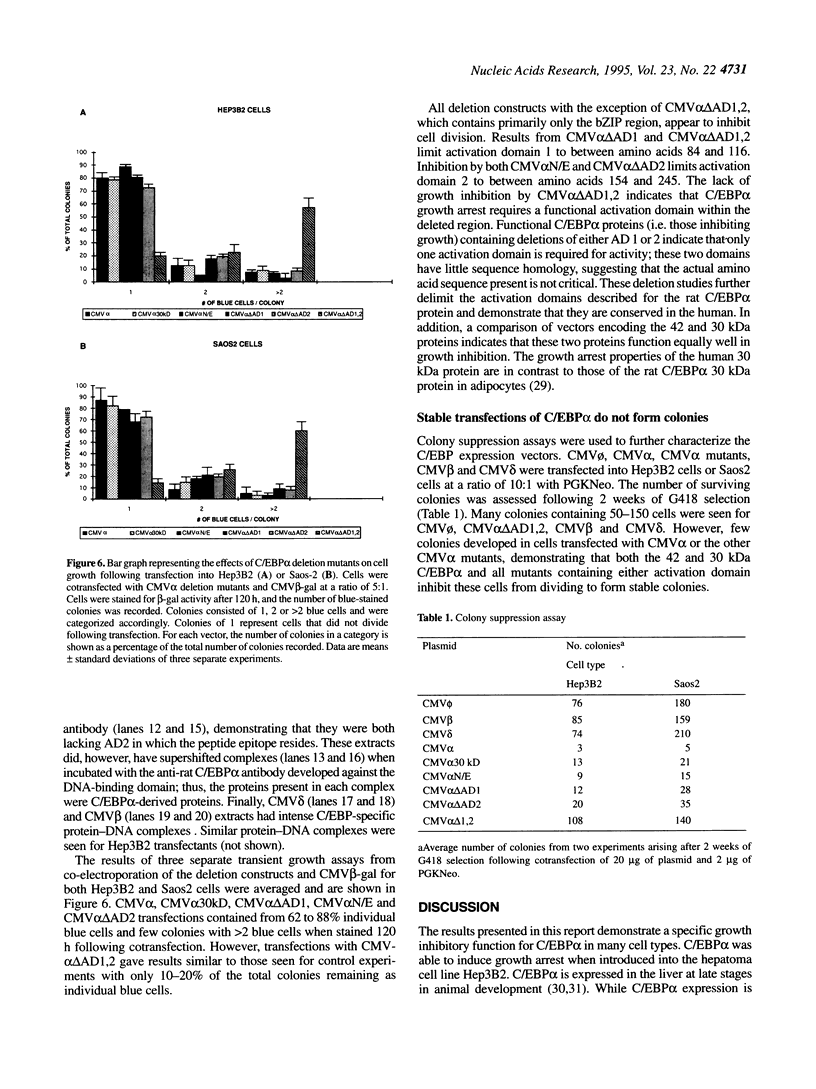
Abstract
The transcription factor CCAAT/enhancer binding protein (C/EBP alpha) is expressed predominantly in differentiated tissues and is able to induce growth arrest and differentiation in preadipocytes. C/EBP alpha expression is high in non-dividing hepatocytes, but decreases during liver regeneration. These observations suggest that C/EBP alpha is inversely related to cell proliferation. To investigate the mechanism of growth inhibition by C/EBP alpha, the response of immortal human cells to cotransfection of a C/EBP alpha expression vector (CMV alpha) and a CMV beta-galactosidase expression vector was examined. Hep3B2, a hepatoma; Saos2, an osteosarcoma deficient for p53 and Rb; and 639, a fibroblast expressing SV40 T-antigen, were examined. Transiently transfected cells were stained for beta-gal activity to monitor their ability to undergo division. The ability of stable transformants to form colonies was also assessed for each cell line. Cells transfected with CMV alpha remained as non-dividing cells while control cells divided to form colonies. Mutations of the C/EBP alpha sequence demonstrated that only a small, previously uncharacterized activation domain was required for antimitotic activity. Our results suggest that C/EBP alpha may play a role in maintaining the quiescent state of hepatocytes and other cells. Furthermore, it appears that the effects of C/EBP alpha are not mediated through p53 or Rb and are not altered by T-antigen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam T., An M. R., Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992 Mar 15;267(8):5021–5024. [PubMed] [Google Scholar]
- Baumann H. Hepatic acute phase reaction in vivo and in vitro. In Vitro Cell Dev Biol. 1989 Feb;25(2):115–126. doi: 10.1007/BF02626167. [DOI] [PubMed] [Google Scholar]
- Baumann H., Morella K. K., Jahreis G. P., Marinković S. Distinct regulation of the interleukin-1 and interleukin-6 response elements of the rat haptoglobin gene in rat and human hepatoma cells. Mol Cell Biol. 1990 Nov;10(11):5967–5976. doi: 10.1128/mcb.10.11.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Buck M., Turler H., Chojkier M. LAP (NF-IL-6), a tissue-specific transcriptional activator, is an inhibitor of hepatoma cell proliferation. EMBO J. 1994 Feb 15;13(4):851–860. doi: 10.1002/j.1460-2075.1994.tb06328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chang C. J., Chen T. T., Lei H. Y., Chen D. S., Lee S. C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990 Dec;10(12):6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Yang V. W., Ntambi J. M., Geiman D. E., Landschulz W. H., Friedman A. D., Nakabeppu Y., Kelly T. J., Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989 Sep;3(9):1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Xanthopoulos K. G., Darnell J. E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, alpha 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzi M., Fleming T. P., Lassar A. B., Weintraub H., Aaronson S. A. MyoD induces growth arrest independent of differentiation in normal and transformed cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8442–8446. doi: 10.1073/pnas.87.21.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Revel M., Kelly J. H. Response of liver genes to acute phase mediators. Ann N Y Acad Sci. 1989;557:310–316. doi: 10.1111/j.1749-6632.1989.tb24023.x. [DOI] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Fey G. H., Gauldie J. The acute phase response of the liver in inflammation. Prog Liver Dis. 1990;9:89–116. [PubMed] [Google Scholar]
- Freytag S. O., Geddes T. J. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science. 1992 Apr 17;256(5055):379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Friedman A. D., McKnight S. L. Identification of two polypeptide segments of CCAAT/enhancer-binding protein required for transcriptional activation of the serum albumin gene. Genes Dev. 1990 Aug;4(8):1416–1426. doi: 10.1101/gad.4.8.1416. [DOI] [PubMed] [Google Scholar]
- Goldberger G., Bing D. H., Sipe J. D., Rits M., Colten H. R. Transcriptional regulation of genes encoding the acute-phase proteins CRP, SAA, and C3. J Immunol. 1987 Jun 1;138(11):3967–3971. [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Herrera R., Ro H. S., Robinson G. S., Xanthopoulos K. G., Spiegelman B. M. A direct role for C/EBP and the AP-I-binding site in gene expression linked to adipocyte differentiation. Mol Cell Biol. 1989 Dec;9(12):5331–5339. doi: 10.1128/mcb.9.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Sugita T., Nishio Y., Hashimoto S., Pawlowski T., Suematsu S., Kishimoto T. Reciprocal expression of NF-IL6 and C/EBP in hepatocytes: possible involvement of NF-IL6 in acute phase protein gene expression. New Biol. 1991 Jan;3(1):63–70. [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Juan T. S., Wilson D. R., Wilde M. D., Darlington G. J. Participation of the transcription factor C/EBP delta in the acute-phase regulation of the human gene for complement component C3. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2584–2588. doi: 10.1073/pnas.90.7.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., Lane M. D. Mouse insulin-responsive glucose transporter gene: characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1990 Jan;87(1):251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S., Akira S., Kishimoto T. A member of the C/EBP family, NF-IL6 beta, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1473–1476. doi: 10.1073/pnas.89.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiss S., Quaiser A., Oren M., Montenarh M. Oligomerization of oncoprotein p53. J Virol. 1988 Dec;62(12):4737–4744. doi: 10.1128/jvi.62.12.4737-4744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989 Mar 31;243(4899):1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- Li X. X., Huang J. H., Rienhoff H. Y., Jr, Liao W. S. Two adjacent C/EBP-binding sequences that participate in the cell-specific expression of the mouse serum amyloid A3 gene. Mol Cell Biol. 1990 Dec;10(12):6624–6631. doi: 10.1128/mcb.10.12.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. T., Lane M. D. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992 Apr;6(4):533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Lin F. T., MacDougald O. A., Diehl A. M., Lane M. D. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein alpha message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Mischoulon D., Rana B., Bucher N. L., Farmer S. R. Growth-dependent inhibition of CCAAT enhancer-binding protein (C/EBP alpha) gene expression during hepatocyte proliferation in the regenerating liver and in culture. Mol Cell Biol. 1992 Jun;12(6):2553–2560. doi: 10.1128/mcb.12.6.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. R., Maire P., Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990 Apr 20;61(2):279–291. doi: 10.1016/0092-8674(90)90808-r. [DOI] [PubMed] [Google Scholar]
- Nerlov C., Ziff E. B. Three levels of functional interaction determine the activity of CCAAT/enhancer binding protein-alpha on the serum albumin promoter. Genes Dev. 1994 Feb 1;8(3):350–362. doi: 10.1101/gad.8.3.350. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Buhrow S. A., Kaestner K. H., Christy R. J., Sibley E., Kelly T. J., Jr, Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1988 Nov 25;263(33):17291–17300. [PubMed] [Google Scholar]
- Ossipow V., Descombes P., Schibler U. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8219–8223. doi: 10.1073/pnas.90.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V., Mancini F. P., Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990 Nov 2;63(3):643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- Roman C., Platero J. S., Shuman J., Calame K. Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is similar to C/EBP and heterodimerizes with C/EBP. Genes Dev. 1990 Aug;4(8):1404–1415. doi: 10.1101/gad.4.8.1404. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Xanthopoulos K. G., Mirkovitch J., Decker T., Kuo C. F., Darnell J. E., Jr Cell-specific transcriptional control of the mouse DNA-binding protein mC/EBP. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4117–4121. doi: 10.1073/pnas.86.11.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti G. P., Levine A. J. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993 Jul;7(10):855–865. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S. J., van Laar T., Kast W. M., Melief C. J., Zantema A., van der Eb A. J. Association between the cellular p53 and the adenovirus 5 E1B-55kd proteins reduces the oncogenicity of Ad-transformed cells. EMBO J. 1990 Aug;9(8):2621–2629. doi: 10.1002/j.1460-2075.1990.tb07444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]