SUMMARY
A carotid artery calcified plaque (CarCP) linkage peak on chromosome 16p (LOD 4.39 at 8.4cM) in European American (EA) families with type 2 diabetes mellitus (T2DM) from the Diabetes Heart Study (DHS) has been refined by fine-mapping and candidate genes and SNPs evaluated for association with subclinical CVD. Fine-mapping was based on 104 SNPs in 937 subjects from 315 families, including 45 SNPs in six candidate genes (CACNA1H, SEPX1, ABCA3, IL32, SOCS1, and KIAA0350). Linkage and association analyses using variance components analysis (SOLAR; adjusting for age, gender, BMI, and T2DM status) refined the original CarCP linkage into two distinct linkage regions (LOD scores: 3.89 at 6.9cM and 4.86 at 16.0cM). Evidence of linkage for coronary calcified plaque (LOD: 2.27 at 19cM) and a vascular calcification principle component (LOD: 3.71 at 16.0cM) was also observed. The strongest evidence for association with CarCP was observed with SNPs in the A2BP1 gene region (rs4337300 p=0.005) with modest evidence of association with SNPs in CACNA1H (p=0.010–0.033). Bayesian Quantitative Trait Nucleotide analysis identified a SNP, rs1358489, with either a functional effect on CarCP or in linkage disequilibrium with a functional SNP. This study refined the 16p region contributing to vascular calcification. Although the causal variants remain to be identified the results are consistent with a linkage peak which is due to multiple common variants, though rare variants cannot be excluded.
Keywords: type 2 diabetes, subclinical cardiovascular disease, fine mapping
INTRODUCTION
Cardiovascular disease (CVD) is the major cause of mortality in Western industrial countries and diabetes is widely recognized as an independent risk factor for the development of atherosclerotic CVD (Kannel & McGee, 1979; Pan et al., 1986; Abbott et al., 1987; Brand et al., 1989; Haffner et al., 1998). Diabetes contributes substantially to the development of premature mortality and morbidity from CVD and atherosclerotic heart disease, and patients with diabetes are at an increased risk of mortality from coronary heart disease (Miettinen et al., 1998). While the relationship between CVD risk and diabetes risk has been extensively documented, the origin of diabetic macrovascular disease remains poorly understood. The risk for CVD and diabetes is widely accepted as being due to genetic and environmental factors.
Numerous reports document that vascular calcification, i.e. vascular calcified plaque, is an excellent surrogate marker of CVD. Coronary artery calcified plaque (CorCP) has long been considered a primary determinant of CVD (Greenland et al., 2004; Terry et al., 2005; Vliegenthart et al., 2005), predicting both prevalent CVD and total mortality in asymptomatic individuals (Raggi et al., 2001; Raggi et al., 2004; Detrano et al., 2008; Greenland et al., 2004; Shemesh et al., 2004). Pathological studies indicate that CVD is a systemic disease, and as such, it is unusual for an individual to have disease localized to a single vascular bed. In addition to coronary artery calcified plaque CorCP, calcified lesions are also commonly seen in the carotid artery and the abdominal aorta (Simon et al., 1995), and the extent of this peripheral arterial calcification correlates with CorCP (Wagenknecht et al., 2004).
Because of the high prevalence of clinical and subclinical CVD in diabetes-affected populations, families with multiple diabetes-affected members provide an enriched environment for the expression of CVD susceptibility genes. To date, most CVD mapping studies have focused on CorCP as the primary measure of CVD. However, we recently reported evidence for linkage of carotid artery calcified plaque (CarCP) to chromosome 16p at 8.4 cM with a logarithm of the odds (LOD) score of 4.39 (support interval tel-15 cM) in 357 pedigrees consisting of European American (EA) subjects with type 2 diabetes mellitus (T2DM) in the Diabetes Heart Study (Bowden et al., 2008).
Here we describe fine-mapping of the chromosome 16p quantitative trait locus (QTL), evaluation of selected candidate genes within 16p and SNPs across the QTL, and explore SNP contributions to variation in measures of subclinical CVD.
MATERIALS AND METHODS
Subjects
The Diabetes Heart Study (DHS) is being conducted in Forsyth County, North Carolina to elucidate the genetic and epidemiological origins of CVD in families affected with T2DM. Ascertainment and recruitment have been described previously (Bowden et al., 2006; Lange et al., 2002; Wagenknecht et al., 2001). Briefly, siblings concordant for T2DM and lacking renal insufficiency were recruited from internal medicine clinics, endocrinology clinics, and community advertising. T2DM was defined as a clinical diagnosis of diabetes after the age of 34 years, in the absence of historical evidence of diabetic ketoacidosis, and active treatment at the time of examination. Unaffected siblings, similar in age to the siblings with T2DM, were also invited to participate, as were any additional diabetes-affected siblings. Individuals with other serious health conditions, such as renal replacement therapy, were not eligible to participate. Recruitment was based upon family structure and there were no inclusions/exclusions based on prior or current evidence of prevalent CVD at the time of recruitment. The sample includes European American (EA) and African-American (AA; approximately 15% of the total) participants. The results reported here are from 937 EA subjects from 315 pedigrees with at least two individuals with T2DM. All protocols were approved by the Institutional Review Board of Wake Forest University School of Medicine, and all participants gave informed consent prior to participation.
Clinical Evaluation
Participant examinations were conducted in the General Clinical Research Center of the Wake Forest University Baptist Medical Center and included interviews for medical history and health behaviors, anthropometric measures, resting blood pressure, a fasting blood draw and a spot urine collection. Laboratory assays included urine albumin and creatinine, total cholesterol, non-high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL), HDL, triglycerides, glycated hemoglobin, fasting glucose and blood chemistries. A detailed medical history was collected with emphasis on CVD. In addition, a resting 12-lead electrocardiogram (ECG) was performed to assess history of clinically significant (past or present) CVD.
CorCP, CarCP and abdominal aortic calcified plaque (AACP) were measured with single and multidetector cardiac CT systems using a standardized protocol based on those implemented in the National Heart, Lung and Blood Institute's (NHLBI's) CARDIA and MESA studies for measuring the coronary arteries (Carr et al., 2005; Detrano et al., 2005). High-resolution B-mode carotid ultrasonography was performed as described previously (Lange et al., 2002) using a 7.5-MHz transducer and a Biosound Esaote (AU5) machine (Biosound Esaote, Inc., Indianapolis, IN, USA). Scans were performed of the near and far walls of the distal 10-mm portion of the common carotid artery at five predefined interrogation angles on each side. The mean value of up to 20 common carotid artery intima-media thickness (IMT) values was reported.
Genotyping
Fine-Mapping
Single nucleotide polymorphisms (SNPs) located within and surrounding the initial chromosome 16p linkage region were selected to fine-map the CarCP locus. All available SNP data for the chromosome 16p linkage interval was extracted from the HapMap database (http://www.hapmap.org/cgi-perl/gbrowse/hapmap_B36/; (Frazer et al., 2007)). Specifically, the genotypes of all HapMap SNPs genotyped in the CEU population were selected, loaded into the Haploview program (Barrett et al., 2005), filtered based on a minor allele frequency threshold of 0.30, and the resulting filtered SNPs binned based on genetic map position with bin boundaries being designated every 0.30 cM. Within each bin, the single SNP with the highest heterozygosity and closest to the center of the bin was selected. The HapMap CEU genotyping data for this final SNP list was loaded into Haploview for evaluation of pairwise linkage disequilibrium (LD) between consecutive SNPs. A pairwise r2<0.30 was used to minimize the LD and to reduce the type 1 error in the subsequent linkage analysis. For SNP pairs that failed to meet the LD criteria, different SNPs were selected from the affected SNP bins. The LD evaluation was reiterated until a complete SNP list was generated where all pairwise r2 values were less than 0.30. Primers for PCR amplification and extension reactions were designed using the MassARRAY Assay Design Software (Sequenom Inc., San Francisco, CA) for 69 SNPs.
Total genomic DNA was purified from whole blood samples obtained from subjects using the PUREGENE DNA isolation kit (Gentra, Inc., Minneapolis, MN). DNA concentration was quantified using standardized fluorometric readings on a Hoefer DyNA Quant 200 fluorometer (Hoefer Pharmacia Biotech Inc., San Francisco, CA). Each sample was diluted to a final concentration of 5 ng/μl.
Genotypes were determined using a MassARRAY SNP Genotyping System (Sequenom Inc., San Diego, CA)(Oeth et al., 2005). This genotyping system uses single-base extension reactions to create allele-specific products that are separated and scored in a matrix-assisted laser desorption ionization/time of flight mass spectrometer. Thirty-six individuals from 31 families served as blind duplicates (duplicated samples given new unique identifiers) to evaluate genotyping accuracy.
Candidate Genes
Six positional candidate genes were chosen within the linked region based on their hypothesized functional role in vascular calcification and CVD. SNPs were selected from the HapMap database (http://www.hapmap.org/cgi-perl/gbrowse/hapmap_B36/; (Frazer et al., 2007)) to capture the genetic variation within each gene plus 5 kb upstream and downstream of each gene. Tagging SNPs for the CEU population were selected using the greedy pair-wise tagging algorithm implemented in the Tagger program (de Bakker et al., 2005) of Haploview (Barrett et al., 2005). Using a minimum minor allele frequency of 0.10 and an r2 threshold of 0.80, genotyping assays for 47 SNPs were successfully designed using the MassARRAY Assay Design Software (Sequenom Inc., San Francisco, CA). Genotypes were determined as described above. Forty-two individuals from 27 families served as blind duplicates (duplicated samples given new unique identifiers) to evaluate genotyping accuracy.
Statistical Analysis
Fine-Mapping
Quantitative traits were transformed to best approximate the distributional assumptions for the variance component QTL linkage analysis (i.e., conditional normality after adjusting for covariates, homogeneity of variance). The reported results represent the analyses of the square root of AACP after adding 10 and the natural logarithms of IMT, CorCP and CarCP after adding 1. All available genotypic data were used to compute the identity-by-descent (IBD) statistics using a Bayesian Markov Chain Monte Carlo approach implemented in the software LOKI (Heath, 1997). Previously, self-reported familial relationships were examined and modified using the genome scan data and the software PREST (McPeek & Sun, 2000). Evidence of linkage to a QTL was tested using the variance component approach implemented in the SOLAR software package (Almasy & Blangero, 1998), adjusting for the covariates age, gender, body mass index (BMI), and diabetes status (where appropriate). Trait-specific LOD scores reported are empirical LOD scores determined with SOLAR by simulation (lodadj procedure) as described (Bowden et al., 2008).
As previously reported (Bowden et al., 2008), strong genetic correlations exist between the measures of subclinical CVD (CorCP, CarCP, AACP and IMT) in the Diabetes Heart Study participants. These correlations between vascular calcified plaque in all three vascular beds suggests that genetic variants contribute to systemic vascular calcification in multiple beds. A principal component (PC) analysis based on the significant genetic correlations between CorCP, CarCP, AACP and IMT indicated that two PCs explain more than 80% of the variation in these four measures. The first principal component was derived from mean of CorCP, CarCP and AACP to reflect vascular calcified plaque across multiple vascular beds (subsequently referred to vascular calcified plaque principal component (VCP-PC)); the second principal component was IMT alone (Bowden et al., 2008). Therefore, in addition to evaluating the CarCP phenotype alone for significant linkage to chromosome 16p, we also analyzed the genome scan data for linkage to calcification in other individual vascular beds and to the VCP-PC. Generation of the principal components has been described previously (Bowden et al., 2008).
Candidate Genes
For each gene, the physical map interval was obtained from the National Center for Biotechnology (NCBI) build 36 of the human genome. The Rutgers Combined Linkage-Physical Map of the Human Genome (Kong et al., 2004) was used to map each gene to the chromosomal sex-averaged genetic positions based on the NCBI build 36 physical map coordinates. The Rutgers Map Interpolator uses smoothed chromosomal maps to interpolate the genetic map positions.
Maximum likelihood allele and genotype frequencies for each SNP were calculated from unrelated probands and were tested for departures from Hardy-Weinberg equilibrium using χ2 tests. Estimates of linkage disequilibrium between SNPs were determined by calculating pairwise D' and r2 statistics in unrelated individuals. As previously reported, the microsatellite markers from a 10 cM genome scan were used to examine and correct self-reported familial relationships (Bowden et al., 2006).
Association between each SNP and each phenotype was tested using variance components methods implemented in SOLAR (Almasy & Blangero, 1998), adjusting for the covariates age, gender, BMI, and diabetes status (where appropriate). For each SNP, the two degree of freedom test of genotypic association with each phenotype was performed as the primary inferential analysis. In cases where there was nominal or trending evidence of association in the two degree of freedom test, genetic models (dominant, additive, and recessive) were computed to assess whether individual models provided greater insight into the trait association. No formal haplotype analyses were performed for the candidate genes. Within each candidate gene, SNPs were selected for genotyping based on their ability to tag the genetic variation within the gene region. Therefore, the genotyped SNPs within a gene were not necessarily contained within a single block of LD.
Bayesian Quantitative Trait Nucleotide (BQTN) Analysis
The BQTN analysis was conducted to identify variant(s) most strongly related to the trait. This approach to perform association is an extension of the classical variance component approach, and estimates not only the main effects of SNP genotypes but also the random effects to unravel the genetic structure of the trait (Almasy & Blangero, 1998; Blangero et al., 2005).
Details of the BQTN analysis are given elsewhere (Blangero et al., 2005; Curran et al., 2005). In short, in a Bayesian framework, the null hypothesis in which there are no fixed QTN effects is compared with a hypothesis in which QTN effects are being estimated. Bayesian information criterion (BIC) is defined with reference to a null model and is used to assess whether the QTN model explains sufficient variation in the phenotype to justify the number of parameters used. BIC difference provides an estimate of the evidence of support of one model over another. For example BIC differences of greater than two units provides evidence of support of one model over another with posterior probability of 75%, and BIC differences greater than 10 units represent support for one model over another with 99% posterior probabilities (Blangero et al., 1999). This approach (BQTN) has been shown to provide accurate determination of functional variants in conditions where all the variants have been identified (Blangero et al., 2005; Blangero et al., 1999; Curran et al., 2005; Soria et al., 2005), however, in those cases where a subset of SNPs have been genotyped, it has also proven extremely useful in identifying a SNP or set of SNPs, that are most likely to be functional or in high LD with a functional variant that has not been typed. To be consistent with the linkage and association results, age, gender and BMI were used as covariates.
RESULTS
The characteristics of the 937 EA participants evaluated in the fine-mapping and candidate gene analyses are presented in Table 1. Overall, these participants have biometric and clinical characteristics consistent with a diabetes-enriched family population: older age (mean age of 62 years in T2DM-affected individuals, mean age of 60 years in unaffected individuals), clinically obese (mean BMI of 32.5 in T2DM-affected individuals, mean BMI of 28.8 in unaffected individuals), and hypertensive (87.7% of T2DM-affected individuals, 66% of unaffected individuals). While the majority of all EA participants (both T2DM-affected and unaffected) have detectable calcified plaque in all three vascular beds (coronary artery, carotid artery, and abdominal aorta), the amount of quantifiable vascular calcium is significantly greater in the diabetes-affected individuals. In addition, the diabetes-affected individuals have lower total cholesterol and LDL cholesterol than their unaffected family members, which is likely due to a treatment effect since 45% of the T2DM-affected individuals were being treated with statins the time of recruitment (Bowden et al., 2005).
Table 1.
Clinical characteristics of the European American study sample.
| T2DM-affected (n=778) | Unaffected (n=159) | |
|---|---|---|
| Age (years) | 61.9±9.2 (61.8) | 59.6±10.4 (59.4) |
| Gender (% female) | 53.6 | 67.3 |
| BMI (kg/m2) | 32.5±6.8 (31.4) | 28.8±5.2 (28.1) |
| Duration of Diabetes (years) | 10.2±6.9 (8.0) | N/A |
| Systolic Blood Pressure (mm Hg) | 139.8±19.0 (138.5) | 135.3±19.3 (133.3) |
| Diastolic Blood Pressure (mm Hg) | 72.8±10.2 (72.0) | 74.3±10.2 (74.0) |
| Diagnosis of Hypertension (%) | 87.7 | 66.0 |
| Medications | ||
| Use of Hypertension Medication (%) | 78.6 | 43.7 |
| Use of Lipid Lowering Medication (%) | 44.3 | 26.8 |
| Insulin (%) | 26.2 | 0 |
| Oral hypoglycemic (%) | 76.2 | 0 |
| Laboratory | ||
| HbA1c (%) | 7.65±1.76 (7.30) | 5.57±0.51 (5.50) |
| Fasting Glucose (mg/dL) | 150.0±57.0 (138.0) | 93.5±11.5 (93.0) |
| Total Cholesterol (mg/dL) | 187.6±43.0 (182.0) | 195.7±34.2 (195.5) |
| HDL Cholesterol (mg/dL) | 42.8±12.0 (41.0) | 48.0±13.4 (46.0) |
| LDL Cholesterol (mg/dL) | 104.2±32.3 (102.0) | 115.0±29.3 (113.0) |
| Triglycerides (mg/dL) | 214.0±145.4 (179.0) | 163.7±78.0 (153.5) |
| Smoking | ||
| Current (%) | 16.3 | 20.9 |
| Past (%) | 42.7 | 35.4 |
| Never (%) | 41.0 | 43.7 |
| Subclinical CVD Measures | ||
| CorCP | 1391.0±2643.7 (357.0) | 444.7±989.5 (35.5) |
| CorCP>0 (%) | 94.8 | 83.0 |
| CarCP | 359.3±723.3 (77.5) | 133.8±355.3 (1.5) |
| CarCP>0 (%) | 77.4 | 55.9 |
| AACP | 3724.0±4518.3 (1937.0) | 2031.9±3447.6 (605.5) |
| AACP>0 (%) | 94.7 | 83.3 |
| IMT (mm) | 0.68±0.13 (0.66) | 0.64±0.11 (0.61) |
Data are presented as mean ± SD (median). CorCP = coronary artery calcified plaque; CarCP = carotid artery calcified plaque; AACP = abdominal aortic calcified plaque; IMT = intima-media thickness
Mapping of Vascular Calcified Plaque Loci
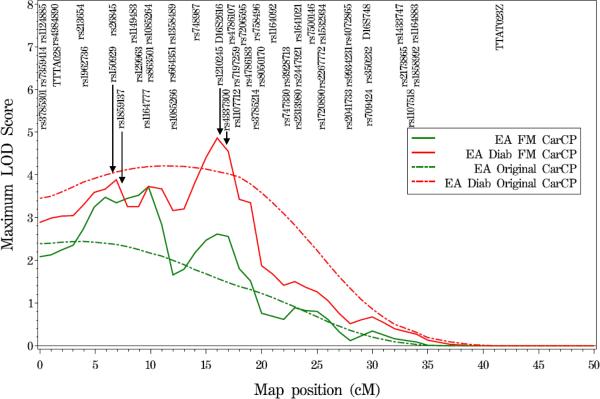
Based on the prior evidence of linkage of CarCP to chromosome 16p in EA T2DM-affected individuals, fine mapping of the region was carried out by genotyping 69 SNPs across the region. Of the 69 SNPs genotyped, eight SNPs failed to reach the genotyping efficiency threshold of 90% and two SNPs were not polymorphic in the DHS EA population. The remaining 59 SNPs (Supplementary Table 1) covered a 30cM region, with an average SNP density of 1 SNP/0.54 cM and the largest interval being 2.29 cM. A variance components QTL linkage analysis combining the initial genome scan microsatellite markers and the 59 additional fine-mapping SNPs was completed in the EA T2DM-affected individuals. As shown in Figure 1A, with fine mapping the prior CarCP linkage peak from the initial genome scan ((Bowden et al., 2008); LOD score of 4.39 at 8.4 cM) resolved into two distinct linkage regions with LOD scores of 3.89 at 6.9 cM (flanked by markers rs150929 and rs1859137) and 4.86 at 16.0 cM (flanked by markers rs12102452 and rs4337300). When all EA subjects (i.e., both T2DM-affected and unaffected) were included in the analysis the maximum LOD scores were 3.72 at 9.8 cM and 2.61 at 16.0 cM (Figure 1A).
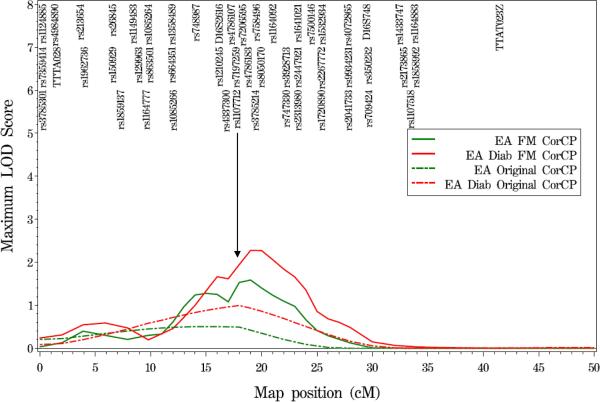
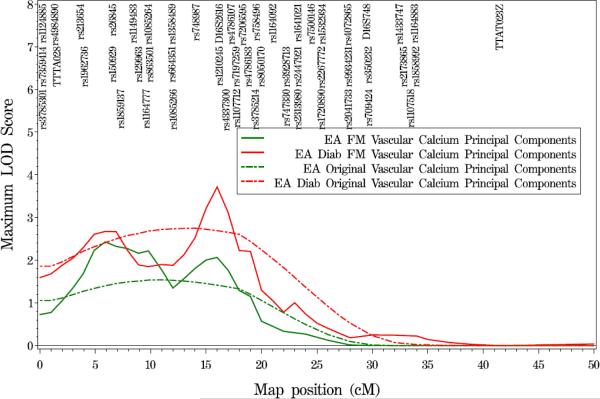
Figure 1.
Variance components QTL linkage analysis of chromosome 16 in EA families and EA T2DM-affected individuals. Results shown here reflect the linkage signals observed before and after fine-mapping. Broken lines represent results from the original genome scan, while solid lines represent the results from fine-mapping. To optimize legibility, not fine-mapping analyses SNPs are displayed on the figures.
(A) carotid artery calcified plaque (CarCP)
(B) coronary artery calcified plaque (CorCP)
(C) vascular calcified plaque principal component
Additional analysis of the genome scan data for linkage to calcification in other individual vascular beds and to the VCP-PC revealed evidence of linkage for CorCP (LOD=2.27 at 19 cM; Figure 1B) and the VCP-PC (LOD=2.67 at 5.9 cM, LOD=3.71 at 16.0 cM; Figure 1C) in analyses restricted to the EA T2DM-affected subjects. When all EA individuals were included in the analyses, these linkage signals remained statistically significant but decreased in magnitude by approximately one LOD unit.
Association Analysis
A total of six positional candidate genes located within the linked region were selected for detailed evaluation. The location and putative function for each gene is shown in Table 2. Forty-seven SNPs located within the six genes were genotyped based on the ability of the SNPs to tag the genetic variation in individuals of European ancestry (HapMap CEU population). Of the 47 SNPs genotyped, two SNPs failed to reach the genotyping efficiency threshold of 90%. The remaining 45 SNPs, along with the 59 SNPs used for fine-mapping, i.e., a total of 104 SNPs, were evaluated for association with CarCP, CorCP, AACP, VCP-PC, and IMT. For all 104 SNPs, the genotyping consensus rate for duplicate DNA samples within and across DNA plates was 100%.
Table 2.
Positional candidate genes on chromosome 16p.
| Name | Gene Symbol | Genetic Location (cM)* | Physical Location (bp)* | Role |
|---|---|---|---|---|
| Calcium Channel, Voltage-Dependent, T Type, Alpha-1H Subunit | CACNA1H | 4.05–4.29 | 1,143,242–1,211,772 | Allows for temporal and spatial control of intracellular calcium and supports regulation of cellular activity |
| Selenoprotein X | SEPX1 | 6.45–6.44 | 1,933,295–1,928,235 | Methionine sulfoxide reductase; functions as an antioxidant |
| ATP-Binding Cassette, Subfamily A, Member 3 | ABCA3 | 7.16–7.05 | 2,330,748–2,265,880 | Involved in regulation of lipid transport and membrane trafficking |
| Interleukin 32 | IL32 | 8.24–8.25 | 3,055,314–3,059,668 | Functions as a cytokine that induces TNFα, IL1β, IL6 and chemokines; plays a role in inflammatory and autoimmune diseases |
| C-type Lectin Domain Family 16, Member A | KIAA0350 | 27.84–28.38 | 10,945,943–11,183,539 | Unknown function; recently implicated in the development of type 1 diabetes |
| Suppressor of Cytokine Signalling 1 | SOCS1 | 28.53 | 11,257,540–11,255,775 | Inhibits signal transduction of some cytokines; abnormal expression of SOCS1 is involved in the development of leukemia, rheumatoid arthritis, liver cirrhosis, and liver cancer; ability to diminish insulin action |
Genetic and physical positions relative to NCBI Build 36.
The strongest evidence for association with the primary phenotype from the linkage analysis, CarCP, was observed with a group of four SNPs in a 1.1 Mb region used in the fine-mapping component of the study (Table 3; p-values ranging from 0.018 to 0.079, overall two degree of freedom test). Three of these associated SNPs (rs1358489, rs7186211, rs748987) are located in an intergenic region upstream of the A2BP1 gene, which encodes the ataxin 2-binding protein. The remaining associated SNP (rs4337300) is located in intron 2 of A2BP1, isoform 4. Evaluation of the mean CarCP scores by genotype indicates that rs1358489 association is most consistent with a recessive model of inheritance, with homozygotes for the C allele having greater detectable CarCP than carriers of the T allele (p=0.005; Table 4A). In contrast, rs748987 appears to follow a dominant model, as individuals carrying at least one copy of the C allele have lower detectable CarCP than individuals who are homozygous for the G allele (p=0.026; Table 4A). While at least one model-specific p-value is significant for each of the two remaining associated SNPs, the mean CarCP values by genotype do not suggest an obvious genetic model (Table 4A). Two additional SNPs located within introns 3 and 13 of A2BP1, respectively, exhibited a trend towards association with calcified plaque in one of the other vascular beds (rs11077123, p=0.091 for CorCP; rs3785214, p=0.065 for AACP; Table 3).
Table 3.
Association analysis of fine-mapping SNPs and positional candidate gene SNPs with quantitative measures of subclinical CVD in European American T2DM-affected individuals.
| p-values | ||||||
|---|---|---|---|---|---|---|
| Region/Gene | SNP | CorCP | CarCP | AACP | VCP-PC | IMT |
| CACNA1H | rs3809635 | 0.969 | 0.010 | 0.024 | 0.069 | 0.163 |
| rs11640796 | 0.079 | 0.127 | 0.955 | 0.073 | 0.577 | |
| rs4347630 | 0.491 | 0.337 | 0.101 | 0.207 | 0.136 | |
| rs4984636 | 0.332 | 0.600 | 0.299 | 0.430 | 0.567 | |
| rs2745167 | 0.505 | 0.916 | 0.982 | 0.779 | 0.172 | |
| rs4984637 | 0.014 | 0.651 | 0.322 | 0.105 | 0.027 | |
| rs1054645 | 0.096 | 0.674 | 0.767 | 0.387 | 0.061 | |
| rs1004041 | 0.033 | 0.896 | 0.675 | 0.295 | 0.101 | |
| rs12934797 | 0.292 | 0.671 | 0.795 | 0.583 | 0.517 | |
|
| ||||||
| Chr16p13.13–13.3 | rs1962736 | 0.077 | 0.884 | 0.296 | 0.389 | 0.474 |
|
| ||||||
| SEPX1 | rs13331553 | 0.590 | 0.499 | 0.458 | 0.635 | 0.283 |
| rs9934331 | 0.373 | 0.869 | 0.432 | 0.610 | 0.461 | |
| rs1003904 | 0.954 | 0.502 | 0.728 | 0.787 | 0.177 | |
| rs2252523 | 0.381 | 0.597 | 0.357 | 0.633 | 0.814 | |
|
| ||||||
| ABCA3 | rs150926 | 0.887 | 0.720 | 0.557 | 0.732 | 0.862 |
| rs323074 | 0.451 | 0.960 | 0.946 | 0.988 | 0.707 | |
| rs323069 | 0.943 | 0.970 | 1.000 | 0.771 | 0.183 | |
| rs323066 | 0.949 | 0.749 | 0.965 | 0.822 | 0.602 | |
|
| ||||||
| Chr16p13.13–13.3 | rs17660212 | 0.643 | 0.766 | 0.670 | 0.644 | 0.008 |
|
| ||||||
| IL32 | rs10438593 | 0.104 | 0.485 | 0.117 | 0.128 | 0.354 |
| rs7188573 | 0.902 | 0.080 | 0.591 | 0.222 | 0.181 | |
| rs1554999 | 0.358 | 0.077 | 0.165 | 0.065 | 0.444 | |
| rs2239301 | 0.153 | 0.602 | 0.941 | 0.624 | 0.462 | |
| rs1555001 | 0.380 | 0.933 | 0.498 | 0.770 | 0.447 | |
|
| ||||||
| Chr16p13.13–13.3 | rs129963 | 0.224 | 0.026 | 0.152 | 0.026 | 0.282 |
| rs11647778 | 0.008 | 0.674 | 0.222 | 0.130 | 0.530 | |
| rs1358489 | 0.148 | 0.018 | 0.142 | 0.241 | 0.209 | |
| rs7186211 | 0.688 | 0.079 | 0.292 | 0.263 | 0.205 | |
| rs748987 | 0.765 | 0.067 | 0.020 | 0.230 | 0.049 | |
| rs4337300 | 0.251 | 0.038 | 0.118 | 0.050 | 0.745 | |
| rs11077123 | 0.091 | 0.531 | 0.725 | 0.404 | 0.823 | |
| rs3785214 | 0.109 | 0.506 | 0.065 | 0.178 | 0.927 | |
| rs2313980 | 0.599 | 0.006 | 0.552 | 0.056 | 0.185 | |
|
| ||||||
| KIAA0350 | rs8055876 | 0.999 | 0.185 | 0.195 | 0.763 | 0.547 |
| rs16957849 | 0.479 | 0.779 | 0.163 | 0.781 | 0.327 | |
| rs17803698 | 0.051 | 0.862 | 0.034 | 0.173 | 0.542 | |
| rs7197758 | 0.297 | 0.942 | 0.139 | 0.690 | 0.980 | |
| rs9940155 | 0.448 | 0.011 | 0.206 | 0.083 | 0.616 | |
| rs723586 | 0.973 | 0.076 | 0.218 | 0.480 | 0.830 | |
| rs7186166 | 0.052 | 0.046 | 0.083 | 0.037 | 0.748 | |
| rs11074945 | 0.192 | 0.231 | 0.099 | 0.098 | 0.154 | |
| rs8062923 | 0.986 | 0.981 | 0.641 | 0.920 | 0.993 | |
| rs725613 | 0.325 | 0.645 | 0.061 | 0.189 | 0.136 | |
| rs9652582 | 0.926 | 0.685 | 0.122 | 0.472 | 0.223 | |
| rs12932833 | 0.463 | 0.908 | 0.401 | 0.716 | 0.982 | |
| rs9926078 | 0.313 | 0.129 | 0.267 | 0.226 | 0.520 | |
| rs12935657 | 0.761 | 0.614 | 0.621 | 0.494 | 0.823 | |
| rs2003400 | 0.490 | 0.635 | 0.445 | 0.513 | 0.997 | |
| rs794426 | 0.950 | 0.076 | 0.470 | 0.353 | 0.762 | |
| rs7204935 | 0.193 | 0.318 | 0.407 | 0.219 | 0.724 | |
| rs27838 | 0.455 | 0.150 | 0.239 | 0.152 | 0.870 | |
| rs767019 | 0.891 | 0.194 | 0.994 | 0.453 | 0.766 | |
| rs11643123 | 0.891 | 0.747 | 0.254 | 0.811 | 0.855 | |
| rs3960630 | 0.791 | 0.180 | 0.700 | 0.406 | 0.698 | |
|
| ||||||
| SOCS1 | rs243327 | 0.146 | 0.057 | 0.087 | 0.096 | 0.116 |
| rs243325 | 0.537 | 0.540 | 0.163 | 0.630 | 0.267 | |
|
| ||||||
| Chr16p13.13–13.3 | rs350232 | 0.560 | 0.455 | 0.080 | 0.895 | 0.651 |
| rs1858992 | 0.923 | 0.388 | 0.083 | 0.366 | 0.506 | |
Results are shown for all positional candidate gene SNPs and only the fine-mapping SNPs with evidence of association with at least one phenotype. P-values determined from the genotypic two degree-of-freedom test. CorCP = coronary artery calcified plaque; CarCP = carotid artery calcified plaque; AACP = abdominal aortic calcified plaque; VCP-PC= vascular calcified plaque principal component; IMT = intima-media thickness
Table 4.
Untransformed mean trait values by genotype (from individuals with both genotype and phenotype information available), model-specific p-values for associated SNPs and Hardy-Weinberg Equilibrium (HWE) assessment.
| (A) Mean carotid artery calcified plaque scores by genotype for associated SNPs in intergenic region of chromosome 16p. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Alleles (Major/Minor) | MAF | Mean ± Standard Deviation | p-values |
HWE (χ2) | |||||
| Overall | Additive | Dominant | Recessive | |||||||
| rs1358489 | T/C | 0.475 | TT (n=158) 363.80±866.55 | TC (n=338) 333.16±678.47 | CC (n=163) 419.80±647.09 | 0.018 | 0.012 | 0.227 | 0.005 | 0.55 |
| rs7186211 | C/T | 0.470 | CC (n=170) 246.36±497.25 | CT (n=314) 390.95±741.06 | TT (n=164) 361.43±714.75 | 0.079 | 0.024 | 0.073 | 0.063 | 0.86 |
| rs748987 | G/C | 0.479 | GG (n=146) 410.11±718.85 | GC (n=348) 351.02±759.90 | CC (n=164) 339.36±634.57 | 0.067 | 0.177 | 0.026 | 0.966 | 0.32 |
| rs4337300 | C/T | 0.463 | CC (n=133) 361.09±695.82 | CT (n=319) 343.47±747.72 | TT (n=181) 378.04±717.61 | 0.038 | 0.080 | 0.863 | 0.012 | 0.92 |
| (B) Mean coronary artery calcified plaque scores by genotype for associated SNPs in CACNA1H. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Alleles (Major/Minor) | MAF | Mean ± Standard Deviation | p-values |
HWE (χ2) | |||||
| Overall | Additive | Dominant | Recessive | |||||||
| rs11640796 | A/G | 0.132 | AA (n=458) 1349.92±2606.97 | AG (n=139) 1353.31±2383.74 | GG (n=14) 1860.96±1984.80 | 0.079 | 0.543 | 0.924 | 0.030 | 0.90 |
| rs4984637 | C/T | 0.199 | CC (n=405) 1482.44±2987.10 | CT (n=171) 1447.82±2106.08 | TT (n=34) 490.68±516.77 | 0.014 | 0.842 | 0.198 | 0.036 | 0.029 |
| rs1054645 | A/G | 0.403 | AA (n=220) 1334.96±2771.22 | AG (n=257) 1572.21±2773.88 | GG (n=108) 1031.06±1498.13 | 0.096 | 0.781 | 0.178 | 0.250 | 0.005 |
| rs1004041 | G/T | 0.467 | GG (n=193) 1134.06±1944.07 | GT (n=248) 1693.03±3238.41 | TT (n=143) 1360.63±2624.12 | 0.033 | 0.924 | 0.187 | 0.107 | 0.022 |
Models are reported relative to the minor allele. MAF = minor allele frequency.
Modest evidence of association was observed between CorCP and SNPs in CACNA1H, which functions as the alpha subunit of a voltage-dependent calcium channel that mediates the relaxation of coronary smooth muscle. As shown in Table 3, two of the nine CACNA1H SNPs were nominally associated with CorCP in the EA T2DM-affected individuals (rs4984637, p=0.014; rs1004041, p=0.033); two additional SNPs exhibited a trend towards association with CorCP (rs11640796, p=0.079; rs1054645, p=0.096). Three of these associated SNPs (rs4984637, rs1054645, rs1004041) were inconsistent with Hardy-Weinberg Equilibrium (p<0.05) due to a deficiency in the observed number of heterozygotes and formed a small LD block (data not shown). Evaluation of the mean CorCP values by genotype for each of the SNPs indicates that rs11640796 and rs4984637 follow a recessive model of inheritance. Homozygotes for the minor allele G of rs11640796 have greater detectable CorCP than carriers of the major allele A (p=0.030; Table 4B). In contrast, homozygotes for the minor allele T of rs4984637 have much less detectable CorCP than carrier of the major allele C (p=0.036; Table 4B). The remaining two SNPs exhibit no significant associations under any of the three genetic models tested and likewise exhibit no apparent trend in the mean CorCP scores by genotype (Table 4B). It should be noted that several of the SNPs in Table 4B are out of HWE if uncorrected for the total number of SNPs in the analysis.
Bayesian Quantitative Trait Nucleotide (BQTN) Analysis
The linkage and SNP association analysis was complemented by application of the BQTN method in an effort to identify the variant(s) most strongly related to the primary traits in this study. The results of this analysis are summarized briefly in Table 5. With BQTN, a posterior probability of ≥75% (0.75) is indicative of positive evidence supporting the functional model over the null model of no effect; ≥95 % (0.95) is indicative of strong support for the functional model; and ≥99% (0.99) is indicative of very strong support for the functional model. The SNP rs1358489 had a posterior probability of 0.898, providing evidence that this SNP warrants consideration as one variant likely to be associated (either functionally or as a tagging SNP) with the CarCP phenotype. A second SNP, rs4337300, narrowly missed meeting the criteria for influencing the VCP-PC (0.749).
Table 5.
Results from Bayesian Quantitative Trait Nucleotide (BQTN) analysis of the SNP data.
| Trait | Gene/Region_SNP | Posterior probability of a functional effect (BQTN analysis)* |
|---|---|---|
| CorCP | N/S | N/S |
| IMT | CACNA1H_rs1004041 | 0.738 |
| CarCP | KIAA0350_rs723586 | 0.496 |
| Chr16p_rs1358489 | 0.898 | |
| AACP | N/S | N/S |
| VCP-PC | Chr16p_rs4337300 | 0.749 |
Posterior probability of 75% (0.75) and greater is indicative of positive evidence of support for the functional model over the null model of no effect; 95 % (0.95) is indicative of strong support favoring the functional model over the null model of no effect; 99% (0.99) is indicative of very strong support of a functional model.
CorCP = coronary artery calcified plaque; IMT = intima-media thickness; CarCP = carotid artery calcified plaque; AACP = abdominal aortic calcified plaque; VCP-PC = vascular calcified plaque principal component
DISCUSSION
We have conducted detailed genetic analyses of subclinical CVD on chromosome 16p in European American families from the Diabetes Heart Study. Fine-mapping and analysis of a set of positional candidate genes for association with quantitative measures of subclinical CVD was completed and accompanied by Bayesian Quantitative Trait Nucleotide analysis. From the fine-mapping efforts, we observed refinement of the initial single linkage peak for CarCP into two distinct signals that maximize at 6.9 cM and 16.0 cM in the EA T2DM-affected individuals and observed an increased maximum LOD score of 4.86. In addition, we observed evidence for linkage to other vascular calcification phenotypes, notably CorCP and the principal component of vascular calcified plaque (VCP-PC). This evidence of linkage was not observed in the original genome scan analysis (Bowden et al., 2008). These new observations suggest that loci within the linked region contribute to calcified plaque in multiple vascular beds. The coincident linkages of CarCP and CorCP in this region of chromosome 16p, coupled with the significant genetic correlation between CarCP and CorCP in the DHS population (0.52±0.11, p<0.05) (Bowden et al., 2008), suggests the same locus is contributing to calcification in multiple vascular beds.
Although, previous studies have focused primarily on the heritability of carotid artery plaque and atherosclerotic lesions (Hunt et al., 2002; Moskau et al., 2005), including our own previous report of a heritability of 0.40±0.08 for CarCP in the DHS EA population (Bowden et al., 2008), to our knowledge the current results provide the first evidence of a QTL for CarCP. A recent genome-wide linkage analysis of carotid artery plaque in National Heart, Lung, Blood Institute (NHLBI) Family Heart Study did not identify any regions of significant or suggestive linkage for carotid artery plaque; however, they did observe suggestive evidence for linkage (LOD=2.43) on chromosome 2p11.2 in the subset of sibling pairs aged 55 years or younger (Pankow et al., 2004). Further, a genome-wide association study for subclinical atherosclerosis in the Framingham population did not include measures of CarCP, however did report significant associations for other measures of subclinical CVD at regions that did not include 16p (O'Donnell et al., 2007). The lack of reproducibility between these studies is not unusual and it is worth noting at this point that the DHS is a novel population sample, being highly enriched for T2DM. The observation that linkage is amplified when analyses were limited to diabetes affected subjects is consistent with our prior observations (Bowden et al 2008). The observation of increased evidence of linkage in diabetic subjects is consistent with a model in which the diabetes environment amplifies the effect of risk polymorphisms.
Analysis of both the fine mapping SNPs and candidate gene SNPs, revealed the most significant evidence for association with the CarCP phenotype was observed with SNPs located in an intergenic region. Three of the associated SNPs are located upstream of the A2BP1 gene, with the fourth SNP being located in intron 2 of A2BP1. However, if one takes into account the multiple comparisons, results are at best marginally significant. That said, the multiple comparisons problem with correlated SNPs and correlated traits is not straightforward and simple Bonferonni corrections are inappropriate (Rice et al., 2008). By using a BQTN approach, the intergenic SNP most strongly associated with CarCP, rs1358489, had a posterior probability of 0.898 for CarCP providing further evidence that this SNP may be one variant with a functional impact on CarCP. In addition, the SNP located in intron 2 of A2BP1 (rs4337300) fell just short of the 0.75 BQTN threshold supporting a functional role, with a further two SNPs in introns 3 and 13 exhibiting a trend towards association with calcified plaque in other vascular beds. Given that A2BP1 encodes the ataxin 2-binding protein 1 and is predominantly expressed in muscle and brain it is difficult to speculate how SNPs in the A2BP1 gene might be involved in the development of vascular calcified plaque. However, intergenic SNPs should not be automatically ignored in linkage studies; polymorphisms in an intergenic region of chromosome 9 have been consistently and reproducibly demonstrated to be significantly associated with T2DM (Saxena et al., 2007; Scott et al., 2007; Zeggini et al., 2007), coronary heart disease (McPherson et al., 2007), myocardial infarction (Helgadottir et al., 2007), and measures of subclinical atherosclerosis (O'Donnell et al., 2007). Other possibilities are that the associated SNPs are not causally responsible for the development of CarCP, but are instead in strong LD with the true causal variant(s), although evidence for this interpretation is limited in the current Involvement of other hypothetical genes and expressed sequences located within the region should also be considered. The associated SNPs are located in an intergenic region between the A2BP1 and FAM86A genes. FAM86A encodes a hypothetical protein and there is additional evidence of a predicted gene (based on expressed sequence data) located within the region. It is therefore possible that the associated SNPs may be involved in the expression of these unknown genes
Six positional candidate genes were also evaluated for association with the quantitative measures of subclinical CVD with the most consistent evidence of association observed with SNPs in the CACNA1H gene and the CorCP phenotype. CACNA1H encodes the alpha -1H subunit of voltage-dependent calcium channels and is involved in the mitogen-activated protein kinase (MAPK) signaling pathway. This complex signal transduction pathway regulates various cellular activities such as apoptosis, proliferation, differentiation and inflammation. As atherosclerosis is a chronic inflammatory condition that begins early in life (Scheuner, 2001) and culminates in the accumulation of plaque in the artery wall, genes involved in the inflammatory process are attractive candidates for vascular calcified plaque formation. Of the nine SNPs evaluated in CACNA1H, rs4984637 and rs1004041 were significantly associated with CorCP and two additional SNPs exhibited a trend towards association with CorCP.
In summary, the Diabetes Heart Study is an extensively phenotyped sample of T2DM-enriched families that provides a unique resource for genetic studies of subclinical CVD. This study reports the first strong evidence for a QTL for CarCP. These loci may be involved more broadly in systemic vascular calcification, as demonstrated by the coincident linkages of CarCP, CorCP and the VCP-PC to this region of chromosome 16p. The current study refined the regions of interest and potential regions for follow-up investigation include the CACNA1H gene and the intergenic region between A2BP1 and FAM86A. As it is now widely acknowledged that common variations frequently contribute relatively modestly to the genetic basis of complex traits (Manolio et al., 2009), many investigators are reassessing the relevance of family studies and their ability to contribute insights into the molecular basis of complex traits. A linkage analysis such as this, which results in good evidence of linkage, may be an excellent candidate for detailed analysis such as exome sequencing to identify rare variants.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by the General Clinical Research Center of the Wake Forest University School of Medicine (Grant M01 RR07122) and by the National Heart, Lung, and Blood Institute (Grants R01 AR48797 to JJC, R01 HL67348 and R01HL092301 to DWB, and F32 HL085989 to ABL).
REFERENCES
- Abbott RD, Donahue RP, Macmahon SW, Reed DM, Yano K. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA. 1987;257:949–952. [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bhalla K, Phillips HA, Crawford J, Mckenzie OL, Mulley JC, Eyre H, Gardner AE, Kremmidiotis G, Callen DF. The de novo chromosome 16 translocations of two patients with abnormal phenotypes (mental retardation and epilepsy) disrupt the A2BP1 gene. J Hum Genet. 2004;49:308–311. doi: 10.1007/s10038-004-0145-4. [DOI] [PubMed] [Google Scholar]
- Blangero J, Goring HH, Kent JW, Jr., Williams JT, Peterson CP, Almasy L, Dyer TD. Quantitative trait nucleotide analysis using Bayesian model selection. Hum Biol. 2005;77:541–559. doi: 10.1353/hub.2006.0003. [DOI] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Iturria SJ, Almasy L. Oligogenic model selection using the Bayesian Information Criterion: linkage analysis of the P300 Cz event-related brain potential. Genet Epidemiol. 1999;17(Suppl 1):S67–72. doi: 10.1002/gepi.1370170712. [DOI] [PubMed] [Google Scholar]
- Bowden DW, Lange LA, Langefeld CD, Brosnihan KB, Freedman BI, Carr JJ, Wagenknecht LE, Herrington DM. The relationship between C-reactive protein and subclinical cardiovascular disease in the Diabetes Heart Study (DHS) Am Heart J. 2005;150:1032–1038. doi: 10.1016/j.ahj.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Bowden DW, Lehtinen AB, Ziegler JT, Rudock ME, Xu J, Wagenknecht LE, Herrington DM, Rich SS, Freedman BI, Carr JJ, Langefeld CD. Genetic epidemiology of subclinical cardiovascular disease in the diabetes heart study. Ann Hum Genet. 2008;72:598–610. doi: 10.1111/j.1469-1809.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden DW, Rudock M, Ziegler J, Lehtinen AB, Xu J, Wagenknecht LE, Herrington D, Rich SS, Freedman BI, Carr JJ, Langefeld CD. Coincident linkage of type 2 diabetes, metabolic syndrome, and measures of cardiovascular disease in a genome scan of the diabetes heart study. Diabetes. 2006;55:1985–1994. doi: 10.2337/db06-0003. [DOI] [PubMed] [Google Scholar]
- Brand FN, Abbott RD, Kannel WB. Diabetes, intermittent claudication, and risk of cardiovascular events. The Framingham Study. Diabetes. 1989;38:504–509. doi: 10.2337/diab.38.4.504. [DOI] [PubMed] [Google Scholar]
- Carr JJ, Nelson JC, Wong ND, Mcnitt-Gray M, Arad Y, Jacobs DR, Jr., Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, Maccluer JW, Collier GR, Kissebah AH, Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- De Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, Mcnitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, Pasternak S, Wheeler DA, Willis TD, Yu F, Yang H, Zeng C, Gao Y, Hu H, Hu W, Li C, Lin W, Liu S, Pan H, Tang X, Wang J, Wang W, Yu J, Zhang B, Zhang Q, Zhao H, Zhou J, Gabriel SB, Barry R, Blumenstiel B, Camargo A, Defelice M, Faggart M, Goyette M, Gupta S, Moore J, Nguyen H, Onofrio RC, Parkin M, Roy J, Stahl E, Winchester E, Ziaugra L, Altshuler D, Shen Y, Yao Z, Huang W, Chu X, He Y, Jin L, Liu Y, Sun W, Wang H, Wang Y, Xiong X, Xu L, Waye MM, Tsui SK, Xue H, Wong JT, Galver LM, Fan JB, Gunderson K, Murray SS, Oliphant AR, Chee MS, Montpetit A, Chagnon F, Ferretti V, Leboeuf M, Olivier JF, Phillips MS, Roumy S, Sallee C, Verner A, Hudson TJ, Kwok PY, Cai D, Koboldt DC, Miller RD, Pawlikowska L, Taillon-Miller P, Xiao M, Tsui LC, Mak W, Song YQ, Tam PK, Nakamura Y, Kawaguchi T, Kitamoto T, Morizono T, Nagashima A, Ohnishi Y, Sekine A, Tanaka T, Tsunoda T, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland P, Labree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Heath SC. Markov chain Monte Carlo segregation and linkage analysis for oligogenic models. Am J Hum Genet. 1997;61:748–760. doi: 10.1086/515506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- Hunt KJ, Duggirala R, Goring HH, Williams JT, Almasy L, Blangero J, O'leary DH, Stern MP. Genetic basis of variation in carotid artery plaque in the San Antonio Family Heart Study. Stroke. 2002;33:2775–2780. doi: 10.1161/01.str.0000043827.03966.ef. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Mcgee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Kong X, Murphy K, Raj T, He C, White PS, Matise TC. A combined linkage-physical map of the human genome. Am J Hum Genet. 2004;75:1143–1148. doi: 10.1086/426405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange LA, Bowden DW, Langefeld CD, Wagenknecht LE, Carr JJ, Rich SS, Riley WA, Freedman BI. Heritability of carotid artery intima-medial thickness in type 2 diabetes. Stroke. 2002;33:1876–1881. doi: 10.1161/01.str.0000019909.71547.aa. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, Mccarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, Mccarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CL, Duvall JA, Ilkin Y, Simon JS, Arreaza MG, Wilkes K, Alvarez-Retuerto A, Whichello A, Powell CM, Rao K, Cook E, Geschwind DH. Cytogenetic and molecular characterization of A2BP1/FOX1 as a candidate gene for autism. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:869–876. doi: 10.1002/ajmg.b.30530. [DOI] [PubMed] [Google Scholar]
- McPeek MS, Sun L. Statistical tests for detection of misspecified relationships by use of genome-screen data. Am J Hum Genet. 2000;66:1076–1094. doi: 10.1086/302800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcpherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boerwinkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen H, Lehto S, Salomaa V, Mahonen M, Niemela M, Haffner SM, Pyorala K, Tuomilehto J. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care. 1998;21:69–75. doi: 10.2337/diacare.21.1.69. [DOI] [PubMed] [Google Scholar]
- Moskau S, Golla A, Grothe C, Boes M, Pohl C, Klockgether T. Heritability of carotid artery atherosclerotic lesions: an ultrasound study in 154 families. Stroke. 2005;36:5–8. doi: 10.1161/01.STR.0000149936.33498.83. [DOI] [PubMed] [Google Scholar]
- O'donnell CJ, Cupples LA, D'agostino RB, Fox CS, Hoffmann U, Hwang SJ, Ingellson E, Liu C, Murabito JM, Polak JF, Wolf PA, Demissie S. Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI's Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S4. doi: 10.1186/1471-2350-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeth P, Beaulieu M, Park C, Kosman D, Mistro GD, Boom DVD, Jurinke C. iPLEX assay: increased plexing efficiency and flexibility for MassARRAY system through single base primer extension with mass-modified terminators. Sequenom Application Note. 2005 [Google Scholar]
- Pan WH, Cedres LB, Liu K, Dyer A, Schoenberger JA, Shekelle RB, Stamler R, Smith D, Collette P, Stamler J. Relationship of clinical diabetes and asymptomatic hyperglycemia to risk of coronary heart disease mortality in men and women. Am J Epidemiol. 1986;123:504–516. doi: 10.1093/oxfordjournals.aje.a114266. [DOI] [PubMed] [Google Scholar]
- Pankow JS, Heiss G, Evans GW, Sholinsky P, Province MA, Coon H, Ellison RC, Miller MB, Qaqish B. Familial aggregation and genome-wide linkage analysis of carotid artery plaque: the NHLBI family heart study. Hum Hered. 2004;57:80–89. doi: 10.1159/000077545. [DOI] [PubMed] [Google Scholar]
- Raggi P, Cooil B, Callister TQ. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J. 2001;141:375–382. doi: 10.1067/mhj.2001.113220. [DOI] [PubMed] [Google Scholar]
- Raggi P, Shaw LJ, Berman DS, Callister TQ. Gender-based differences in the prognostic value of coronary calcification. J Womens Health (Larchmt) 2004;13:273–283. doi: 10.1089/154099904323016437. [DOI] [PubMed] [Google Scholar]
- Rice TK, Schork NJ, Rao DC. Methods for handling multiple testing. Adv Genet. 2008;60:293–308. doi: 10.1016/S0065-2660(07)00412-9. [DOI] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, De Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Scheuner MT. Genetic predisposition to coronary artery disease. Curr Opin Cardiol. 2001;16:251–260. doi: 10.1097/00001573-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh J, Morag-Koren N, Goldbourt U, Grossman E, Tenenbaum A, Fisman EZ, Apter S, Itzchak Y, Motro M. Coronary calcium by spiral computed tomography predicts cardiovascular events in high-risk hypertensive patients. J Hypertens. 2004;22:605–610. doi: 10.1097/00004872-200403000-00024. [DOI] [PubMed] [Google Scholar]
- Simon A, Giral P, Levenson J. Extracoronary atherosclerotic plaque at multiple sites and total coronary calcification deposit in asymptomatic men. Association with coronary risk profile. Circulation. 1995;92:1414–1421. doi: 10.1161/01.cir.92.6.1414. [DOI] [PubMed] [Google Scholar]
- Soria JM, Almasy L, Souto JC, Sabater-Lleal M, Fontcuberta J, Blangero J. The F7 gene and clotting factor VII levels: dissection of a human quantitative trait locus. Hum Biol. 2005;77:561–575. doi: 10.1353/hub.2006.0006. [DOI] [PubMed] [Google Scholar]
- Terry JG, Carr JJ, Tang R, Evans GW, Kouba EO, Shi R, Cook DR, Vieira JL, Espeland MA, Mercuri MF, Crouse JR., 3rd Coronary artery calcium outperforms carotid artery intima-media thickness as a noninvasive index of prevalent coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2005;25:1723–1728. doi: 10.1161/01.ATV.0000173418.42264.19. [DOI] [PubMed] [Google Scholar]
- Vliegenthart R, Oudkerk M, Hofman A, Oei HH, Van Dijck W, Van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Bowden DW, Carr JJ, Langefeld CD, Freedman BI, Rich SS. Familial aggregation of coronary artery calcium in families with type 2 diabetes. Diabetes. 2001;50:861–866. doi: 10.2337/diabetes.50.4.861. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Langefeld CD, Carr JJ, Riley W, Freedman BI, Moossavi S, Bowden DW. Race-specific relationships between coronary and carotid artery calcification and carotid intimal medial thickness. Stroke. 2004;35:e97–99. doi: 10.1161/01.STR.0000127081.99767.1d. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, Mccarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.