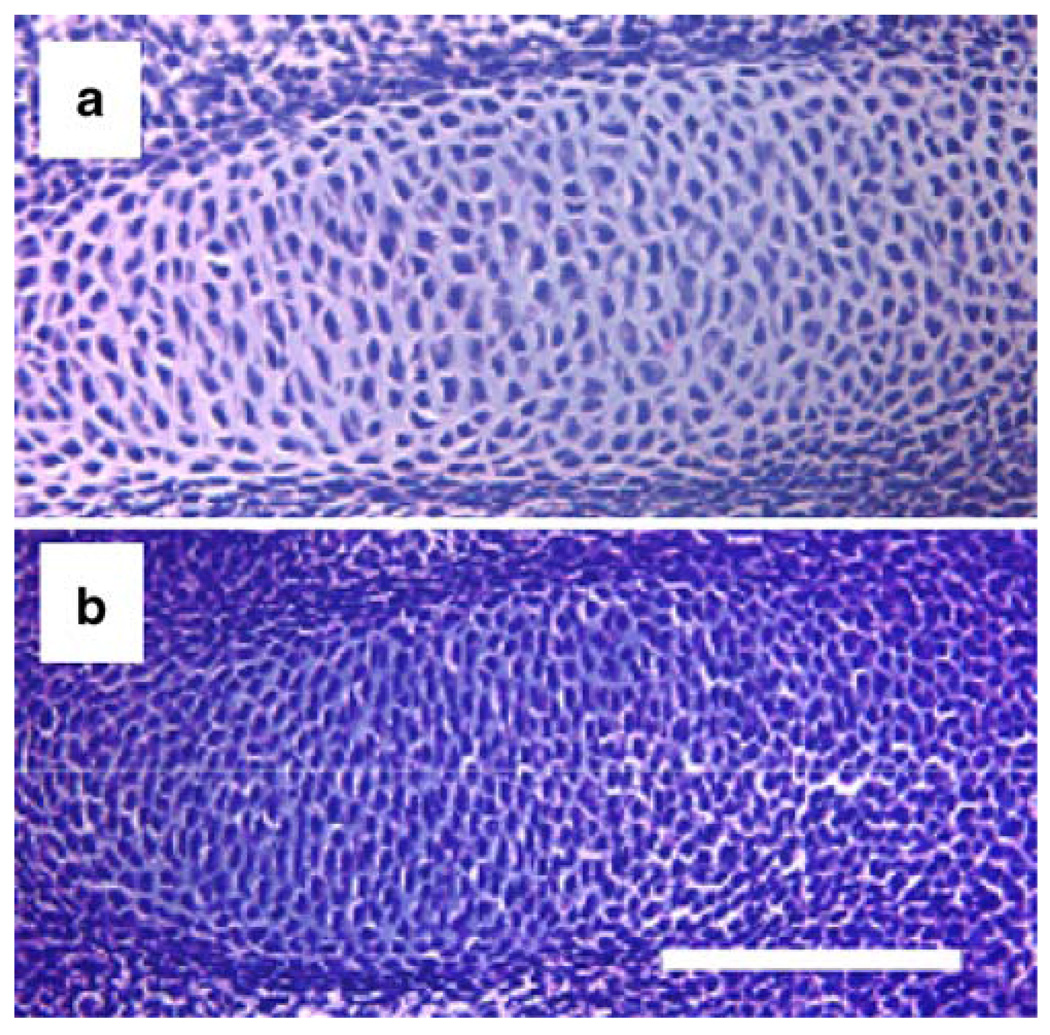
Histological sections of the distal epiphysis of newborn control (a) and Col2a1-Cre;HIF-1αf/f (b) mouse radiuses stained with hematoxylin and eosin. The mutant growth plate is severely misshapen, and its center is dramatically hypocellular as a consequence of massive central cell death. Bar 100 µm
HIF-1α increases the cartilaginous matrix and thus drives the mesenchymal cells to differentiate into chondrocytes. Embryonic mesenchymal condensations that exclude blood vessels are highly hypoxic and express HIF-1α in both the limb bud and the axial skeleton (Amarilio et al. 2007; Provot et al. 2007). Moreover, a hypoxia-inducible reporter mouse line (5XHRE-LacZ reporter) shows activation of the reporter in mesenchymal condensations (Provot et al. 2007). In a conditional knockout mouse line in which HIF-1α is inactivated in the limb bud mesenchyme (Prx1-Cre;HIF-1αf/f), a lack of HIF-1α delays the differentiation of the mesenchymal cells into chondrocytes (Fig. 5; Amarilio et al. 2007; Provot et al. 2007). Prx1 is a homeobox gene that is expressed predominantly in the mesenchyme (ten Berge et al. 1998). Prx1-Cre mice express Cre-recombinase largely in the limb bud mesenchyme, starting from embryonic day 9.5, before any condensation forms (Logan et al. 2002). Analysis of Prx1-Cre;HIF-1αf/f mice has shown that HIF-1α is not required for the formation of precartilaginous condensations but has a non-redundant and critical role in the differentiation of mesenchymal cells into chondrocytes (Fig. 5; Amarilio et al. 2007; Provot et al. 2007). Lack of HIF-1α in the limb bud mesenchyme causes a considerable delay in cartilage formation and in joint formation (Fig. 5; Robins et al. 2005; Amarilio et al. 2007; Provot et al. 2007; Xu et al. 2007). These findings demonstrate the positive role of HIF-1α in chondrocyte differentiation and establish its essential role in endochondral bone development.
Fig. 5.
Histological sections of the digital ray of E13.5 control (a) and Prx1-Cre;HIF-1αf/f (b) mouse autopods stained with hematoxylin and eosin. Differentiated cuboidal chondrocytes are present in the control and undifferentiated mesenchymal cells in the mutant autopod. Bar 100 µm
The role of hypoxia and HIF-1α in cell differentiation is tissue-specific, because HIF-1α maintains the stem cells in an undifferentiated state, inhibits the differentiation of mesenchymal cells into osteoblasts, adipocytes, and myocytes, but stimulates the differentiation of trophoblastic cells and dopaminergic neurons and chondrocytes (Morrison et al. 2000; Studer et al. 2000; Jogi et al. 2002; Yun et al. 2002, 2005; Salim et al. 2004; Cowden Dahl et al. 2005; Gustafsson et al. 2005; Lin et al. 2006; Sainson and Harris 2006; Jeong et al. 2007; Simon and Keith 2008).
HIF-1α and joint development
The HIF-1α protein and vascular endothelial growth factor-A (VEGF-A) mRNA are particularly abundant in the highly hypoxic developing joints, possibly because the avascular perichondrium surrounding them is thickened (Provot et al. 2007). Even after the joint space has formed, the articular chondrocytes are significantly more hypoxic than the rest of the cartilage (Provot et al. 2007). Since a lack of HIF-1α in the limb bud mesenchyme delays joint development without altering the thickening of the perichondrium (Amarilio et al. 2007; Provot et al. 2007), we can conclude that thickening of the perichondrium precedes joint formation and is likely to be critical for joint development.
GDF5, Wnt14, and Noggin are essential regulators of joint development (Brunet et al. 1998; Storm and Kingsley 1999; Hartmann and Tabin 2001; Kingsley 2001; Guo et al. 2004). Interestingly, microarray experiments have shown that brief exposure to 1% O2 does not induce expression of the mRNA of any of these factors in ex vivo metatarsal explants (Provot et al. 2007), indicating that they are unlikely to be direct transcriptional targets of HIF-1α. Similar results have been obtained with primary chondrocytes briefly cultured under hypoxic conditions (Provot et al. 2007). Since chondrogenesis and joint formation are tightly coupled (Kornak and Mundlos 2003), a delay in early chondrogenesis secondary to the lack of HIF-1α might impair joint formation. Because of the pronounced expression of HIF-1α in the prospective joint, however, delayed joint formation associated with the loss of HIF-1α may not be the only consequence of a delay in early chondrogenesis.
Role of post-translational modifications of collagens in mediating the survival and differentiation functions of HIF-1α in growth plate chondrocytes
A variety of mechanisms can be invoked downstream of HIF-1α in its role as a survival and differentiation factor, including the regulation of VEGF expression and the modulation of metabolic pathways and autophagy (Provot and Schipani 2007; Khatri and Schipani 2008). Recent experimental evidence nevertheless indicates that the regulation of the post-translational modification of collagens, especially that of prolyl 4-hydroxylation, could be one of the modalities by which HIF-1α influences chondrocyte survival and differentiation. Collagen P4Hs bind O2 much more efficiently than the HIF-P4Hs that trigger HIF-1α degradation (Hirsilä et al. 2003), suggesting that collagen P4Hs can still function at low O2 levels. The genes for the collagen P4H α(I) and α(II) subunits (P4HaI and P4HaII) are targets of HIF-1α-dependent hypoxia in chondrocytes and other cell types (Takahashi et al. 2000; Hofbauer et al. 2003; Grimmer et al. 2006; Provot et al. 2007). Proper extracellular matrix accumulation is not only essential for organ development, but also promotes cell differentiation and survival through specific cell-matrix interactions (Svoboda 1998; Egerbacher and Haeusler 2003). HIF-1α may thus operate as a survival and differentiation factor in chondrocytes, improving the efficiency of post-translational modifications of collagen type II and, in so doing, promoting the formation of a proper extracellular matrix. A defect in the post-translational hydroxylation of collagens leads to a decrease in extracellular matrix and an increase in under-hydroxylated collagens, which may in turn trigger an unfolded protein response (Pacifici and Iozzo 1988; Zhang and Kaufman 2006; Tsang et al. 2007) and be a cause of the delayed chondrogenesis observed in mice that lack HIF-1α in the limb bud mesenchyme. The positive effect of HIF-1α on matrix accumulation in chondrocytes is consistent with the role of hypoxia in promoting fibrosis under pathological conditions (Higgins et al. 2008).
Hypoxia and HIF-1α may also modulate matrix formation by chondrocytes by up-regulating the expression of Sox9 (Robins et al. 2005; Amarilio et al. 2007), a master regulator of chondrogenesis (Huang et al. 2000; Smits et al. 2001; Akiyama et al. 2002; Lefebvre and Smits 2005). In mouse bone marrow stromal cells in particular, hypoxia brings about an increase in the accumulation of HIF-1α and in Sox9 transcription (Robins et al. 2005). Similar findings have been reported in limb bud micromass cultures (Amarilio et al. 2007) but not in primary chondrocytes or ex vivo metatarsal explants (Provot et al. 2007). In addition to HIF-1α, HIF-2α has important roles in cartilage biology. HIF-2α, instead of HIF-1α, has been shown to be essential for hypoxia-enhanced matrix synthesis and SOX9 expression in human articular chondrocytes (Lafont et al. 2007). Furthermore, HIF-2α and HIF-1α have opposing effects in the regulation of autophagy in human and murine articular chondrocytes, HIF-2α acting as a brake on the autophagy-accelerator function of HIF-1α (Bohensky et al. 2009).
Future perspectives
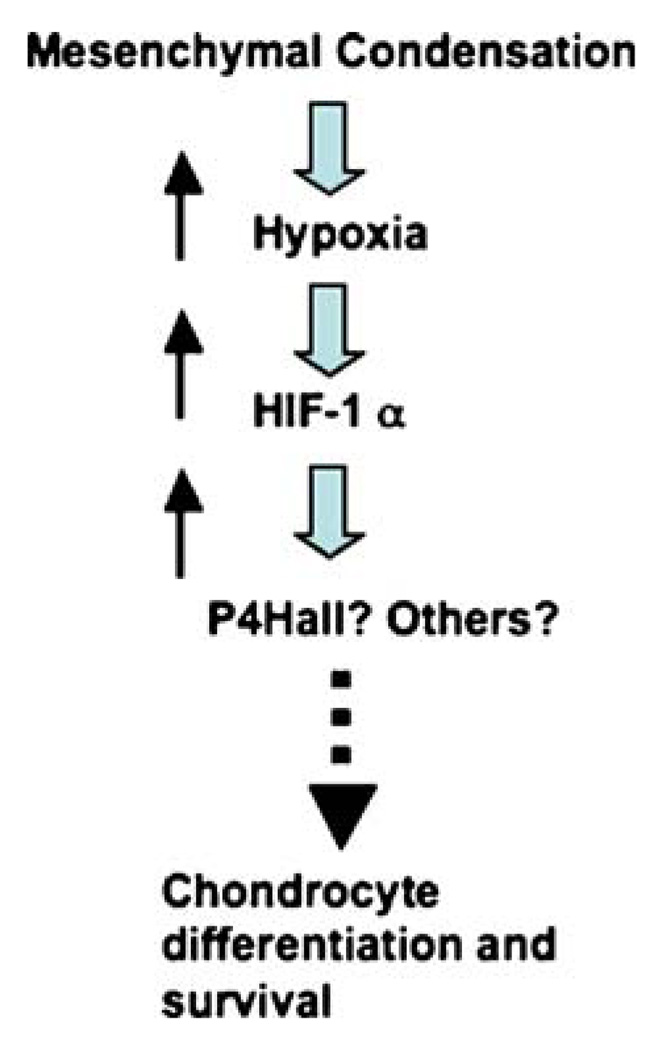
This review summarizes our current knowledge of the role of hypoxia and HIF-1α in matrix formation and remodeling and of its importance in mediating the survival and differentiation functions of HIF-1α in developing cartilage and joints. The investigation of whether the regulation of collagen P4Hs is the main mechanism adopted by this transcription factor to control matrix accumulation in the developing growth plate is now of importance (Fig. 6). Studies of the modulation of matrix accumulation by hypoxia and HIF-1α in cartilage and joints could significantly expand our understanding of both cellular adaptation to hypoxia under physiological conditions and cartilage and joint homeostasis.
Fig. 6.
Model for HIF-1α-dependent regulation of early chondrocyte differentiation and survival
Contributor Information
Johanna Myllyharju, Email: johanna.myllyharju@oulu.fi, Oulu Center for Cell Matrix Research, Biocenter Oulu and Department of Medical Biochemistry and Molecular Biology, University of Oulu, 90014 Oulu, Finland.
Ernestina Schipani, Endocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston MA 02114, USA.
References
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, deCrombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1α regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am J Respir Crit Care Med. 1998;158:1921–1928. doi: 10.1164/ajrccm.158.6.9804076. [DOI] [PubMed] [Google Scholar]
- tenBerge D, Brouwer A, Korving J, Martin JF, Meijlink F. Prx1 and Prx2 in skeletogenesis: roles in the craniofacial region, inner ear and limbs. Development. 1998;125:3831–3842. doi: 10.1242/dev.125.19.3831. [DOI] [PubMed] [Google Scholar]
- Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, Srinivas V. Regulation of autophagy in human and murine cartilage. Hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009;60:1406–1415. doi: 10.1002/art.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl 4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Simon MC. Hypoxia-inducible factor: roles in development, physiology, and disease. Cell Death Differ. 2008;15:619–620. doi: 10.1038/cdd.2008.11. [DOI] [PubMed] [Google Scholar]
- Chi J-T, Wang Z, Nuyten DSA, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland Å, Børresen-Dale A-L, Giaccia A, Longaker MT, Hastie T, Yang GP, vandeVijver MJ, Brown PO. Gene expression programs in response to hypoxia. PLOS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdury R, Hardy A, Schofield CJ. The human oxygen sensing machinery and its manipulation. Chem Soc Rev. 2008;37:1308–1319. doi: 10.1039/b701676j. [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet P, Simon MC. Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, Giaccia AJ. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- Durmowicz AG, Parks WC, Hyde DM, Mecham RP, Stenmark KR. Persistence, re-expression, and induction of pulmonary arterial fibronectin, tropoelastin, and type I procollagen mRNA expression in neonatal hypoxic pulmonary hypertension. Am J Pathol. 1994;145:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- Egerbacher M, Haeusler G. Integrins in growth plate cartilage. Pediatr Endocrinol Rev. 2003;1:2–8. [PubMed] [Google Scholar]
- Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition. The role of HIF-1α, HIF-2α, and other pathways. J Biol Chem. 2006;281:15215–15226. doi: 10.1074/jbc.M511408200. [DOI] [PubMed] [Google Scholar]
- Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian Y-M, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le Q-T, Chi J-TA, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le Q-T, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fähling M, Perlewitz A, Doller A, Thiele B-J. Regulation of collagen prolyl 4-hydroxylase and matrix metalloproteinases in fibrosarcoma cells by hypoxia. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:119–126. doi: 10.1016/j.cca.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Fähling M, Mrowka R, Steege A, Nebrich G, Perlewitz A, Persson PB, Thiele B-J. Translational control of collagen prolyl 4-hydroxylase-α (I) gene expression under hypoxia. J Biol Chem. 2006;281:26089–26101. doi: 10.1074/jbc.M604939200. [DOI] [PubMed] [Google Scholar]
- Falanga V, Martin TA, Takagi H, Kirsner RS, Helfman T, Pardes J, Ochoa MS. Low oxygen tension increases mRNA levels of α1(I) procollagen in human dermal fibroblasts. J Cell Physiol. 1993;157:408–412. doi: 10.1002/jcp.1041570225. [DOI] [PubMed] [Google Scholar]
- Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Grimmer C, Balbus N, Lang U, Aigner T, Cramer T, Müller L, Swoboda B, Pfander D. Regulation of type II collagen synthesis during osteoarthritis by prolyl-4-hydroxylases: possible influence of low oxygen tension. Am J Pathol. 2006;169:491–502. doi: 10.2353/ajpath.2006.050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M, Hu C-J, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of HIF-2α results in anemia. Proc Natl Acad Sci USA. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/β-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Heikkinen J, Risteli M, Wang C, Latvala J, Rossi M, Valtavaara M, Myllylä R. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J Biol Chem. 2000;275:36158–36163. doi: 10.1074/jbc.M006203200. [DOI] [PubMed] [Google Scholar]
- Hewitson KS, McNeill LA, Riordan MV, Tian Y-M, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, Ratcliffe PJ, Pugh CW, Schofield CJ. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of CTGF is directly mediated by HIF-1. Am J Physiol Renal Physiol. 2004;287:F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsilä M, Koivunen P, Günzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- Hofbauer K-H, Gess B, Lohaus C, Meyer HE, Katschinski D, Kurtz A. Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur J Biochem. 2003;270:4515–4522. doi: 10.1046/j.1432-1033.2003.03846.x. [DOI] [PubMed] [Google Scholar]
- Holster T, Pakkanen O, Soininen R, Sormunen R, Nokelainen M, Kivirikko KI, Myllyharju J. Loss of assembly of the main basement membrane collagen, type IV, but not fibril-forming collagens and embryonic death in collagen prolyl 4-hydroxylase I null mice. J Biol Chem. 2007;282:2512–2519. doi: 10.1074/jbc.M606608200. [DOI] [PubMed] [Google Scholar]
- Horino Y, Takahashi S, Miura T, Takahashi Y. Prolonged hypoxia accelerates the posttranscriptional process of collagen synthesis in cultured fibroblasts. Life Sci. 2002;71:3031–3045. doi: 10.1016/s0024-3205(02)02142-2. [DOI] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Hu C-J, Wang L-Y, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhou X, Lefebvre V, deCrombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9′s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lanie WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Ivan M, Haberberger T, Gervasi DC, Michelson KS, Günzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG., Jr Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AV, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jeong C-H, Lee H-J, Cha J-H, Kim JH, Kim KR, Kim J-H, Yoon D-K, Kim K-W. Hypoxia-inducible factor-1α inhibits selfrenewal of mouse embryonic stem cells in vitro via negative regulation of the leukemia inhibitory factor-STAT3 pathway. J Biol Chem. 2007;282:13672–13679. doi: 10.1074/jbc.M700534200. [DOI] [PubMed] [Google Scholar]
- Jögi A, Øra I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Påhlman S. Hypoxia alters gene expression in human neuroblastoma cells towards an immature and neural crest-like phenotype. Proc Natl Acad Sci USA. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Karsenty G. The complexities of skeletal biology. Nature. 2003;423:316–318. doi: 10.1038/nature01654. [DOI] [PubMed] [Google Scholar]
- Khatri R, Schipani E. About the importance of being desulfated. Genes Dev. 2008;22:2750–2754. doi: 10.1101/gad.1735508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T, Roth U, Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxiainducible factor-1 in rat hepatocytes. Blood. 1999;94:4177–4185. [PubMed] [Google Scholar]
- Kingsley DM. Genetic control of bone and joint formation. Novartis Found Symp. 2001;232:213–222. doi: 10.1002/0470846658.ch15. [DOI] [PubMed] [Google Scholar]
- Kivirikko KI, Pihlajaniemi T. Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv Enzymol Relat Areas Mol Biol. 1998;72:325–398. doi: 10.1002/9780470123188.ch9. [DOI] [PubMed] [Google Scholar]
- Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, Giaccia AJ. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–887. [PubMed] [Google Scholar]
- Kornak U, Mundlos S. Genetic disorders of the skeleton: a developmental approach. Am J Hum Genet. 2003;73:447–474. doi: 10.1086/377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkola L, Hieta R, Kivirikko KI, Myllyharju J. Identification and characterization of a third human, rat, and mouse collagen prolyl 4-hydroxylase isoenzyme. J Biol Chem. 2003;278:47685–47693. doi: 10.1074/jbc.M306806200. [DOI] [PubMed] [Google Scholar]
- Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2α is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain—a hypoxic switch. Science. 2002a;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002b;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Siemann DW, Koch CJ, Lord EM. Direct relationship between radiobiological hypoxia in tumors and monoclonal antibody detection of EF5 cellular adducts. Int J Cancer. 1996;67:372–378. doi: 10.1002/(SICI)1097-0215(19960729)67:3<372::AID-IJC11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res Part C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Lin Q, Lee Y-J, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olsen EN, Tabin CJ. Expression of Cre recombinase in the developing mouse limb bud driven by a Prx1 enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- Lord EM, Harwell L, Koch CJ. Detection of hypoxic cells by monoclonal antibody recognizing 2-nitroimidazole adducts. Cancer Res. 1993;53:5721–5726. [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyharju J. Intracellular post-translational modifications of collagens. In: Brinckmann J, Müller PK, Nothbom H, editors. Topics in current chemistry. vol 247. Heidelberg: Springer; 2005. pp. 115–147. Collagen, primer in structure, processing and assembly. [Google Scholar]
- Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med. 2008;40:402–417. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibro-genesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- Olsen BR. Role of cartilage collagens in formation of the skeleton. Ann N Y Acad Sci. 1996;785:124–130. doi: 10.1111/j.1749-6632.1996.tb56250.x. [DOI] [PubMed] [Google Scholar]
- Ostadal B, Kolar F, Pelouch V, Widimsky J. Ontogenetic differences in cardiopulmonary adaptation to chronic hypoxia. Physiol Res. 1995;44:45–51. [PubMed] [Google Scholar]
- Pacifici M, Iozzo RV. Remodeling of the rough endoplasmic reticulum during stimulation of procollagen secretion by ascorbic acid in cultured chondrocytes. A biochemical and morphological study. J Biol Chem. 1988;263:2483–2492. [PubMed] [Google Scholar]
- Payne SL, Hendrix MJC, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- Perhonen M, Wang W, Han X, Ruskoaho H, Takala TE. Righ ventricular collagen type III and IV gene expression increases during early phases of endurance training in hypobaric hypoxic condition. Basic Res Cardiol. 1997;92:299–309. doi: 10.1007/BF00788942. [DOI] [PubMed] [Google Scholar]
- Pollard PJ, Loenarz C, Mole DR, McDonough MA, Gleadle JM, Schofield CJ, Ratcliffe PJ. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1α. Biochem J. 2008;416:387–394. doi: 10.1042/BJ20081238. [DOI] [PubMed] [Google Scholar]
- Provot S, Schipani E. Fetal growth plate: a developmental model of cellular adaptation to hypoxia. Ann N Y Acad Sci. 2007;1117:26–39. doi: 10.1196/annals.1402.076. [DOI] [PubMed] [Google Scholar]
- Provot S, Zinyk D, Gunes Y, Khatri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E. HIF-1α regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2006;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautavuoma K, Takaluoma K, Passoja K, Pirskanen A, Kvist AP, Kivirikko KI, Myllyharju J. Characterization of three fragments that constitute the monomers of the human lysyl hydroxylase isoenzymes 1–3. The 30-kDa N-terminal fragment is not required for lysyl hydroxylase activity. J Biol Chem. 2002;277:23084–23091. doi: 10.1074/jbc.M112077200. [DOI] [PubMed] [Google Scholar]
- Rautavuoma K, Takaluoma K, Sormunen R, Myllyharju J, Kivirikko KI, Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc Natl Acad Sci USA. 2004;101:14120–14125. doi: 10.1073/pnas.0404966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen H, Sipilä L, Vapola M, Sormunen R, Salo AM, Uitto L, Mercer DK, Robins SP, Risteli M, Aszodi A, Fässler R, Myllylä R. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. J Cell Sci. 2006;119:625–635. doi: 10.1242/jcs.02780. [DOI] [PubMed] [Google Scholar]
- Sainson RC, Harris AL. Hypoxia-regulated differentiation: let's step it up a Notch. Trends Mol Med. 2006;12:141–143. doi: 10.1016/j.molmed.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–40016. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- Schipani E. Hypoxia and HIF-1α in chondrogenesis. Semin Cell Dev Biol. 2005;16:539–546. doi: 10.1016/j.semcdb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Schwartz NB, Domowicz M. Chondrodysplasias due to proteoglycan defects. Glycobiology. 2002;12:57R–68R. doi: 10.1093/glycob/12.4.57r. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Nejfelt MK, Chi SM, Antonarkos SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro IM, Mansfield KD, Evans SM, Lord EM, Koch CJ. Chondrocytes in the endochondral growth cartilage are not hypoxic. Am J Physiol. 1997;272:C1134–C1143. doi: 10.1152/ajpcell.1997.272.4.C1134. [DOI] [PubMed] [Google Scholar]
- Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot AJvander, Zuurmond AM, Bardoel AF, Wijmenga C, Pruijs HE, Sillence DO, Brinckmann J, Abraham DJ, Black CM, Verzijl N, DeGroot J, Hanemaaijer R, TeKoppele JM, Huizinga TWJ, Bank RA. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- Slot AJvander, Zuurmond AM, vandenBogaerdt AJ, Ulrich MMW, Middelkoop E, Boers W, Ronday HK, DeGroot J, Huizinga TWJ, Bank RA. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004;23:251–257. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–334. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, Crombrugghe Bde, Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999;209:11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KK. Chondrocyte-matrix attachment complexes mediate survival and differentiation. Microsc Res Tech. 1998;43:111–122. doi: 10.1002/(SICI)1097-0029(19981015)43:2<111::AID-JEMT4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tajima R, Kawaguchi N, Horino Y, Takahashi Y, Toriyama K, Inou K, Torii S, Kitagawa Y. Hypoxic enhancement of type IV collagen secretion accelerates adipose conversion of 3T3-L1 fibroblasts. Biochim Biophys Acta. 2001;1540:179–187. doi: 10.1016/s0167-4889(01)00114-8. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Takahashi S, Shiga Y, Yoshimi T, Miura T. Hypoxic induction of prolyl 4-hydroxylase α (I) in cultured cells. J Biol Chem. 2000;275:14139–14146. doi: 10.1074/jbc.275.19.14139. [DOI] [PubMed] [Google Scholar]
- Takaluoma K, Hyry M, Lantto J, Sormunen R, Bank RA, Kivirikko KI, Myllyharju J, Soininen R. Tissue-specific changes in the hydroxylysine content and cross-links of collagens and alterations in fibril morphology in lysyl hydroxylase 1 knockout mice. J Biol Chem. 2007a;282:6588–6596. doi: 10.1074/jbc.M608830200. [DOI] [PubMed] [Google Scholar]
- Takaluoma K, Lantto J, Myllyharju J. Lysyl hydroxylase 2 is a specific telopeptide hydroxylase, while all three isoenzymes hydroxylate collagenous sequences. Matrix Biol. 2007b;26:396–403. doi: 10.1016/j.matbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Tamamori M, Ito H, Hiroe M, Marumo F, Hata RI. Stimulation of collagen synthesis in rat cardiac fibroblasts by exposure to hypoxic culture conditions and suppression of the effect by natriuretic peptides. Cell Biol Int. 1997;21:175–180. doi: 10.1006/cbir.1997.0130. [DOI] [PubMed] [Google Scholar]
- Tsang KY, Chan D, Cheslett D, Chan WC, So CL, Melhado IG, Chan TW, Kwan KM, Hunziker EB, Yamada Y, Bateman JF, Cheung KM, Cheah KS. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol. 2007;5:e44. doi: 10.1371/journal.pbio.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Luosujärvi H, Heikkinen J, Risteli M, Uitto L, Myllylä R. The third activity for lysyl hydroxylase 3: galactosylation of hydroxylysyl residues in collagens in vitro. Matrix Biol. 2002;21:559–566. doi: 10.1016/s0945-053x(02)00071-9. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia inducible factor is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1α and hypoxia-inducible factor-2α in HEK293T cells. Cancer Res. 2005;65:3299–3306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1α and HIF-2α (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2α target gene in Hep3B and Kelly cells. FASEB J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- Xu Y, Malladi P, Chiou M, Bekerman E, Giaccia AJ, Longaker MT. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- Yu F, White SB, Zhao Q, Lee FS. HIF-1α is regulated by stimulus sensitive proline hydroxylation. Proc Natl Acad Sci USA. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005;25:3040–3055. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol. 2006;172:69–91. doi: 10.1007/3-540-29717-0_3. [DOI] [PubMed] [Google Scholar]