Abstract
Gurfinkel and colleagues (2006) recently found that healthy adults dynamically modulate postural muscle tone in the body axis during anti-gravity postural maintenance and that this modulation is inversely correlated with axial stiffness. Our objective in the present study was to investigate whether dynamic modulation of axial postural tone can change through training. We examined whether teachers of the Alexander Technique (AT), who undergo “long-term” (3-year) training, have greater modulation of axial postural tone than matched control subjects. In addition, we performed a longitudinal study on the effect of “short-term” (10-week) AT training on the axial postural tone of individuals with low back pain (LBP), since short term AT training has previously been shown to reduce LBP. Axial postural tone was quantified by measuring the resistance of the neck, trunk and hips to small (±10°), slow (1°/s) torsional rotation during stance. Modulation of tone was determined by the torsional resistance to rotation (peak-to-peak, phase-advance, and variability of torque) and axial muscle activity (EMG). Peak-to-peak torque was lower (~50%), while phase-advance and cycle-to-cycle variability were enhanced for AT teachers compared to matched control subjects at all levels of the axis. In addition, LBP subjects decreased trunk and hip stiffness following short-term AT training compared to a control intervention. While changes in static levels of postural tone may have contributed to the reduced stiffness observed with the AT, our results suggest that dynamic modulation of postural tone can be enhanced through long-term training in the AT, which may constitute an important direction for therapeutic intervention.
Keywords: Muscle Tone, Posture, Alexander Technique, Low Back Pain, Motor Processes
1. Introduction
In the absence of external support, tonic activation of skeletal muscles is necessary to maintain the relative positions of body segments and to prevent the body from collapsing against gravity. Such ongoing subconscious muscular activity is referred to as “postural tone.” Tonic muscular activity is assessed clinically as the resistance to passive joint rotation, typically in the limbs (Foster, 1892). However, because the clinician commonly supports the limb being examined, resistance to joint rotation does not explicitly reflect the state of postural tone, as skeletal muscles must be engaged in anti-gravity postural support for postural tone to manifest.
While postural tone consists, in part, of low-level stable activity—typically a few percent of maximal voluntary contraction (Berardelli, Sabra, & Hallett, 1983; Gurfinkel, et al., 2006; Masani, et al., 2009)—this baseline activity can be modulated dynamically to adapt to changes in joint position and load. Postural tone can be modulated in 2 different ways: 1) resistive, in which the activity of stretched muscles increases via the tonic stretch reflex (Sherrington & Liddell, 1924), and 2) plastic. Plastic tone modulation consists of yielding to movement via the lengthening reaction, in which the activity of stretched muscles decreases, and assisting movement via the shortening reaction in which the activity of shortening muscles increases (Sherrington, 1909, 1915).
Postural tone may appear to be rigidly and stably controlled, but tonic activity must be modulated dynamically for movement to be coordinated. Any time one part of the body moves, postural tone in both that and other parts of the body must be modulated to prevent resisting the movement and to maintain static equilibrium. Thus, modulation of postural tone can provide the body with both mechanical and operational flexibility for different types of movements.
While many studies have examined tonic responses to stretch by applying low-frequency rotations to an isolated joint while the subject is relaxed or voluntarily maintaining a specified level of muscle activity (e.g. Burne, Carleton, & O’Dwyer, 2005; Cathers, O’Dwyer, & Neilson, 2004; Katz & Rondot, 1978; Woolacott & Burne, 2006; Xia & Rymer, 2004; Zhang & Rymer, 1997), few studies have examined how tonic activity is modulated while an individual maintains the body in an anti-gravity posture. Postural tone is highly sensitive to the individual’s state (Hultborn, 2001), e.g., the level of background activity (Cathers, et al., 2004; Zhang & Rymer, 1997) or presence of “reinforcement” (Andrews, Neilson, & Lance, 1973; Mark, 1963; Walsh, 1992), and, thus, the modulation of postural tone might differ during active postural maintenance.
Gurfinkel et al. (Gurfinkel, et al., 2006) quantified tonic reactions of healthy, unsupported, standing subjects to very slow and small torsional rotations of the body axis, where sustained tonic activity is necessary to stabilize the spine and support the body against gravity (Lucas & Bresler, 1960). This study showed that postural tone is dynamically modulated by lengthening and shortening reactions (Gurfinkel, et al., 2006). The extent of this modulation differed markedly across these healthy individuals and was inversely correlated with torsional stiffness—subjects with a higher level of modulation had lower axial stiffness.
Over a long timescale, postural tone must undergo changes, for instance during pregnancy or physical growth. Long-term changes in tone in healthy individuals are typically presumed to result from alterations to the “static” baseline level of tonic activity. However, it is also possible that long-term changes occur to its dynamic modulation. The extent to which the natural plastic modulation of postural tone observed by Gurfinkel and colleagues can be changed through learning is not known. We hypothesized that long-term changes in the dynamic modulation of postural tone can be achieved through training in healthy adults.
One intervention that may enhance the dynamic modulation of postural tone is the Alexander Technique (AT), which is a method for consciously altering habitual postural behavior (Alexander, 1923). With the AT, the teacher guides the subject and instructs verbally so as to alter their positional and tensional patterns, in particular to achieve elongation along the spine during posture and throughout movement. Relevant to the present study, the AT distinguishes between “fixed” and “dynamic” qualities of muscular tension and aims to achieve the latter through training (de Alcantara, 1996; Jones, 1976). Because the AT principally addresses postural tension along the body axis (head, neck and back), its influence on postural tone can be assessed with our protocol (Gurfinkel, et al., 2006).
In the study reported here, we examined the effects of the AT on postural tone by measuring the torque necessary to torsionally rotate axial segments of standing subjects over a short distance, at a very slow speed (Gurfinkel, et al., 2006). To examine the effects of long-term (3-year) training, we compared responses of AT teachers to healthy control subjects. Because our measure of resistance reflects both tonic baseline activity as well as its dynamic modulation, we used torque resistance, variability, phase-advance and electromyography to identify dynamic modulation. We also examined whether short-term (10-week) training in the AT alters axial tone in subjects with low back pain (LBP), as changes in axial tone could underlie the substantial reduction in back pain reported with this intervention (Little, et al., 2008).
2. Methods
2.1 Protocols
This study comprised two protocols: Protocol 1 quantified “long-term” changes in postural tone by comparing subjects proficient in the AT (AT teachers) with matched healthy control subjects; Protocol 2 longitudinally studied the effect of “short-term” AT training on subjects with idiopathic LBP.
2.2 Subjects
A total of 37 subjects between the ages of 21–60 were enrolled into the study. Each subject provided informed consent following procedures approved by the Oregon Health & Science University Institutional Review Board.
2.2.1 AT teachers
Fourteen AT teachers (4 male, 10 female), who had completed training programs certified by the American Society for the Alexander Technique (AmSAT) and its international affiliates, were recruited for this study from a locally held, national AT symposium. AT teachers were selected because they undergo extensive training (80% of the 3-year 1600 hour training is devoted to practical proficiency in the AT). All teachers were free of musculoskeletal pain at the time of testing. The majority of female subjects reflected the gender bias of AT teachers. AT teachers had a mean age of 41.6±8.4 years, height of 170.3±6.3 cm, and weight of 69.4±11.0 kg. All AT teachers underwent axial torque measurement. Three teachers who were able to participate in longer testing sessions also underwent EMG measurement.
2.2.2 Control subjects
Fifteen healthy control subjects (4 male, 11 female) with no history of musculoskeletal pain were recruited to match the population of AT teachers. The mean age, height and weight of this group was not significantly different from the AT teacher sample population: 38.5± 11.1 years (F(1,27)=0.711, p = 0.41), 166.4 ± 6.0 cm (F(1,27)=2.950, p = 0.10) and 68.1± 10 kg (F(1,27)=0.100, p = 0.75).
2.2.3 Low-Back Pain subjects
Eight LBP subjects (3 male, 5 female) were recruited for this study. All subjects with LBP were examined by a physical therapist to ensure they met the following inclusion criteria: 1) episodes of LBP for longer than 6 months; 2) a score on the Oswestry Disability Questionnaire (Fairbanks, Daview, Mbaot, & O’Brien, 1980) of at least 5%; 3) no previous back pain related surgery; 4) no pain radiating below the knee; 5) normal lower body sensation, strength, and reflexes; 6) no increased pain with 15° of head or trunk rotation in both directions. The LBP subjects had a mean age of 34.4 ± 8.7 years, height of 171.9±7.7 cm, weight of 75.5 ± 11.0 kg and Oswestry score of 11.8±5.9%.
2.3 Axial rotation apparatus
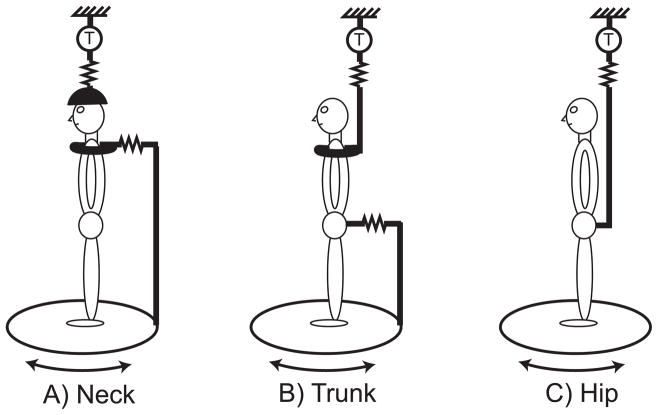
Axial and proximal postural tone were quantified by measuring the force required to slowly twist an axial body segment (i.e., measured stress to imposed strain). This method has been detailed previously (Gurfinkel, et al., 2006) and generates reproducible measurements that primarily reflect myogenic forces, rather than resistance from passive osteo-ligamentous structures. Subjects stood blindfolded on a horizontal platform that rotated about a vertical axis approximately aligned with the spine. The subject’s pelvis, shoulders, or head was attached with a harness or helmet to an external rigid steel frame while the hips or shoulders were attached to a platform under the subject’s feet. This platform rotated so as to twist the body axis at the level of the trunk, neck or hips (Fig. 1). From the neutral position (i.e., whole body facing forward), the platform was rotated ±10° at 1 °/s, alternating between counterclockwise and clockwise directions. One complete cycle lasted 40 s, and each trial included 5 continuous cycles (200 s duration). The magnitude and velocity of rotation were chosen to minimize the sense of movement and to avoid provoking phasic stretch reflex responses or voluntary reactions. Subjects experienced only a vague sense of movement during a platform rotation.
Figure 1.
Three configurations of the twisting apparatus. Subjects stood on a platform that rotates the feet and lower segment together while the upper segment is fixed above to a rigid frame, via a suspension system (zigzag lines) and torque sensor (T). A) Neck: shoulders affixed to the rotating platform with head fixed above. B) Trunk: pelvis affixed to rotating platform with shoulders fixed above. C) Hip: feet rotated with platform with pelvis fixed above.
Platform position was measured by a precision optical encoder. The reaction force to torsional rotation of the body axis was measured via a torque sensor located between the rigid frame and the uppermost body fixation. A counterbalanced suspension system between the torque sensor and the rigid frame ensured that the upper body fixation restricted rotation only around the vertical axis (stiffness for axial rotation was 590 Nm/° vs. 0.25 N/cm for x, y and z translations). A hinge joint allowed anterior-posterior translation of the lower fixation relative to the platform in order to minimize any interference with normal motion during stance and not provide postural support.
2.4 Experimental procedure
During experimentation, body attachments to the external frame were adjusted to yield zero torque for a subject’s initial standing position (Fig. 1). Each subject was instructed to stand relaxed and not intervene. It was emphasized that subjects should not resist or voluntarily help the movement. Subjects were not informed about details of the imposed movement, e.g., which segment would be axially rotated or even that the platform would rotate at all, and were kept naive by wearing a blindfold that prevented them from seeing movement during the trial. In addition, subjects wore all body fixations throughout the experiment and were unaware which were attached to the platform and torque sensor. Platform position and torque signals were sampled digitally at 50 Hz.
2.4.1 Testing for protocol 1
Trunk, hip, and neck torque were measured in a single testing session in AT teachers and matched control subjects.
2.4.2 Testing for protocol 2
Trunk and hip torque were measured in LBP subjects over 5 testing sessions. Neck torque was not measured in this subject group. The first 3 testing sessions took place at 2-week intervals, which provided 3 independent measurements of ‘baseline’ axial stiffness and intra-subject repeatability. The 8 LBP subjects were then randomized into 2 groups, 4 receiving a series of AT training, and the other 4 receiving a control intervention prior to retesting. In both groups, the assigned intervention was given for a period of 10 weeks. The fourth testing session took place within 2 days after the first intervention. Subjects then crossed over and received the other intervention for 10 weeks, and the fifth testing session took place within 2 days after completion of the intervention.
2.5 Interventions with LBP subjects
The LBP subjects were informed that the study aimed to compare two different interventions. Subjects received twenty 45-minute sessions of each intervention, given individually, two sessions/week for 10 weeks. One LBP subject completed AT training and subsequent testing but did not complete the control intervention for personal reasons.
2.5.1 AT intervention
Training in the AT was given by an AmSAT-certified teacher using standard procedures detailed elsewhere (Alexander, 1923; Cacciatore, Horak, & Henry, 2005; de Alcantara, 1996). Postures and movements performed in lessons include sitting down in a chair, standing up from a chair, bending the knees, rising onto the toes, and lying supine. Unlike typical physical exercise, these movements are generally performed slowly, without repetition, and with attention. The lessons did not specifically address pain or practice axial rotation.
2.5.2 Control intervention
The control intervention matched the attention, time, touch, and movement occurring in AT training. It was given by a single physical therapist, although specifically did not include physical therapy per se. Subjects were told that this intervention assessed coordination and targeted problematic body areas with light massage. Subjects were asked to make movements similar to those in the AT, such as standing from a chair and rising onto toes. To control for touch and lying down in the AT, subjects received gentle light-touch massage while lying supine that focused on neck and back regions.
2.6 Data processing
To determine if differences in torsional resistance were due to the dynamic modulation of postural tone, we examined the peak-to-peak torque, variability, phase-advance and EMG modulation during twisting, which all reflect dynamic tonic modulation (Gurfinkel, et al., 2006; Sherrington, 1909; Xia & Rymer, 2004). Before further processing, torque data were low-pass filtered (2 Hz).
2.6.1 Peak-to-peak torque
Peak-to-peak torque magnitude was assessed as the difference in the maximal resistive torque during clockwise and counterclockwise rotation within each cycle and averaging across the 5 cycles comprising each trial.
2.6.2 Cycle-to-cycle variability
Torque variation across cycles reflects the extent that active processes contribute to the torque magnitude (Xia & Rymer, 2004). Muscle under constant activation has relatively consistent length-tension behavior across cycles (cf. Fig 3 in Gurfinkel et al., 2006). Active control processes introduce an additional source of variation and increase cycle-to-cycle variability, which we assessed by the standard deviation in the torque zero-crossing time across cycles. The torque zero-crossing times were determined for crossings in the clockwise and counterclockwise directions relative to start of the cycle. Standard deviations of the zero-crossing times were computed separately for each direction of crossing and then averaged to determine the cycle-to-cycle variability for a trial.
2.6.3 Torque phase-advance
Torque phase-advance manifested as a zero-crossing of torsional resistive torque prior to the return of the platform to the center (i.e., straight-ahead) position. Such a phase-advance indicates that the subject’s torque neutral position shifted each half cycle in the direction of platform displacement. While some torque phase-advance could result from passive properties of axial tissues, moderate shifts in the neutral position imply a redistribution of muscular forces. In addition, phase-advance has been found to correlate with dynamic modulation of baseline tonic EMG in axial musculature (Gurfinkel, et al., 2006).
Torque data were averaged across the 5 cycles within each trial, yielding the mean torque at each point in the cycle. Note that there are two values of phase-advance per cycle, when the platform approaches center from the clockwise and counterclockwise directions. For each half cycle, the time difference between the first torque zero-crossing in the same direction as the platform rotation and time the platform reached neutral position was determined. The counterclockwise and clockwise time differences were averaged together and expressed as a percentage of the total cycle duration (40 s), yielding the phase-advance for the trial. A positive phase-advance corresponds to torque reaching zero before the platform returned to the central position. A phase-advance of 25% corresponds to the torque neutral position occurring ¼ way through the cycle, at 10° of platform rotation. Constraining the torque and platform rotation zero-crossings to be in the same direction (i.e., both from positive to negative or vice versa, see Fig. 3) ensured positive work performed by the subject on average (i.e., assisting platform rotation) had a phase-advance > 25%. The average shift in the neutral position per half cycle was determined by multiplying the phase-advance by 10°/25%.
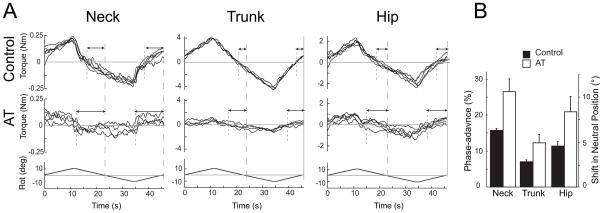
Figure 3.
A) Representative phase-advance at each level for AT teachers and control subjects. Each graph represents a single trial with cycles overlaid. Torque zero-crossing is indicated by short-dashed lines; platform zero-crossing by long-dashed lines. Arrows depict phase-advance. Control traces: phase-advance for the neck=14.5%, trunk=5.3% and hip=7.7%; AT traces: phase-advance for neck=24.0%, trunk=12.0% and hip=10.2%, and. B) Mean phase-advance at each level for AT teachers and control subjects (±SEM). The left axis indicates the advance as a % of the full 40s, while the right axis indicates the corresponding shift in the torque-neutral position from the central platform position per half cycle (i.e., the platform angular displacement at zero-torque).
2.6.4 EMG measurement
Coherent modulation of EMG baseline activity with axial rotation indicates that tone is being dynamically regulated. We assessed EMG activity during axial rotation using bipolar Ag-AgCl surface electrodes placed 2 cm apart, oriented parallel to the muscle fibers. A reference electrode was placed on the subject’s clavicle. EMG signals were recorded bilaterally from external oblique, internal oblique, multifidus at the level of L4, and the medial heads of longissimus at the level of L1. Raw EMG activity was amplified (x1000) and sampled at 2000 Hz. Off-line, EMG activity was band-pass filtered (50–400 Hz), rectified and integrated by convolving with a 3 s wide boxcar function. Modulation depth was calculated by computing the difference between the maximum and minimum of the integrated EMG over each cycle, averaging across cycles and dividing by the muscle’s background activity. Background activity was calculated as the mean integrated EMG over the 5 s prior to onset of the first rotation cycle.
2.7 Statistics
2.7.1 Statistics for protocol 1
Differences in peak-to-peak torque magnitude between AT teachers and matched controls were assessed for significance with a separate 1-way ANOVA for each axial level, as torque magnitude differs across axial levels (Gurfinkel, et al., 2006). Phase-advance was examined across these two subject groups using a 2-way ANOVA (group × level). Difference in variability across populations was examined with an F-test by comparing the variance of the time of torque zero-crossings between populations. The relationship between peak-to-peak torque magnitude and torque phase-advance was observed to obey a power law relationship. This was assessed by linear regression on the log of both values and computing Pearson’s correlation coefficient.
2.7.1 Statistics for protocol 2
Repeatability of torque values in LBP subjects was assessed across the baseline period using a within-subjects, repeated measures ANOVA for the hip and trunk. The 3 baseline measurements were then averaged to obtain an overall pre-intervention baseline. Post-AT measurements were obtained from Testing Session 4 for LBP subjects receiving AT first and from Testing Session 5 for those receiving AT as the second intervention. Post-control intervention measurements were obtained from Testing Session 4 for LBP subjects receiving the control as the first intervention and from Testing Session 5 for those receiving the control intervention second. Significant effects of intervention on LBP subjects were assessed by comparing the torque values of the average baseline to the post-control and post-AT measurements using a within-subject, repeated measures ANOVA. The effect of intervention order was examined by a 1-way ANOVA on the difference between post-AT and post-control intervention between the AT-first and AT-second group.
All statistical tests were conducted with a significance level of α = 0.05. Measurements are given with ± standard deviation unless otherwise noted.
3. Results
We first present the results of healthy control subjects and AT teachers (protocol 1) followed by the effect of AT training on LBP subjects (protocol 2).
3.1 AT teachers vs. matched controls
3.1.1 Torsional resistance
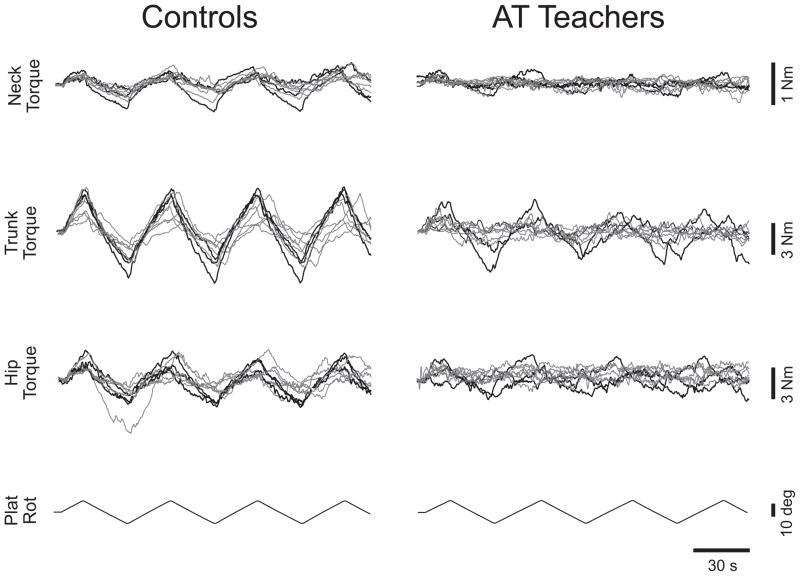
In general, resistive torque increased with increasing platform excursion. The torsional resistance in AT teachers and healthy control subjects in response to ±10° torsional rotation of the neck, trunk, or hips is shown in Fig 2. Upwards deflection of the Plat Rot record (lowest trace) corresponds to counterclockwise rotation of the platform, while upward deflection of torque traces corresponds to clockwise resistance. At each level of the body axis, there was up to a fourfold variation in peak torque magnitude across subjects, but comparatively little variation within subjects across different cycles.
Figure 2.
Torsional resistance of AT teachers and matched controls. Single neck, trunk and hip trials overlaid from 9 subjects (6 female, gray lines; 3 male, black lines) from each group. Upwards Plat Rot deflection indicates CCW platform rotation; upwards Torque deflection indicates resistance to CCW rotation.
In general, the population of AT teachers had lower resistance to axial rotation than control subjects. The mean maximal peak-to-peak resistance of AT teachers (Table 1) was approximately half that of matched control subjects at all axial levels and these differences were statistically significant (neck: F(1,26)=13.7, p < 0.001; trunk: F(1,27)=19.9, p < 0.001; hip: F(1,26)=6.6, p < 0.02).
Table 1.
Peak-to-peak torque magnitude for AT teachers and matched controls.
| Neck Torque (Nm) | Trunk Torque (Nm) | Hip Torque (Nm) | ||||
|---|---|---|---|---|---|---|
| mean± SD | range | mean± SD | range | mean± SD | range | |
| Control Subjects | 0.48±0.24 | 0.21–0.82 | 5.00±1.80 | 2.74–7.62 | 3.07±1.66 | 1.00–6.78 |
| AT Teachers | 0.21±0.11 *** | 0.05–0.47 | 2.29±1.42 *** | 0.57–5.40 | 1.71±0.96 ** | 0.77–3.54 |
p< 0.02
p<0.001
3.1.2 Cycle-to-cycle variability
During axial rotation, AT teachers had greater cycle-to-cycle variation than control subjects, as shown by a greater standard deviation in the torque zero-crossing time across cycles (Table 2). These differences in the variability were statistically significant at all axial levels (neck F=5.49, p<0.05; trunk F=14.1, p <0.001; hip F=6.28, p<0.01).
Table 2.
Variability in zero-crossing time
| Neck SD (s) | Trunk SD (s) | Hip SD (s) | |
|---|---|---|---|
| Control Subjects | 1.8±1.6 | 1.0±0.39 | 1.7±1.5 |
| AT Teachers | 4.2±1.9 * | 3.6±2.6 *** | 4.3±3.0 ** |
p< 0.05
p<0.01
p<0.001
3.1.3 Phase-advance
Although the phase-advance differed across levels in both groups, AT teachers had a greater phase-advance than control subjects for the neck (AT=26.5±12.9%; control = 15.7±2.2%), trunk (AT =12.3±8.4%; control=7.0±2.1%), and hip (AT=20.9±15.4%; control=11.4±4.9%). A 2-way ANOVA showed a significant effect of both subject type (F(1,59)=11.3, p < 0.001) and axial level (F(2,59)=3.8, p < 0.05). The interaction, however, was not significant (F(2,59)=0.33, p = 0.72), suggesting there was no differential effect of the AT on phase-advance across segmental levels. Representative examples of phase-advance are provided in Fig 3A for AT teachers and healthy control subjects. Fig 3B shows the mean phase-advance of each group and corresponding shift in torque-neutral position.
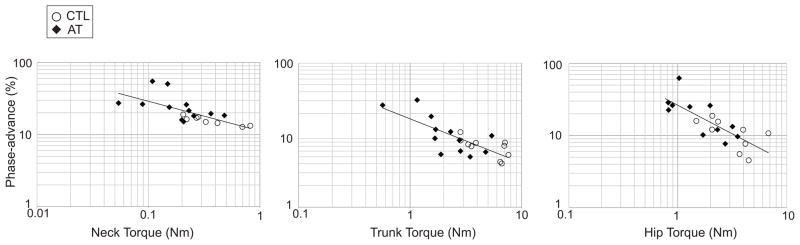
If adaptation of postural tone during axial rotation underlies both the decreased torque magnitude and increased phase-advance observed in AT teachers, we would expect an inverse correlation between torque and phase-advance. While previous studies found linear correlations between both variables with EMG modulation, the relationship between phase-advance and torque magnitude has not been examined. We observed an inverse power relationship between phase-advance and torque magnitude at all axial levels as evidenced by the linear relationships in the log-log plots in Figure 4. The linear regression on the log10(phase-advance) vs. log10(torque-magnitude) was significant for the neck (p = 0.001, R2=0.47), trunk (p < 0.001, R2=0.59) and hip (p < 0.001, R2=0.60).
Figure 4.
Relationship between torque magnitude and phase-advance. Data is plotted on log-log plots for AT teachers (filled diamonds) and matched control subjects (open circles) at the 3 levels tested. The linear regressions correspond to an inverse power law relationship for the neck of phase = torque −0.412+1.05, for the trunk of phase = torque−0.611+1.23, and for the hip of phase = torque−0.793+1.42 (see section 3.1.3).
3.1.4 EMG responses of AT teachers to axial rotation
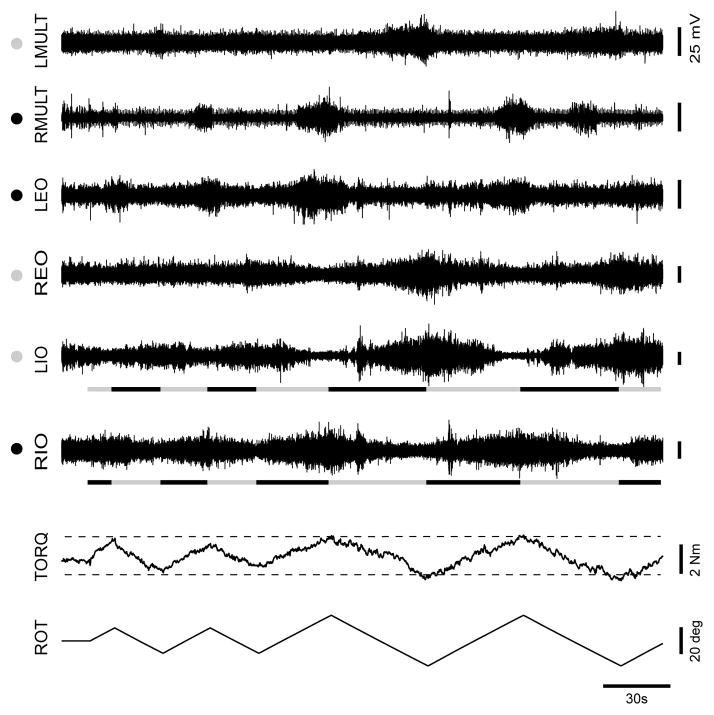
We observed pronounced EMG modulation synchronized to platform rotation in the muscle recordings of the 3 AT teachers who underwent EMG recording (Fig. 5). In general, EMG activity increased during muscle shortening and decreased during lengthening, relative to the background level, consistent with the tonic lengthening and shortening reactions. In the AT teachers, the average modulation depth was 78.4±27.7% for external obliques, 60.3±7.6% for internal obliques, 16.1 ±23.3% for longissimus and 39.8±23.3% for multifidus. For comparison, in healthy subjects the identical protocol produced a modulation depth between 21.5% and 32.1% across trunk muscles (Gurfinkel, et al., 2006). The mechanical plasticity imparted by tonic modulation can be seen from an AT teacher in Fig. 5, in which a doubling in rotation during the last two cycles (from ±10° to ±20°) caused a disproportionately small increase in torque magnitude, likely due to the coincident increase in modulation of tonic activity.
Figure 5.
Modulation of AT teacher EMG activity during torsional rotation. Upwards ROT deflection indicates CCW platform rotation. EMG activity is shown for left multifidus (LMULT), right multifidus (RMULT), left external oblique (LEO), right external oblique (REO) left internal oblique (LIO) and right internal oblique (RIO). Muscle lengthening and shortening is indicated for LIO and RIO by the lines underneath (black = shortening; grey = lengthening). Circles on left indicate whether each muscle lengthened (light circles) or shortened (black circles) during CCW platform rotation.
3.2 AT training in subjects with low-back pain
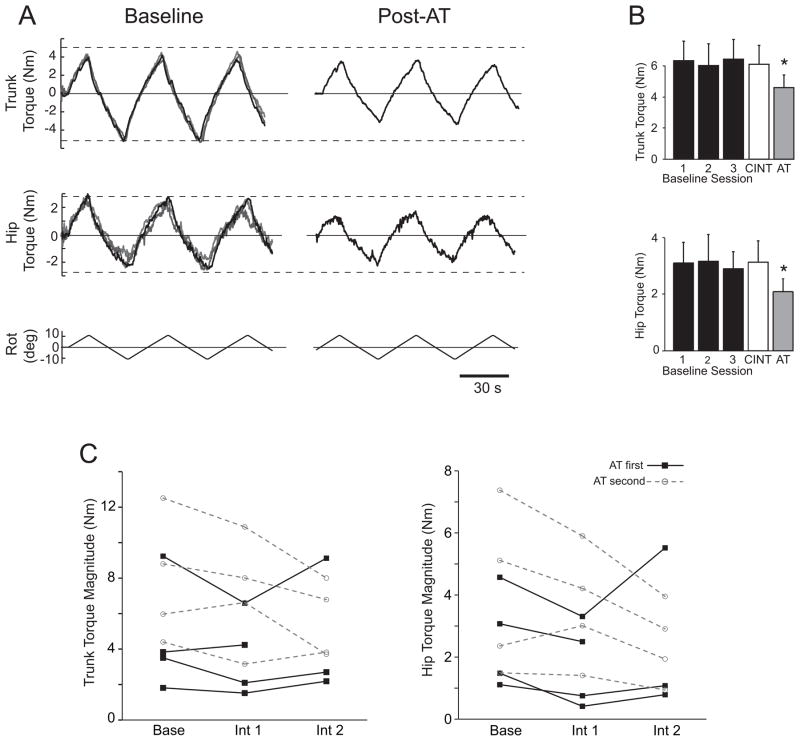
There were no significant differences in the torsional resistance of LBP subjects across baseline sessions for the trunk (F(2,14)=0.707, p=0.71) or the hip (F(2,14)=1.53, p=0.263), consistent with the lack of a practice effect (Table 3). Figure 6A shows the torque resistance of a representative LBP subject across the baseline period and following AT lessons. The group means for each testing session is shown in Fig 6B.
Table 3.
Peak-to-peak (mean±SD) torque magnitude for subjects with low-back pain before and after intervention.
| Baseline 1 | Baseline 2 | Baseline 3 | Baseline (avg) | Control Intervention | AT Lessons | |
|---|---|---|---|---|---|---|
| Trunk mean (Nm) | 6.33 ± 3.56 | 6.02 ± 3.95 | 6.43 ± 3.60 | 6.26 ± 3.61 | 6.10 ± 3.45 | 4.60 ± 2.31 *† |
| Trunk range (Nm) | 2.06–11.34 | 1.71–12.97 | 1.66–13.22 | 1.81–12.51 | 2.19–10.89 | 1.52–8.00 |
| Hip mean (Nm) | 3.10 ± 2.07 | 3.16 ± 2.68 | 2.90 ± 1.70 | 3.06 ± 2.19 | 3.13 ± 2.13 | 2.08 ± 1.29**†† |
| Hip range (Nm) | 0.98–6.16 | 1.10–8.62 | 0.79–5.34 | 1.11–7.38 | 0.79–5.90 | 0.42–3.95 |
Different from Baseline:
p<0.05,
p<0.01
Different from Control Intervention:
p<0.05,
p<0.01
Figure 6.
Resistance to torsional rotation in low-back pain subjects before and after intervention. A) Hip and trunk resistance from a single subject over the baseline sessions (three traces overlaid left) and the post-AT intervention measurement (right). B) Mean (±SEM) peak-to-peak torque magnitude for low-back pain subjects during the 3 baseline sessions, post-control intervention (CINT) and post-AT intervention (AT). * indicates a significant difference (p < 0.05) from the baseline period and the control intervention. C) Peak-to-peak torque magnitude of individual low-back pain subjects during baseline and after intervention. Data is shown for each subject for the mean of the baseline period (Base), after the first intervention (Int 1), and after the second intervention (Int 2). Filled squares with solid lines indicate subjects who received the AT first, while open circles with dashed lines indicate subjects who received the AT second.
The torsional resistance decreased relative to baseline following the AT intervention for both the trunk (F(1,7)=9.13, p<0.05) and hip (F(1,7)=10.5, p<0.01), but there was no change from baseline following the control intervention (trunk F(1,6)=2.56, p=0.16; hip F(1,6)=0.48, p=0.51). In addition, torsional resistance was significantly lower following AT lessons than the control intervention (trunk F(1,6)=7.86, p <0.05; hip F(1,6)=14.4, p<0.01).
Figure 6C shows the peak-to-peak torque magnitudes for all LBP subjects during the baseline period and after each intervention. There was no significant effect of intervention order for the trunk (F(1,5)=0.079, p=0.79) or the hips (F(1,5)=0.131, p=0.732) and, for most subjects, torsional resistance was lowest following AT lessons for both the trunk (n = 6) and hip (n = 7). Subjects with higher resistive torque levels during the baseline period showed greater reductions in magnitude.
4. Discussion
4.1 Increase in dynamic tonic regulation in AT teachers
It has been suggested that postural tone is governed by a ‘conservative’ process (Lestienne & Gurfinkel, 1988), resistant to long-term changes in order to provide a consistent postural framework over time. In view of this supposed conservatism, it would not be surprising if tonic regulation is difficult to change through intervention. Nevertheless, our results suggest that postural tone can be altered though training within an individual over a period of months to years.
AT teachers had substantially lower torsional resistance than the matched control subjects in the present study or that reported previously in untrained healthy adults (e.g. 0.64±0.31 Nm for the neck, 5.1±1.9 Nm for the trunk, and 3.2±1.7 Nm for the hips; Gurfinkel, et al., 2006). Reductions in axial stiffness, mediated by tonic activity, could hypothetically result from a reduction in the baseline level of activity (e.g. reduced co-contraction) or from a change to the mechanism by which tone changes dynamically. Several observations in AT teachers suggest the latter possibility contributed to their lower stiffness.
First, AT teachers had greater cycle-to-cycle variability than control subjects, and often displayed resistance that was not monotonically increasing with displacement (see Fig 2), which are both associated with the modulation of active muscle contraction (Gurfinkel, et al., 2006; Xia & Rymer, 2004).
Second, axial torque acted to assist platform rotation for some AT teachers (antiphasic in our figures). This implies the subjects applied net positive work to the apparatus and that muscle activity was modulated throughout the cycle.
Third, the mean phase-advance in AT teachers corresponded to a shift in torque-neutral position of 10.6°, 4.9° and 8.6° towards platform displacement, for the neck, trunk, and hip respectively, per half-cycle (10° of platform rotation). Over the whole cycle, the average AT teacher’s neutral position shifted by twice the above values, which was near the extent of rotation for the neck and hip, suggesting these regions remained near static equilibrium throughout. However, passive axial stiffness for this magnitude displacement is not negligible (e.g. 2.3 Nm/10° for the lumbar region; McGill, Seguin, & Bennett, 1994) and the postural tone required for spinal stabilization in an upright posture (Lucas & Bresler, 1960) would additionally elevate intrinsic stiffness (Sinkjaer, Toft, Andreassen, & Hornemann, 1988). This implies changes in muscular activation during the twisting cycle were necessary to counteract the restoring torques to rotation.
Fourth, AT teachers shifted their torque-neutral position by 4.3°, 2.1°, and 4.0° more than control subjects for the neck, trunk and hip respectively, per half cycle (i.e., 20–40% of the rotational excursion). This angular difference is larger than the shift in neutral position that occurs without EMG modulation (Gurfinkel, et al., 2006) and exceeds that necessary to measure joint stiffness in general (McGill, Seguin, & Bennett, 1994; Mirbagheri, Barbeau, & Kearney, 2000). We conclude that this increased shift is too large to result from nonlinear, passive properties of muscle and therefore reflects a greater redistribution of muscle activation during twisting (i.e., modulation of postural tone) in AT teachers compared to control subjects.
Finally, the significant inverse power-law relationship between phase-advance and torque magnitude suggests that dynamic modulation explains the majority of torque variation across both populations, and in particular, the lower resistance with long-term AT training. The prominent EMG modulation observed in AT teachers through lengthening and shortening reactions supports this conclusion.
It is important to note that alterations to static baseline levels of postural tone may have also contributed to the reduced stiffness associated in AT teachers. Either reducing overall tonic levels by decreasing ‘antagonistic’ activity that is not directed against gravity, or by redistributing the activity to have smaller torsional moment arms (e.g., more medially located) would decrease torsional resistance. However, changes in baseline levels of tone alone cannot explain the greater phase-advance and cycle-to-cycle variability observed in AT teachers. Because the slow velocity of our perturbation does not allow us to measure intrinsic stiffness in the presence of modulation, further studies are necessary to determine the effect of the AT on baseline levels of postural tone.
4.2 Short-term AT training in LBP subjects
We found that individual LBP subjects decreased axial stiffness by 29%, on average, following short-term AT training. The reduction in axial resistance observed longitudinally following AT, but not the control intervention, supports the conclusion that AT training is responsible for the low resistance observed in AT teachers compared to age-matched control subjects. The short-term training of LBP subjects had a smaller effect, however (AT teachers had a 52% lower stiffness than controls), presumably due to the much shorter duration of training by LBP subjects. While the decrease in resistance in LBP subjects likely results from similar mechanisms to long-term training, because of the small sample size and heterogeneity of LBP subjects, it was not possible to determine whether dynamic modulation of axial tone was increased.
The observed decrease in axial stiffness following AT lessons could underlie the reduction in back pain reported with short-term lessons in the AT (Little, et al., 2008). The pathology of idiopathic LBP is controversial, however, and it is not clear how stiffness and pain are related. One view is that LBP results from inadequate spinal stability and that increasing axial stiffness can reduce pain by stabilizing the spine (McGill, 1998; Panjabi, 1992). Another view is that pain results from increased loading on axial tissues due to excessive axial stiffness (Marras, Ferguson, Burr, Davis, & Gupta, 2004; van Dieen, Cholewicki, & Radebold, 2003; van Dieen, Selen, & Cholewicki, 2003). That the AT reduces both stiffness and pain in LBP subjects supports the latter view. However, it might be important to have sufficient baseline levels of tone to stabilize the spine and avoid injury, but also sufficient dynamic modulation to prevent excessive loading during movement.
The high variation in axial stiffness across LBP subjects could result from distinct subgroups of LBP patients, such as “stiff” and “flexible” sub-categories (Moffroid, Haugh, Henry, & Short, 1994; Van Dillen, et al., 2003). It is interesting that the subjects with the highest axial resistance had the largest stiffness reduction following AT lessons. It is not clear whether the further stiffness reduction in LBP subjects with low baseline resistance was clinically beneficial or acted to increase pain. Studies have not examined whether there is a differential clinical effect of the AT across LBP subgroups.
4.3 Effect of dynamic modulation on coordination
While correlations between torsional resistance and motor performance in Parkinson’s disease (Franzen, et al., 2009) and between spasticity and movement disabilities in neurological patients have been reported (Cooney, Sanders, Concha, & Buczek, 2006; Mirbagheri, Tsao, & Rymer, 2004), little is known about the influence of tonic regulation on motor performance in neurologically healthy individuals. Dynamic modulation of postural tone might act to impart “flexibility” to anti-gravity support during self-initiated movement to minimize co-contraction and stiffness—reconciling posture with movement. It is possible that other, previously described effects of the AT, such as improved balance (Cacciatore, Horak, & Henry, 2005; Dennis, 1999), greater respiratory capacity (Austin & Ausubel, 1992), and altered sit-to-stand strategy (Cacciatore, Horak, & Gurfinkel, 2005; Jones, Gray, Hanson, & Oconnell, 1959), might result from lower axial stiffness or enhanced dynamic modulation following AT training.
While tonic shortening reactions have been reported by a number of authors in human subjects (Andrews & Burke, 1973; Andrews, et al., 1973; Angel, 1982, 1983; Berardelli & Hallett, 1984; Katz & Rondot, 1978; Rondot, 1991; Walsh, 1975), fewer reports have been made of lengthening reactions (Denny-Brown, 1960; Gurfinkel, et al., 2006), and in the latter, under very limited circumstances. In contrast, we observed lengthening reactions in the majority of muscles recorded in all 3 AT teachers who were subjected to EMG recordings. These lengthening reactions might relate to an emphasis on muscle lengthening in the AT (Alexander, 1923) and could be particularly important in reducing tonic opposition to changes in posture.
4.4 Physiological basis of increased modulation of tone
The physiological basis of the AT-related changes in tone is not known, but could relate to neural plasticity at the spinal or supraspinal level. While tonic lengthening and shortening reactions occur in spinal animals (Sherrington, 1909) and training can influence spinal circuitry (Meyer-Lohmann, Christakos, & Wolf, 1986; Nielsen, Crone, & Hultborn, 1993; Segal & Wolf, 1994; Wolpaw & Tennissen, 2001) anecdotal evidence suggests the participation of higher levels of the nervous system. While both increased dynamic modulation and altered baseline levels of postural tone are consistent with the aims of the AT to achieve a dynamically adaptive elongated posture along the body axis (Alexander, 1923), the AT claims that conscious motor and bodily attention (i.e., the AT concept of “direction”) is essential to producing the desired adaptability of muscle tension. This emphasis on conscious attention may suggest that higher brain levels contribute to the AT-related increase in tonic modulation. Notably, this conscious motor attention is considered distinct from voluntary movement (Macdonald, 1989). Descending commands might serve as “reinforcement” (Andrews, et al., 1973; Mark, 1963; Walsh, 1975) in facilitating changes in tone.
While we observed low axial stiffness with the AT, it does not aim to produce a “low-tone”, overly compliant or floppy postural state and incorporates resistance, as well as compliance, in training (de Alcantara, 1996; Macdonald, 1989). Additionally, the AT has been observed to minimize spinal movement during load changes (Cacciatore, Horak, & Gurfinkel, 2005). Thus, the AT might facilitate both types of tonic modulation (yielding and resistive).
5. Conclusion
We have found that AT teachers, who undergo long-term training, and short-term AT training in LBP subjects are associated with decreased axial stiffness. Our results suggest dynamic modulation of postural tone is enhanced in AT teachers, and that this contributes to lower axial stiffness. The increased variability of axial tone and shift in neutral position in AT teachers cannot be explained by simple reduction in static background levels of muscle tone. Short-term AT training in LBP subjects also reduced axial stiffness similar to, but less than, the AT teachers. Future studies are necessary to understand the influence of static and dynamic tonic regulation on coordination and pain.
Acknowledgments
We would like to thank Shoshana Kaminitz for sharing her insight into the Alexander Technique, Dr. Brian Day, Dr. Kathleen Ballard and Amy Peters for their thoughtful comments on the manuscript, and Rebecca Robbins for providing the Alexander Technique lessons. TWC was supported by NIH F32 HD-008520. We would also like to thank the FM Alexander Trust for their financial support to write up the manuscript.
References
- Alexander FM. Constructive conscious control of the individual. New York: EP Dutton & Company; 1923. [Google Scholar]
- Andrews CJ, Burke D. Quantitative study of the effect of L-dopa and phenoxybenzamine on the rigidity of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1973;36:321–328. doi: 10.1136/jnnp.36.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews CJ, Neilson PD, Lance JW. Comparison of stretch reflexes and shortening reactions in activated normal subjects with those in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1973;36:329–333. doi: 10.1136/jnnp.36.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel RW. Shortening reaction in patients with cerebellar ataxia. Ann Neurol. 1982;11:272–278. doi: 10.1002/ana.410110307. [DOI] [PubMed] [Google Scholar]
- Angel RW. Muscular contractions elicited by passive shortening. Adv Neurol. 1983;39:555–563. [PubMed] [Google Scholar]
- Austin JH, Ausubel P. Enhanced respiratory muscular function in normal adults after lessons in proprioceptive musculoskeletal education without exercises. Chest. 1992;102:486–490. doi: 10.1378/chest.102.2.486. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Hallett M. Shortening reaction of human tibialis anterior. Neurology. 1984;34:242–245. doi: 10.1212/wnl.34.2.242. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Sabra AF, Hallett M. Physiological mechanisms of rigidity in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1983;46:45–53. doi: 10.1136/jnnp.46.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne JA, Carleton VL, O’Dwyer NJ. The spasticity paradox: movement disorder or disorder of resting limbs? J Neurol Neurosurg Psychiatry. 2005;76:47–54. doi: 10.1136/jnnp.2003.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciatore TW, Horak FB, Gurfinkel VS. Differences in the coordination of sit-to-stand in teachers of the Alexander Technique. Gait Posture. 2005;21:S128. doi: 10.1016/j.gaitpost.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciatore TW, Horak FB, Henry SM. Improvement in automatic postural coordination following alexander technique lessons in a person with low back pain. Phys Ther. 2005;85:565–578. [PMC free article] [PubMed] [Google Scholar]
- Cathers I, O’Dwyer N, Neilson P. Variation of magnitude and timing of wrist flexor stretch reflex across the full range of voluntary activation. Exp Brain Res. 2004;157:324–335. doi: 10.1007/s00221-004-1848-7. [DOI] [PubMed] [Google Scholar]
- Cooney KM, Sanders JO, Concha MC, Buczek FL. Novel biomechanics demonstrate gait dysfunction due to hamstring tightness. Clin Biomech (Bristol, Avon) 2006;21:59–66. doi: 10.1016/j.clinbiomech.2005.08.014. [DOI] [PubMed] [Google Scholar]
- de Alcantara P. Indirect procedures : a musician’s guide to the Alexander Technique. Oxford ; New York: Clarendon Press; 1996. [Google Scholar]
- Dennis RJ. Functional reach improvement in normal older women after Alexander Technique instruction. J Gerontol A. 1999;54:M8–11. doi: 10.1093/gerona/54.1.m8. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D. Diseases of the basal ganglia. Their relation to disorders of movement. Lancet. 1960;2:1099–1105. doi: 10.1016/s0140-6736(60)92186-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks JC, Daview JB, Mbaot JC, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- Foster M. The Central Nervous System. 6. III 1892. A Text Book of Physiology. [Google Scholar]
- Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson’s disease. Exp Neurol. 2009;219:430–438. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol. 2006;96:2678–2687. doi: 10.1152/jn.00406.2006. [DOI] [PubMed] [Google Scholar]
- Hultborn H. State-dependent modulation of sensory feedback. J Physiol. 2001;533:5–13. doi: 10.1111/j.1469-7793.2001.0005b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FP. Freedom to change: the development and science of the Alexander Technique. London: Mouritz; 1976. [Google Scholar]
- Jones FP, Gray FE, Hanson JA, Oconnell DN. An experimental study of the effect of head balance on patterns of posture and movement in man. Journal of Psychology. 1959;47:247–258. [Google Scholar]
- Katz R, Rondot P. Muscle reaction to passive shortening in normal man. Electroencephalogr Clin Neurophysiol. 1978;45:90–99. doi: 10.1016/0013-4694(78)90345-0. [DOI] [PubMed] [Google Scholar]
- Lestienne FG, Gurfinkel VS. Posture as an organizational structure based on a dual process: a formal basis to interpret changes of posture in weightlessness. Prog Brain Res. 1988;76:307–313. doi: 10.1016/s0079-6123(08)64517-3. [DOI] [PubMed] [Google Scholar]
- Little P, Lewith G, Webley F, Evans M, Beattie A, Middleton K, Barnett J, Ballard K, Oxford F, Smith P, Yardley L, Hollinghurst S, Sharp D. Randomised controlled trial of Alexander technique lessons, exercise, and massage (ATEAM) for chronic and recurrent back pain. BMJ. 2008;337:a884. doi: 10.1136/bmj.a884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas DB, Bresler B. Biomechanics Lab Report no. 40. University of California, San Francisco; 1960. Stability of the ligamentous spine. [Google Scholar]
- Macdonald P. The Alexander Technique as I See It. Brighton, UK: Rahula Books; 1989. [Google Scholar]
- Mark RF. Tonic Stretch Reflexes in the Calf Muscles of Normal Human Subjects. Nature. 1963;199:50–52. doi: 10.1038/199050a0. [DOI] [PubMed] [Google Scholar]
- Marras WS, Ferguson SA, Burr D, Davis KG, Gupta P. Spine loading in patients with low back pain during asymmetric lifting exertions. Spine J. 2004;4:64–75. doi: 10.1016/s1529-9430(03)00424-8. [DOI] [PubMed] [Google Scholar]
- Masani K, Sin VW, Vette AH, Thrasher AT, Kawashima N, Morris A, Preuss R, Popovic MR. Postural reactions of the trunk muscles to multi-directional perturbations in sitting. Clin Biomech. 2009;24:176–182. doi: 10.1016/j.clinbiomech.2008.12.001. [DOI] [PubMed] [Google Scholar]
- McGill S, Seguin J, Bennett G. Passive stiffness of the lumbar torso in flexion, extension, lateral bending, and axial rotation. Effect of belt wearing and breath holding. Spine. 1994;19:696–704. doi: 10.1097/00007632-199403001-00009. [DOI] [PubMed] [Google Scholar]
- McGill SM. Low back exercises: evidence for improving exercise regimens. Phys Ther. 1998;78:754–765. doi: 10.1093/ptj/78.7.754. [DOI] [PubMed] [Google Scholar]
- McGill SM, Seguin J, Bennett G. Passive stiffness of the lumbar torso in flexion, extension, lateral bending, and axial rotation. Effect of belt wearing and breath holding. Spine. 1994;19:696–704. doi: 10.1097/00007632-199403001-00009. [DOI] [PubMed] [Google Scholar]
- Meyer-Lohmann J, Christakos CN, Wolf H. Dominance of the short-latency component in perturbation induced electromyographic responses of long-trained monkeys. Exp Brain Res. 1986;64:393–399. doi: 10.1007/BF00340475. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Barbeau H, Kearney RE. Intrinsic and reflex contributions to human ankle stiffness: variation with activation level and position. Exp Brain Res. 2000;135:423–436. doi: 10.1007/s002210000534. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Tsao CC, Rymer WZ. Abnormal intrinsic and reflex stiffness related to impaired voluntary movement. Conf Proc IEEE Eng Med Biol Soc. 2004;7:4680–4683. doi: 10.1109/IEMBS.2004.1404296. [DOI] [PubMed] [Google Scholar]
- Moffroid MT, Haugh LD, Henry SM, Short B. Distinguishable groups of musculoskeletal low back pain patients and asymptomatic control subjects based on physical measures of the NIOSH Low Back Atlas. Spine. 1994;19:1350–1358. doi: 10.1097/00007632-199406000-00008. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66:116–121. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5:383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Rondot P. The shadow of movement. J Neurol. 1991;238:411–419. doi: 10.1007/BF00314646. [DOI] [PubMed] [Google Scholar]
- Segal RL, Wolf SL. Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Exp Neurol. 1994;130:202–213. doi: 10.1006/exnr.1994.1199. [DOI] [PubMed] [Google Scholar]
- Sherrington C. On plastic tonus and proprioceptive reflexes. Quart J Exper Physiol. 1909;2:109–156. [Google Scholar]
- Sherrington C. Postural activity of muscle and nerve. Brain. 1915;38:191–234. [Google Scholar]
- Sherrington C, Liddell EGT. Reflexes in Response to Stretch (Myotatic Reflexes) Royal Society of London Proceedings Series B. 1924;96:212. [Google Scholar]
- Sinkjaer T, Toft E, Andreassen S, Hornemann B. Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. J Neurophysiol. 1988;60:1110–1121. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- van Dieen JH, Cholewicki J, Radebold A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine. 2003;28:834–841. [PubMed] [Google Scholar]
- van Dieen JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13:333–351. doi: 10.1016/s1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. J Orthop Sports Phys Ther. 2003;33:126–142. doi: 10.2519/jospt.2003.33.3.126. [DOI] [PubMed] [Google Scholar]
- Walsh EG. Proceedings of the Physiological Society. 1975. Shortening reactions in the human forearm; pp. 116P–117. [Google Scholar]
- Walsh EG. The Physiology of Normality, Hypotonocity, Spasticity and Rigidity. London: Mac Keith; 1992. Muscles, Masses and Motion. [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Woolacott AJ, Burne JA. The tonic stretch reflex and spastic hypertonia after spinal cord injury. Exp Brain Res. 2006;174:386–396. doi: 10.1007/s00221-006-0478-7. [DOI] [PubMed] [Google Scholar]
- Xia R, Rymer WZ. The role of shortening reaction in mediating rigidity in Parkinson’s disease. Exp Brain Res. 2004;156:524–528. doi: 10.1007/s00221-004-1919-9. [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Rymer WZ. Simultaneous and nonlinear identification of mechanical and reflex properties of human elbow joint muscles. IEEE Trans Biomed Eng. 1997;44:1192–1209. doi: 10.1109/10.649991. [DOI] [PubMed] [Google Scholar]