Abstract
In Archaea, dolichol phosphates have been implicated as glycan carriers in the N-glycosylation pathway, much like their eukaryal counterparts. To clarify this relation, highly sensitive liquid chromatography/mass spectrometry was employed to detect and characterize glycan-charged phosphodolichols in the haloarchaeon Haloferax volcanii. It is reported that Hfx. volcanii contains a series of C55 and C60 dolichol phosphates presenting saturated isoprene subunits at the α and ω positions and sequentially modified with the first, second, third and methylated fourth sugar subunits comprising the first four subunits of the pentasaccharide N-linked to the S-layer glycoprotein, a reporter of N-glycosylation. Moreover, when this glycan-charged phosphodolichol pool was examined in cells deleted of agl genes encoding glycosyltransferases participating in N-glycosylation and previously assigned roles in adding pentasaccharide residues one-four, the composition of the lipid-linked glycans was perturbed in the identical manner as was S-layer glycoprotein N-glycosylation in these mutants. By contrast, the fifth sugar of the pentasaccharide, identified as mannose in this study, is added to a distinct dolichol phosphate carrier. This represents the first evidence that in Archaea, as in Eukarya, the oligosaccharides N-linked to glycoproteins are sequentially assembled from glycans originating from distinct phosphodolichol carriers.
Keywords: Archaea, dolichol phosphate, Haloferax volcanii, N-glycosylation, S-layer glycoprotein
INTRODUCTION
N-glycosylation is a post-translational modification performed by all three domains of life, namely Eukarya, Bacteria and Archaea (Helenius and Aebi, 2004; Eichler and Adams, 2005; Szymanski and Wren, 2005; Weerapana and Imperiali, 2006). Presently, the pathway of N-glycosylation is best understood in higher Eukarya, where the oligosaccharide covalently linked to select Asn residues of a target protein is first assembled from seven soluble nucleoside-activated sugars sequentially added to a dolichol pyrophosphate carrier on the cytoplasmic face of the endoplasmic reticulum (ER) membrane. The charged lipid carrier is then flipped to face the ER lumen, at which point seven additional sugars, derived from individually charged and flipped phosphodolichol carriers, are added. Once assembled, the 14-meric oligosaccharide is transferred to the protein target (Burda and Aebi, 1999; Helenius and Aebi, 2004).
In contrast to the detailed delineation of the eukaryal N-glycosylation pathway, much less is known of this post-translational modification in Archaea. As in the ER membrane, glycan-charged phosphodolichol species have been detected in the archaeal plasma membrane. Moreover, evidence exists assigning glycan-charged phosphodolichols roles in archaeal N-glycosylation, in analogy to the function these lipids serve in the parallel eukaryal pathway (Burda and Aebi, 1999; Helenius and Aebi, 2004). For example, the identical methylated hexasaccharide moiety as attached to the Methanothermus fervidus surface (S)-layer glycoprotein is found on a dolichol pyrophosphate carrier in this species (Hartmann and Konig, 1989; Kärcher et al., 1993). Likewise, the sulfated polysaccharide moiety N-linked to the Halobacterium salinarum S-layer glycoprotein and flagellin was detected on dolichol phosphate intermediates, while C60 dolichol phosphate species bearing glucose, mannose and N-acetylglucosamine units have also been observed in this organism (Mescher et al., 1976; Lechner et al., 1985; Sumper, 1987; Wieland et al., 1980; Wieland et al., 1985). Pulse-chase radiolabeling of Hbt. salinarum cells revealed the gradual transfer of the radiolabel from a lipid precursor to the S-layer glycoprotein (Wieland et al., 1980), while the incorporation of radiolabeled glucose into Haloferax volcanii glycoproteins was shown to proceed through a glucose-containing phosphopolyisoprenol intermediate (Zhu et al., 1995). Indeed, Hfx. volcanii membranes were reported to contain C55 and C60 dolichol phosphate charged with an α-linked mannosyl-(β1-4)-galactosyl group, and, to lesser extents, with a sulfated or phosphorylated dihexose and with a tetrasaccharide (comprising the hexoses, mannose and galactose, and the deoxyhexose, rhamnose), as well as with monosaccharides at radiochemical levels (Kuntz et al., 1997). Not all of these phosphodolichol-charged glycans have, however, been detected on Hfx. volcanii glycoproteins (Abu-Qarn et al., 2007; Magidovich et al., 2010; Mengele and Sumper, 1992; Sumper et al., 1990).
Thus, while glycan-charged phosphodolichols are implicated in archaeal N-glycosylation, numerous questions remain unanswered. Are those glycans found on dolichol phosphate carriers in Archaea, presumably destined to decorate target proteins, assembled from soluble, activated monosaccharides, from monosaccharides transferred from individual dolichol phosphate carriers or from both, as in Eukarya? If so, what is the relative contribution of each monosaccharide population in generating oligosaccharide-charged phosphodolichols? Is the protein-targeted oligosaccharide fully pre-assembled on a single phosphodolichol carrier in the archaeal cytoplasm or does assembly of the phosphodolichol-charged oligosaccharide involve steps that transpire on both sides of the membrane? Finally, one can also ask whether assembly of the N-linked glycan includes the addition of sugar subunits to a glycan already transferred from its phosphodolichol carrier to the protein target. Based on advances in describing the archaeal pathway of N-glycosylation made in the last five years (for review, see Yurist-Doutsch et al., 2008a; Calo et al., 2010), it may now be possible to address these and related questions.
In Hfx. volcanii, a series of Agl (archaeal glycosylation) proteins has been shown to participate in the assembly and attachment of a pentasaccharide decorating select Asn residues of the S-layer glycoprotein, a reporter of N-glycoslation in this species (Abu-Qarn and Eichler, 2006; Abu-Qarn et al., 2007; Abu-Qarn et al., 2008; Magidovich et al., 2010; Yurist-Doutsch et al., 2008b; Yurist-Doutsch et al., 2010; Kaminski et al., 2010). The involvement of each of these proteins in the N-glycosylation process was demonstrated by examining the N-linked glycan profile of the S-layer glycoprotein isolated from Hfx. volcanii strains deleted of each of these agl genes, relative to the parent strain. As such, AglJ, AglG, AglI, AglE and AglD were shown to participate in the introduction of the five sugar subunits comprising the S-layer glycoprotein-bound pentasaccharide (Abu-Qarn et al., 2007; Abu-Qarn et al., 2008; Yurist-Doutsch et al., 2008b; Kaminski et al., 2010) while AglB was shown to be the oligosaccharyltransferase, responsible for delivery of the glycan to the S-layer glycoprotein (Abu-Qarn and Eichler, 2006; Abu-Qarn et al., 2007).
Now, to address the involvement of glycan-charged phosphodolichols in the biosynthesis of the Hfx. volcanii N-linked pentasaccharide, liquid chromatography/mass spectrometry (LC/MS) was employed to define the sugar profiles of phosphodolichol-linked glycan carriers in a Hfx. volcanii parent strain as well as from cells deleted of different agl genes. It is reported that Hfx. volcanii contains a series of dolichol phosphate molecules sequentially modified with the one, two, three, and four sugar subunits corresponding to the first four subunits of the pentasaccharide found on the Slayer glycoprotein. The fifth pentasaccharide subunit, mannose, is, by contrast, derived from its own phosphodolichol carrier. These findings thus not only provide the first direct evidence for the sequential assembly of an oligosaccharide on a dolichol carrier prior to the addition of that glycan to an archaeal protein but also that the assembly of N-linked oligosaccharides in Archaea involves glycans originating from distinct dolichol carriers, as occurs in Eukarya.
RESULTS
Hfx. volcanii contains a population of C55 and C60 dolichol phosphate molecules
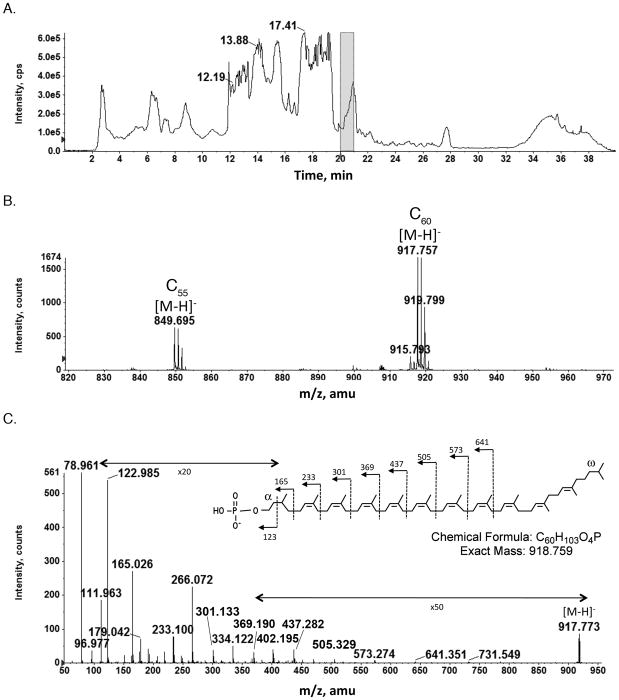
In the present study, a total Hfx. volcanii lipid extract was subjected to normal phase liquid chromatography coupled with mass spectrometry. Figure 1A shows the total ion chromatogram of the NPLC-ESI/MS in the negative ion mode. The mass spectrum averaged from those acquired during the retention time of 20–21 min (Fig 1B) shows prominent ion peaks of m/z 849.695 (this and all reported values are for the monoisotopic ion peaks, unless otherwise stated) and m/z 917.757, corresponding to the [M-H]− ions of the C55 and C60 dolichol phosphates with two saturated isoprene units, respectively. These measured ion masses are in agreement with the calculated values of m/z 849.690 for the [M-H]− ion of the C55 dolichol phosphate and m/z 917.752 for the [M-H]− ion of the C60 dolichol phosphate. In addition, a very minor peak corresponding to C50 dolichol phosphate (m/z 781.671) was observed. MS/MS was performed on the [M-H]− ion at m/z 917.7 of C60 dolichol phosphate (Fig 1C); the obtained fragmentation pattern is consistent with the chemical structure previously described (Kuntz et al., 1997), with the saturated isoprene units at both the α and the ω positions. The same saturation pattern also held true for C55 dolichol phosphate (not shown). This is in contrast to the C55 undecaprenol involved in N-glycosylation in Bacteria, where theα position is unsaturated and the longer dolichols involved in eukaryal N-glycosylation (C70–C110), where theα position is saturated (Jones et al., 2009). The presence of two saturated isoprene units in archaeal dolichol phosphate is quite remarkable, considering that Bacteria contain only unsaturated polyprenol phosphate, while in eukaryal dolichols, only the α-isoprenes are saturated (Burda and Aebi, 1999). Recently, the long-sought reductase for converting polyprenol to dolichol in eukaryotic cells has been identified (Cantagrel et al., 2010). At present, no archaeal polyprenol reductase has been described.
Fig 1.
Normal phase LC/MS identification of dolichol phosphate from the total lipid extract of Hfx. volcanii. A. Total ion chromatogram of the NP-LC/MS analysis in the negative ion mode. B. The [M-H]− ions of C55 and C60 dolichol phosphate detected at m/z 849.695 and 917.757, indicated by C55 and C60, respectively. The mass spectrum shown is averaged from spectra acquired during the 20–21 min window, indicated by the shaded area in A. C. MS/MS of the [M-H]− ion of C60 dolichol phosphate. The inset shows the predicted chemical structure of dolichol phosphate (according to Kuntz et al., 1997) and the MS/MS fragmentation scheme.
Apart from the complete pentasaccharide, Hfx. volcanii contains dolichol phosphates charged with the same glycan series as found N-linked to the S-layer glycoprotein
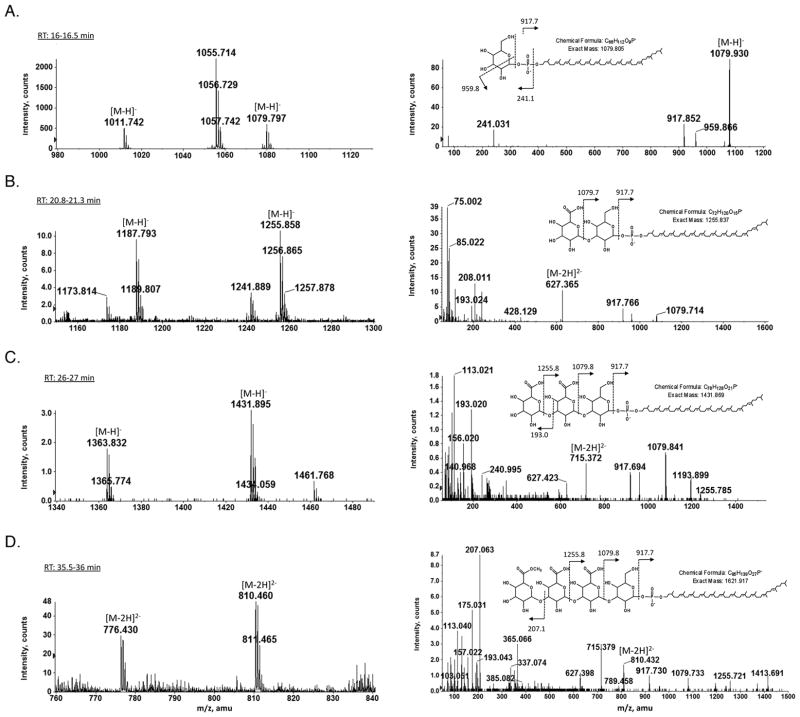
Earlier studies revealed the Hfx. volcanii S-layer glycoprotein to be modified by a N-linked pentasaccharide comprising a hexose, two hexuronic acids, a methyl ester of hexuronic acid and a final hexose (Abu-Qarn et al., 2007; Magadovich et al., 2010). In addition, S-layer glycoprotein-derived peptides have also been shown to be modified by glycans comprising the first, the first two, the first three and the first four sugar subunits of the N-linked pentasaccharide (Abu-Qarn et al., 2007; Abu-Qarn et al., 2008; Magidovich et al., 2010; Yurist-Doutsch et al., 2008b; Yurist-Doutsch et al., 2010). To assess whether similar glycans also decorate Hfx. volcanii dolichol phosphate, phosphodolichol linked glycans were profiled by NPLC/MS in the total lipid extract of Hfx. volcanii. Fig 2 (left panels) reveals the presence of dolichol phosphate species modified by a glycan comprising one to four saccharides, while MS/MS analysis confirmed the nature of the sugars added to the C60 dolichol phosphate (Fig 2, right panels). Specifically, the fraction eluting during the retention time of 16–16.5 min contains C55 and C60 dolichol phosphate species modified by a hexose (peaks at m/z 1011.724 and 1079.797, respectively; Fig 2A). In addition, a major peak at the m/z 1055.714, corresponding to a previously described sulfoglycolipid (Sprott et al., 2003), was also observed. The fraction eluting during the retention time of 20.8–21.3 min contains C55 and C60 dolichol phosphate species modified by a hexose and a hexuronic acid (peaks at m/z 1187.793 and 1255.858, respectively; Fig 2B), while the fraction eluting during the retention time of 26–27 min contains C55 and C60 dolichol phosphate species modified by a hexose and two hexuronic acids (peaks at m/z 1363.832 and 1431.895, respectively; Fig 2C). Finally, the fraction eluting during the retention time of 35.5–36 min contains C55 and C60 dolichol phosphate species modified by a hexose, two hexuronic acids and a methyl ester of hexuronic acid (their doubly charged ions [M-2H]2- are observed at m/z 766.43 and 810.46, respectively; Fig 2D). The structure of the C60 phosphodolichol-linked tetrasacchride was verified by MS/MS (Fig 2D, right panel). The most prominent ion at m/z 207 in the MS/MS spectrum derived from the [M-2H]2- ion at m/z 810.46 is derived from the terminal (i.e. the fourth) sugar. Its methanol-less (32 Da) ion, shown at m/z 175, provides additional evidence for this sugar subunit being a methyl ester of hexuronic acid.
Fig 2.
Normal phase LC/MS/MS identification of mono-, di-, tri-, and tetrasacchride-charged dolichol phosphate from the total Hfx. volcanii lipid extract. The left panels show the [M-H]− ions of (A) hexose-modified, (B) hexuronic acid-hexose-modified and (C) dihexuronic-hexose-modified C55 and C60 phosphodolichol. The left panel of (D) shows the doubly charged [M-2H]2- ions of methyl ester of hexuronic acid-dihexuronic acid-hexose-modified C55 and C60 phosphodolichol, detected at m/z 766.43 and 810.46, respectively. RT refers to retention time. The right panels show the MS/MS spectra of the [M-H]− ion of (A) hexose-modified, (B) hexuronic acid-hexose-modified and (C) dihexuronic acid-hexose-modified C60 phosphodolichol. The right panel of (D) shows the MS/MS spectrum of doubly charged [M-2H]2- ions of methyl ester of hexuronic acid-dihexuronic acid-hexose-modified C60 phosphodolichol. The inset in each right panel shows the chemical structure of the glycan-charged C60 phosphodolichol and the MS/MS fragmentation scheme of the [M-H]− ion (or the [M-2H]2- ions in (D)). The arrows indicating ×20 and ×50 reflect magnification of the ion peaks in the corresponding region of the m/z values on the graph.
Finally, although the S-layer glycoprotein is ultimately modified by a N-linked pentasaccharide, no pentasaccharide-modified dolichol phosphate species was detected. Instead, only dolichol phosphate species sequentially charged with the first four saccharides comprising the pentasaccharide N-linked to the Hfx. volcanii S-layer glycoprotein were observed.
Hfx. volcanii cells lacking components of the N-glycosylation machinery present dolichol phosphates void of or bearing truncated glycans
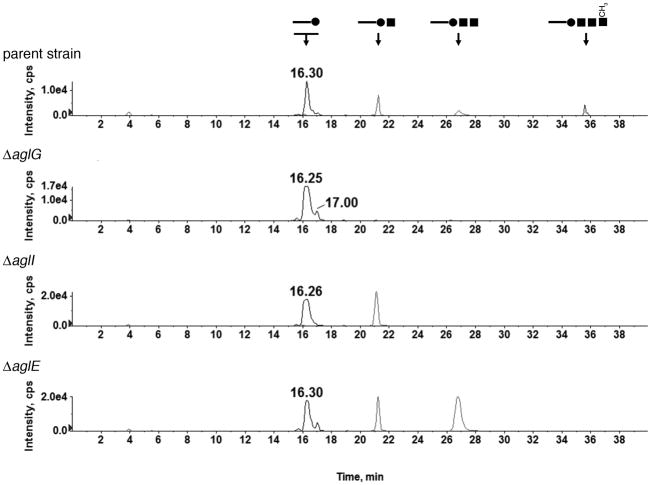
To assess whether the various glycan-charged phosphodolichols described in the previous section are involved in the N-glycosylation of the S-layer glycoprotein, the dolichol phosphate-derived species from Hfx. volcanii cells deleted of aglG, aglI, aglE and aglD were considered. Previous efforts implicated the products of these genes, predicted glycosyltransferases, in the respective addition of the second, third, fourth and fifth saccharides of the pentasaccharide decorating the S-layer glycoprotein, although direct biochemical proof for such activity has yet to be provided (Abu-Qarn et al., 2007; Abu-Qarn et al., 2008; Yurist-Doutsch et al., 2008b).
When the total lipid extract from cells lacking AglG was analyzed by NP-LC/MS/MS as above, only hexose-modified C55 and C60 phosphodolichols were detected (Fig 3). Likewise, cells deleted of aglI or aglE presented dolichol phosphate species containing mono- and disaccharides and mono-, di- and trisaccharides, respectively. Consistent with these results, a recent study (Kaminski et al., 2010) showed that in the absence of AglJ, involved in adding the first sugar subunit of the S-layer glycoprotein N-linked pentasaccharide, the level of a hexose-modified phosphodolichol species was significantly decreased, relative to what is seen in the parent strain.
Fig 3.
LC/MS profiling of glycan-charged phosphodolichols in the parent and agl mutant strains. The presence or absence of mono-, di-, tri- and tetra-sacchride-modified phosphodolichols in each strain was revealed by generating extracted ion chromatograms. The [M-H]− ion at m/z 1079.8, the [M-2H]2- ion at m/z 627.4, the [M-3H]3- ion at m/z 476.6, and the [M-3H]3- ion at m/z 540.0 were selected for monitoring the mono-, di-, tri-, and tetracchride-modified phosphodolichols. Each of these 4 ions represents the highest-abundance charge state observed by ESI/MS of the individual glycan-modified C60 phosphodolichol species. Above each peak, schematic representation of the linked glycan is shown. The full circles correspond to hexose, the full squares correspond to hexuronic acid and the open square corresponds to the methyl ester of hexuronic acid. Note that the monosaccharide-modified phosphodolichol pool comprises several species.
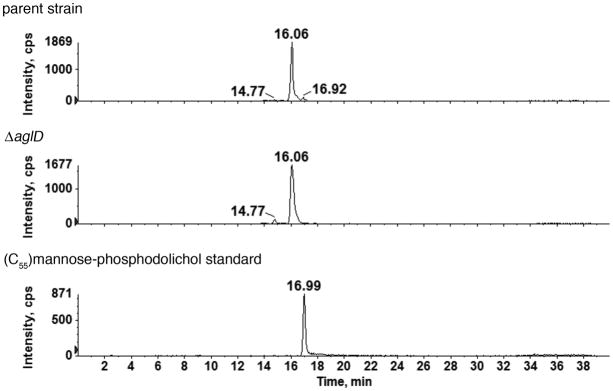
When dolichol phosphate-derived species from cells lacking AglD, implicated in adding the fifth and final subunit of the S-layer glycoprotein N-linked pentasaccharide, were compared with those of the parent strain, a very different effect was seen than observed in cells lacking AglG, AglI or AglE. Hfx. volcanii membranes include three hexose-modified phosphodolichol species retained at 14.77, 16.06 and 16.92 min. Of these, only the hexose-modified phosphodolichol with a retention time of 16.92 min was eliminated in the aglD deletion strain (Fig 4). This implies that AglD is a dolichol phosphate hexose synthase that catalyzes the addition of a hexose residue to the lipid carrier. To identify the monosaccharide apparently added to the dolichol phosphate carrier by AglD, a mannose-charged C55 phosphodolichol standard was examined as above. The mannose-modified phosphodolichol peak eluted at 16.99 min, just as was that peak missing from cells lacking AglD. Thus, the fifth and final pentasaccharide subunit, added to its own dolichol phosphate carrier and previously reported to be a hexose (Abu-Qarn et al., 2007), is now identified as mannose.
Fig 4.
The absence of AglD eliminates mannose-modified phosphodolichol. Normal phase LC extracted ion chromatograms (EIC) of the dolichylphosphate-hexose [M-H]− ion at m/z 1079.8 from the parent strain (upper panel) and the ΔaglD (middle panel) are shown. The peaks at different retention times reflect the existence of three different dolichylphosphate-hexose species (Kaminski et al., 2010). The 16.92 min peak is eliminated in the mutant, as compared with the parent strain, suggesting AglD to be specific for the formation of the third monosaccharide-modified phosphodolichol species. The monosaccharide-modified phosphodolichol peak affected by the absence of AglD is retained at the position of a mannose-modified phosphodolichol standard (16.99 min; lower panel). The identities of the two other monosaccharide-modified phosphodolichols with retention times of 14.77 and 16.06 min, respectively, remain to be determined. While the results shown address C55 dolichol phosphate, similar results were obtained with C60 dolichol phosphate (not shown).
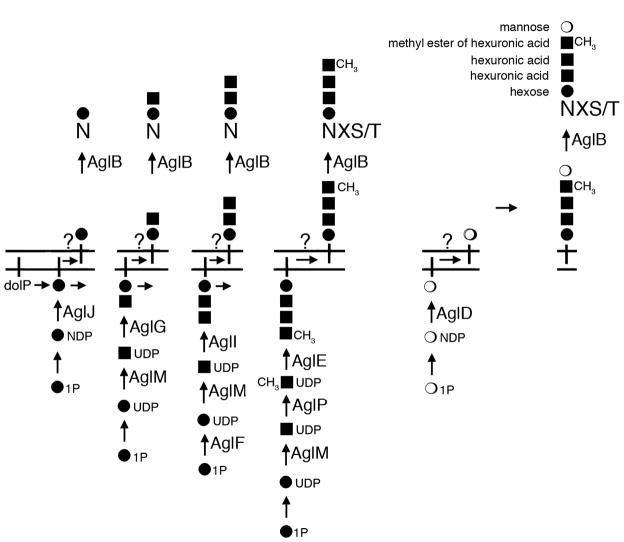
In conclusion, it is proposed that those enzymes previously assigned roles in adding the first four saccharide subunits of the pentasaccharide N-linked to the Hfx. volcanii S-layer glycoprotein sequentially attach their respective sugar substrates to a common dolichol phosphate carrier. By contrast, AglD, previously implicated in adding the fifth saccharide (now identified as mannose) to the N-linked glycan decorating the S-layer glycoprotein, adds its substrate to a distinct dolichol phosphate carrier.
DISCUSSION
It has been long known that Archaea contain glycan-bearing phosphodolichols and, like their eukaryal counterparts (Burda and Aebi, 1999), roles for these lipids in N-glycosylation were postulated (Mescher et al., 1976; Mescher and Storminger, 1978; Lechner et al., 1985a; Lechner et al., 1985b; Sumper, 1987; Wieland et al., 1980; Wieland et al., 1985). Accordingly, dolichol phosphates charged with either the identical or slightly modified versions of the glycans decorating glycoproteins in Hbt. salinarum and M. fervidus were reported (cf. Lechner and Wieland, 1989). Since little or nothing is known of the N-glycosylation process in these species, much related to the involvement of dolichol phosphates in N-glycosylation in Archaea remained a matter of speculation. The recent identification of a series of agl genes involved in the N-glycosylation of the Hfx. volcanii S-layer glycoprotein (for review, see Yurist-Doutsch et al., 2008a; Calo et al., 2010), however, now makes it possible to address the precise role of dolichol phosphates as putative glycan carriers in the archaeal version of this post-translational modification.
Kuntz et al. (1997) first reported the presence of C55 and C60 dolichol phosphates saturated at both the α and the ω positions in Hfx. volcanii, including glycan-modified species. The Hfx. volcanii dolichol phosphate pool was reportedly modified by mannosyl-galactosyl groups, and to a lesser extent, by sulfated or phosphorylated dihexosyl moieties and by a tetrasaccharide that includes mannose, galactose and rhamnose, sugars not detected as components of the N-linked glycans reported to decorate the S-layer glycoprotein at the time (Sumper et al., 1990; Sumper and Mengele, 1992). However, given the revision of the originally reported composition of the glycan N-linked to the Hfx. volcanii S-layer glycoprotein from a string of linear glucose residues (as well as a glucose-, idose- and galactose-containing polysaccharide) (Sumper et al., 1990; Sumper and Mengele, 1992) to a pentasaccharide comprising two hexoses, two hexuronic acids and a methyl ester of hexuronic acid (Abu-Qarn et al., 2007; Magidovich et al., 2010), the present study revisited the composition of glycans decorating dolichol phosphates in Hfx. volcanii in an attempt to link these glycan-charged lipids to the N-glycosylation process.
In recent work from our group (Kaminski et al., 2010), AglJ was shown to add the first hexose subunit of the N-linked S-layer glycoprotein pentasaccharide to a dolichol phosphate carrier. In the present report, it was revealed that the next three subunits of the pentasaccharide are sequentially added to that AglJ-generated monosaccharide-charged carrier, through the respective actions of AglG, AglI and AglE. Since no hexuronic acid-charged phosphodolichol was detected, it seems that pentasaccharide subunits two and three are added from soluble, activated species. Likewise, the methyl ester of hexuronic acid found at position four of the N-linked pentasaccharide is added to the existing trisaccharide-charged phosphodolichol from a soluble methylated hexuronic acid species, since neither a dolichol phosphate modified with only a methyl ester of hexuronic acid nor a tetrasaccharide-charged phosphodolichol bearing a hexuronic acid at position four was detected. These observations, moreover, offer support to the earlier assignment of the nucleoside-hexose dehydrogenase, AglM, shown to catalyze the in vitro conversion of UDP-glucose to UDP-glucuronic acid and likely involved in the biogenesis of pentasaccharide subunits two, three and four, and of AglP, the SAM-dependent methyltransferase responsible for modifying the fourth subunit of the pentasaccharide subunit, as being soluble enzymes (Magidovich et al., 2010; Yurist-Doutsch et al., 2010).
In contrast to the sequential assembly of the first four pentasaccharide subunits onto a common dolichol phosphate, the fifth subunit of the pentasaccharide, mannose, was detected on its own distinct lipid carrier. The finding that AglD, involved in the addition of the fifth pentasaccharide subunit, acts in a manner seemingly independent of the other Agl proteins involved in generating the oligosaccharide decorating the Hfx. volcanii S-layer glycoprotein is not unexpected, given that aglD is the only gene not found in the agl gene island present in the Hfx. volcanii genome (Yurist-Doutsch and Eichler, 2009). Thus, the observation that the same sugar subunits are found on both dolichol phosphate carriers and the S-layer glycoprotein, four of which are sequentially added to the lipid carrier in the same order as found on the modified protein, together with the fact that deletion of agl genes compromised dolichol phosphate glycosylation in a manner reminiscent of the effects of the same gene deletions on Hfx. volcanii S-layer glycoprotein N-glycosylation (Abu-Qarn et al., 2007; Abu-Qarn et al., 2008; Yurist-Doutsch et al., 2008b), directly links dolichol phosphates, acting as mono- and oligosaccharide carriers, to the archaeal N-glycosylation process (Fig 5).
Fig 5.
The working model of the Hfx. volcanii N-glycosylation pathway. Select Asn residues of the Hfx. volcanii S-layer glycoprotein are modified by a pentasaccharide comprising a hexose, 2 hexuronic acids, a methyl ester of hexuronic acid and a terminal mannose residue. Based on the findings of the present study and earlier reports (16,17,23–25,33), a working model of the Hfx. volcanii N-glycosylation pathway is provided. AglJ, AglG, AglI, AglE and AglD are assigned roles in either modifying dolichol phosphates or adding sugars to dolichol phosphate-bound sugars. AglB serves as the oligosaccharyltransferase, while AglF, AglP and AglM serve various sugar processing roles. At present, the flippase(s) responsible for delivering the lipid-charged glycans across the plasma membrane remain to be defined and are indicated by question marks. dolP, dolichol phosphate; NDP, nucleoside diphosphate.
The confirmed involvement of archaeal dolichol phosphate sugar carriers in Hfx. volcanii N-glycosylation provides novel insight into the mechanism of this post-translational modification. Earlier work had shown that Agl proteins involved in adding sugars found on the pentasaccharide N-linked to the Hfx. volcanii S-layer glycoprotein are membrane proteins oriented towards the cytoplasm, pointing to dolichol phosphate sugar charging as occurring within the confines of the cell (Plavner and Eichler, 2008). Hence, as no pentasaccharide-charged phosphodolichol species could be detected, it is possible that transfer of pentasaccharide subunit five occurs directly onto the S-layer glycoprotein-linked tetrasaccharide. Indeed, tetrasaccharide-modified S-layer glycoprotein-derived peptides have been observed (Abu-Qarn et al., 2007; Magidovich et al., 2010). Alternatively, transfer of the fifth sugar subunit from its own dolichol phosphate carrier to the lipid-linked tetrasaccharide and subsequent transfer to the S-layer glycoprotein may occur too rapidly to be detected here. This explanation for our inability to detect a Hfx. volcanii pentasaccharide-modified phosphodolichol species is unlikely, since such an entity was readily observed upon examination of the dolichol phosphate pool of another halophilic archaea originating from the Dead Sea, namely Haloarcula marismortui (Z.G. et al., in preparation). To determine whether the fifth and final subunit of the pentasaccharide is added to the tetrasaccharide-charged phosphodolichol to yield a potentially short-lived pentasaccharide-charged lipid carrier or directly to the tetrasaccharide-modified S-layer glycoprotein, additional biochemical studies are required. It is, however, clear that methylation of the fourth pentasaccharide subunit is important for addition of pentsaccharide subunit five, since no N-linked pentasaccharide is detected in the ΔaglP mutant, where methylation of the fourth pentasaccharide subunit fails to occur (Magidovich et al., 2010). On the other hand, the actions of AglP are not essential for modification of the S-layer glycoprotein by the tetrasaccharide formed in the absence of this methyltransferase.
In conclusion, despite considerable progress made in understanding archaeal N-glycosylation in recent years (Yurist-Doutsch et al., 2008a; Calo et al., 2010), many questions still remain unanswered. For instance, why does N-glycosylation in some Archaea, such as Hfx. volcanii, rely on dolichol phosphate while other species rely on dolichol pyrophosphate or both, as in the case of Hbt. salinarum (31)? What is/are the flippase(s) involved in N-glycosylation in Hfx. volcanii? Finally, do the glycan-modified phosphodolichol species originally reported by Kuntz et al. (1997) participate in any Hfx. volcanii post-translation modification? Continued examination of the Hfx. volcanii N-glycosylation pathway will likely provide answers to these and other outstanding questions.
EXPERIMENTAL PROCEDURES
Strains and growth conditions
The Hfx. volcanii parent strain WR536 (H53) and the same strain deleted of aglG, aglI, aglE or aglD were grown in complete medium containing 3.4 M NaCl, 0.15 M MgSO4•7H20, 1 mM MnCl2, 4 mM KCl, 3 mM CaCl2, 0.3 % (w/v) yeast extract, 0.5 % (w/v) tryptone, 50 mM Tris-HCl, pH 7.2, at 40°C (Mevarech and Werczberger, 1985). The preparation of Hfx. volcanii strains deleted of aglG, aglI, aglE and aglD was previously reported (Abu-Qarn and Eichler, 2006; Abu-Qarn et al., 2008; Yurist-Doutsch et al., 2008).
Isolation of the Hfx. volcanii lipid fraction
The total lipid contents of the Hfx. volcanii parent strain and of Hfx. volcanii ΔaglG, ΔaglI, ΔaglE and ΔaglD cells were extracted as follows. Cells were harvested (8,000 g, 30 min, 4°C) and frozen at −20°C until extraction was performed. At that point, the pelleted cells (15 g) were thawed, resuspended in 20 mL double-distilled water (DDW) and DNase (1.7 μg/ml; Sigma, St. Louis, MO) and stirred overnight at room temperature. Methanol and chloroform were added to the cell extract to yield a methanol:chloroform:cell extract ratio of 2:1:0.8. After stirring for 24 h at room temperature, the mixture was centrifuged (1,075 g, 30 min, 4°C). The clarified supernatants were collected, combined and filtered through glass wool. Chloroform and DDW were added to the filtrate to yield a chloroform:DDW:filtrate ratio of 1:1:3.8, in a separating funnel. After separation, the lower clear organic phase, containing the total lipid extract, was collected into a round bottom flask and evaporated in a rotary evaporator at 35°C. For analysis of the dolichol phosphate-derived species, the total lipid extracts were subjected to normal phase LC/MS analysis without pre-fractionation.
Liquid Chromatography/Mass Spectrometry (LC/MS) and Tandem Mass Spectrometry (MS/MS)
Normal phase LC-ESI/MS of lipids was performed using an Agilent 1200 Quaternary LC system coupled to a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems, Foster City, CA). An Ascentis Si HPLC column (5 μm, 25 cm × 2.1 mm) was used. Mobile phase A consisted of chloroform/methanol/aqueous ammonium hydroxide (800:195:5, v/v/v). Mobile phase B consisted of chloroform/methanol/water/ aqueous ammonium hydroxide (600:340:50:5, v/v/v/v). Mobile phase C consisted of chloroform/methanol/water/aqueous ammonium hydroxide (450:450:95:5, v/v/v/v). The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min and held at 100% C for 3 min, and finally returned to 100% A over 0.5 min and held at 100% A for 5 min. The total LC flow rate was 300 μl/min. The post-columnsplitter diverted ~10% of the LC flow to the ESI source of the Q-Star XL mass spectrometer, with MS settings as follows: IS = −4500 V, CUR = 20 psi, GS1 = 20 psi, DP = −55 V, and FP = −150 V. For MS/MS, collision-induced dissociation (CID) was performed with collision energy ranging from 40 V to 70 V (laboratory frame of energy) and with nitrogen as the collision gas. Data acquisition and analysis were performed using the instrument’s Analyst QS software.
Acknowledgments
J.E. is supported by the Israel Science Foundation (grant 30/07). The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant number GM-069338 from NIH. L.K. is the recipient of a Negev-Zin Associates Scholarship.
References
- Abu-Qarn M, Eichler J. Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol Microbiol. 2006;61:511–525. doi: 10.1111/j.1365-2958.2006.05252.x. [DOI] [PubMed] [Google Scholar]
- Abu-Qarn M, Yurist-Doutsch S, Giordano A, Trauner A, Morris HR, Hitchen P, et al. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J Mol Biol. 2007;14:1224–1236. doi: 10.1016/j.jmb.2007.10.042. [DOI] [PubMed] [Google Scholar]
- Abu-Qarn M, Giordano A, Battaglia F, Trauner A, Morris HR, Hitchen P, et al. Identification of AglE, a second glycosyltransferase involved in N-glycosylation of the Haloferax volcanii S-layer glycoprotein. J Bacteriol. 2008;190:3140–3146. doi: 10.1128/JB.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- Calo D, Kaminski L, Eichler J. Protein glycosylation in Archaea: Sweet and extreme. Glycobiology. 2010;20:1065–1079. doi: 10.1093/glycob/cwq055. [DOI] [PubMed] [Google Scholar]
- Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer A, Bluemel P, Cegielska J, Houliston SR, Swistun D, Ali BR, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CRH, Freeze HH, Morava E, Al-Gazali L, Gleeson JG. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:1–15. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler J, Adams MWW. Posttranslational protein modification in Archaea. Microbiol Mol Biol Rev. 2005;69:393–425. doi: 10.1128/MMBR.69.3.393-425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E, Konig H. Uridine and dolichyl diphosphate activated oligosaccharides are intermediates in the biosynthesis of the S-layer glycoprotein of Methanothermus fervidus. Arch Microbiol. 1989;151:274–281. [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Jones MB, Rosenberg JN, Betenbaugh MJ, Krag SS. Structure and synthesis of polyisoprenoids used in N-glycosylation across the three domains of life. Biochim Biophys Acta. 2009;1790:485–494. doi: 10.1016/j.bbagen.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski L, Abu-Qarn M, Guan Z, Naparstek S, Ventura VV, Raetz CRH, Hitchen PG, Dell A, Eichler J. AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein. J Bacteriol. 2010 doi: 10.1128/JB.00705-10. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärcher U, Schröder H, Haslinger E, Allmaier G, Schreiner R, Wieland F, et al. Primary structure of the heterosaccharide of the surface glycoprotein of Methanothermus fervidus. J Biol Chem. 1993;268:26821–26826. [PubMed] [Google Scholar]
- Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R. Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology. 1997;7:897–904. doi: 10.1093/glycob/7.7.897. [DOI] [PubMed] [Google Scholar]
- Lechner J, Wieland F. Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem. 1989;58:173–194. doi: 10.1146/annurev.bi.58.070189.001133. [DOI] [PubMed] [Google Scholar]
- Lechner J, Wieland F, Sumper M. Biosynthesis of sulfated saccharides N-glycosidically linked to the protein via glucose. Purification and identification of sulfated dolichyl monophosphoryl tetrasaccharides from halobacteria. J Biol Chem. 1985a;260:860–866. [PubMed] [Google Scholar]
- Lechner J, Wieland F, Sumper M. Transient methylation of dolichyl oligosaccharides is an obligatory step in halobacterial sulfated glycoprotein biosynthesis. J Biol Chem. 1985b;260:8984–8989. [PubMed] [Google Scholar]
- Magidovich H, Yurist-Doutsch S, Konrad Z, Ventura VV, Hitchen PG, Dell A, Eichler J. AglP is a S-adenosyl-L-methionine-dependent methyltransferase that participates in the N-glycosylation pathway of Haloferax volcanii. Mol Microbiol. 2010;76:190–199. doi: 10.1111/j.1365-2958.2010.07090.x. [DOI] [PubMed] [Google Scholar]
- Mengele R, Sumper M. Drastic differences in glycosylation of related S-layer glycoproteins from moderate and extreme halophiles. J Biol Chem. 1992;267:8182–8185. [PubMed] [Google Scholar]
- Mescher MF, Hansen U, Strominger JL. Formation of lipid-linked sugar compounds in Halobacterium salinarium. Presumed intermediates in glycoprotein synthesis. J Biol Chem. 1976;251:7289–7294. [PubMed] [Google Scholar]
- Mescher MF, Strominger JL. Glycosylation of the surface glycoprotein of Halobacterium salinarium via a cyclic pathway of lipid-linked intermediates. FEBS Lett. 1978;89:37–41. doi: 10.1016/0014-5793(78)80517-1. [DOI] [PubMed] [Google Scholar]
- Mevarech M, Werczberger R. Genetic transfer in Halobacterium volcanii. J Bacteriol. 1985;162:461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavner N, Eichler J. Defining the topology of the N-glycosylation pathway in the halophilic archaeon Haloferax volcanii. J Bacteriol. 2008;190:8045–8052. doi: 10.1128/JB.01200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott GD, Larocque S, Cadotte N, Dicaire CJ, McGee M, Brisson JR. Novel polar lipids of halophilic eubacterium Planococcus H8 and archaeon Haloferax volcanii. Biochim Biophys Acta. 2003;1633:179–188. doi: 10.1016/j.bbalip.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Sumper M. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta. 1987;906:69–79. doi: 10.1016/0304-4157(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Sumper M, Berg E, Mengele R, Strobel I. Primary structure and glycosylation of the S-layer protein of Haloferax volcanii. J Bacteriol. 1990;172:7111–7118. doi: 10.1128/jb.172.12.7111-7118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski CM, Wren BW. Protein glycosylation in bacterial mucosal pathogens. Nat Rev Microbiol. 2005;3:225–237. doi: 10.1038/nrmicro1100. [DOI] [PubMed] [Google Scholar]
- Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- Wieland F, Dompert W, Bernhardt G, Sumper M. Halobacterial glycoprotein saccharides contain covalently linked sulphate. FEBS Lett. 1980;120:110–114. doi: 10.1016/0014-5793(80)81058-1. [DOI] [PubMed] [Google Scholar]
- Wieland F, Paul G, Sumper M. Halobacterial flagellins are sulfated glycoproteins. J Biol Chem. 1985;260:15180–15185. [PubMed] [Google Scholar]
- Yurist-Doutsch S, Eichler J. Manual annotation, transcriptional analysis and protein expression studies reveal novel genes in the agl cluster responsible for N-glycosylation in the halophilic archaeon Haloferax volcanii. J Bacteriol. 2009;191:3068–3075. doi: 10.1128/JB.01838-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Chaban B, VanDyke D, Jarrell KF, Eichler J. Sweet to the extreme: Protein glycosylation in Archaea. Mol Microbiol. 2008a;68:1079–1084. doi: 10.1111/j.1365-2958.2008.06224.x. [DOI] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Abu-Qarn M, Battaglia F, Morris HR, Hitchen PG, Dell A, Eichler J. aglF, aglG and aglI, novel members of a gene cluster involved in the N-glycosylation of the Haloferax volcanii S-layer glycoprotein. Mol Microbiol. 2008b;69:1234–1245. doi: 10.1111/j.1365-2958.2008.06352.x. [DOI] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Magidovich H, Ventura VV, Hitchen PG, Dell A, Eichler J. N-glycosylation in Archaea: On the coordinated actions of Haloferax volcanii AglF and AglM. Mol Microbiol. 2010;75:1047–1058. doi: 10.1111/j.1365-2958.2009.07045.x. [DOI] [PubMed] [Google Scholar]
- Zhu BC, Drake RR, Schweingruber H, Laine RA. Inhibition of glycosylation by amphomycin and sugar nucleotide analogs PP36 and PP55 indicates that Haloferax volcanii beta-glucosylates both glycoproteins and glycolipids through lipid-linked sugar intermediates: evidence for three novel glycoproteins and a novel sulfated dihexosyl-archaeol glycolipid. Arch Biochem Biophys. 1995;319:355–364. doi: 10.1006/abbi.1995.1305. [DOI] [PubMed] [Google Scholar]