Abstract
Substantia nigra pars reticulata (SNr) GABAergic neurons are key output neurons of the basal ganglia. Given the role of these neurons in motor control, it is important to understand factors that regulate their firing rate and pattern. One potential regulator is hydrogen peroxide (H2O2), a reactive oxygen species that is increasingly recognized as a neuromodulator. We used whole-cell current clamp recordings of SNr GABAergic neurons in guinea-pig midbrain slices to determine how H2O2 affects the activity of these neurons and to explore the classes of ion channels underlying those effects. Elevation of H2O2 levels caused an increase in the spontaneous firing rate of SNr GABAergic neurons, whether by application of exogenous H2O2 or amplification of endogenous H2O2 through inhibition of glutathione peroxidase with mercaptosuccinate. This effect was reversed by flufenamic acid (FFA), implicating transient receptor potential (TRP) channels. Conversely, depletion of endogenous H2O2 by catalase, a peroxidase enzyme, decreased spontaneous firing rate and firing precision of SNr neurons, demonstrating tonic control of firing rate by H2O2. Elevation of H2O2 in the presence of FFA revealed an inhibition of tonic firing that was prevented by blockade of ATP-sensitive K+ (KATP) channels with glibenclamide. In contrast to guinea-pig SNr neurons, the dominant effect of H2O2 elevation in mouse SNr GABAergic neurons was hyperpolarization, indicating a species difference in H2O2-dependent regulation. Thus, H2O2 is an endogenous modulator of SNr GABAergic neurons, acting primarily through presumed TRP channels in guinea-pig SNr, with additional modulation via KATP channels to regulate SNr output.
Keywords: basal ganglia, diffusible messenger, GABA, hydrogen peroxide, reactive oxygen species, TRP channels
Introduction
The GABAergic neurons of the substantia nigra pars reticulata (SNr) comprise one of the major output nuclei of the basal ganglia, and convey information from the basal ganglia network through projections that target the thalamus and superior colliculus, as well as other nuclei including the pedunculopontine nucleus and the mesencephalic locomotor region (Beckstead and Frankfurter, 1982; Deniau and Chevalier, 1992; Redgrave et al., 1992; Mana and Chevalier, 2001; Takakusaki et al., 2003; Cebrián et al., 2005; Lee and Tepper, 2007a; Nambu, 2007). Identifying intrinsic membrane conductances and extrinsic factors that influence the excitability of these neurons is therefore important for understanding regulation of movement by the basal ganglia.
Substantia nigra pars reticulata GABAergic neurons are spontaneously active in vivo and in vitro (Wilson et al., 1977; Deniau et al., 1978; Guyenet and Aghajanian, 1978; Nakanishi et al., 1987; Lacey et al., 1989; Yung et al., 1991; Stanford and Lacey, 1996; Richards et al., 1997; Atherton and Bevan, 2005; Lee and Tepper, 2007b; Zhou et al., 2008). A variety of conductances contribute to this tonic firing, and spontaneous activity persists in the absence of synaptic input indicating that it is intrinsically generated (Atherton and Bevan, 2005). Tonic firing can be modulated, however, by synaptic input as well as by activation of membrane conductances that cause changes in firing rate and pattern (Rick and Lacey, 1994; Stanford and Lacey, 1996; Shen and Johnson, 2006; Zhou et al., 2006, 2008; Ibáñez-Sandoval et al., 2007). Among the important membrane conductances in SNr GABAergic neurons are those mediated by transient receptor potential (TRP) channels (Lee and Tepper, 2007b; Zhou et al., 2008). A number of TRP channel subfamilies are expressed in the CNS (Clapham et al., 2003, 2005), and the canonical TRP type-3 (TRPC3) channel has been identified as a regulator of SNr GABAergic neuron excitability in neonatal mice (Zhou et al., 2008). Activation of TRPC3 channels in SNr neurons increases the firing rate of these cells and contributes to the tonic depolarization that maintains their spontaneous firing (Zhou et al., 2008, 2009). In addition, TRP channel activation may underlie a depolarizing plateau potential observed in these neurons (Lee and Tepper, 2007b). A potential opponent of TRP channel activity is ATP-sensitive K+ (KATP) channels, which can cause membrane hyperpolarization and suppress firing in SNr GABAergic neurons (Schwanstecher and Panten, 1993; Stanford and Lacey, 1996; Dunn-Meynell et al., 1998).
The emerging neuromodulator hydrogen peroxide (H2O2) can activate both some TRP channels and KATP channels (Ichinari et al., 1996; Herson and Ashford, 1997; Tokube et al., 1998; Hara et al., 2002; Avshalumov and Rice, 2003; Avshalumov et al., 2005; Bao et al., 2005; Freestone et al., 2009). Through these effects, H2O2 has been shown to be an important neuromodulator in basal ganglia neurons, including striatal medium spiny neurons (MSNs), which are depolarized by H2O2 through a TRP channel-dependent mechanism (Bao et al., 2005), and dopaminergic (DAergic) neurons of the substantia nigra pars compacta (SNc), which are hyperpolarized by activation of KATP channels (Avshalumov et al., 2005). Whether H2O2 has a neuromodulatory action on SNr GABAergic neurons is unknown. Here, we investigated regulation of SNr GABAergic neuron activity by H2O2 using whole-cell current clamp recordings of visualized SNr GABAergic neurons in guinea-pig and mouse midbrain slices. In marked contrast to the inhibitory effect of H2O2 on SNc DAergic neurons, we found that SNr GABAergic neurons in guinea pig are excited by H2O2. Pharmacological methods implicated TRP channels as probable targets of H2O2 signaling in these neurons. However, SNr GABAergic neurons recorded from mouse are inhibited by H2O2. These results reveal a new mechanism regulating basal ganglia output via H2O2-dependent modulation of SNr neuron firing.
Materials and Methods
Slice preparation
Whole-cell recordings were obtained in midbrain slices containing the substantia nigra (SN) from adult male guinea pigs (Hartley, 150–250 g) or mice (C57BL/6, 120 days). All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the New York University School of Medicine Institutional Animal Care and Use Committee. Procedures for preparation of midbrain slices were similar to those described previously (Avshalumov et al., 2005; Lee and Tepper, 2007a, b). Briefly, animals were deeply anesthetized with 50 mg/kg pentobarbital administered i.p., then transcardially perfused with ice-cold solution containing (in mM): 225 sucrose; 2.5 KCl; 0.5 CaCl2; 7 MgCl2; 28 NaHCO3; 1.25 NaH2PO4; 7 glucose; 1 ascorbate; and 3 pyruvate, equilibrated with 95% O2/5% CO2. The brain was quickly removed, trimmed to a block containing the midbrain, and sectioned at 300 μm in the same medium using a Leica VT1200S vibrating blade microtome (Leica Microsystems, Bannockburn, IL, USA). Slices were immediately transferred to warmed (34°C) recovery medium containing (in mM): 125 NaCl; 2.5 KCl; 1.25 NaH2PO4; 25 NaHCO3; 1 MgCl2; 2 CaCl2; 25 glucose; 1 ascorbate; 3 pyruvate; and 0.4 myo-inositol, equilibrated with 95% O2/5% CO2, which gradually cooled to room temperature over the next hour; slices were maintained in this medium until use. Physiological recording was conducted in a submersion recording chamber, with slices continuously superfused at 1.4 mL/min with artificial cerebrospinal fluid (aCSF) at 32°C containing (in mM): 124 NaCl; 3.7 KCl; 26 NaHCO3; 2.4 CaCl2; 1.3 MgSO4; 1.3 KH2PO4; and 10 glucose, equilibrated with 95% O2/5% CO2.
Visualized whole-cell recording
Neurons were visualized at 40× using a water-immersion objective on an Olympus BX51WI microscope equipped with infrared differential interference contrast (IR-DIC) optics (Olympus America, Center Valley, PA, USA). Pipettes were constructed from 1.5 mm o.d. borosilicate capillary tubing (World Precision Instruments, Sarasota, FL, USA) using a Sutter P-97 Flaming/Brown micropipette puller (Sutter Instrument Company, Novato, CA, USA) and filled with a solution containing (in mM): 129 potassium gluconate, 11 KCl, 10 HEPES, 2 MgCl2, 10 EGTA, 3 Na2-ATP, and 0.3 Na3-GTP, which was titrated to a pH of 7.3 with KOH. In some experiments, the pipette backfill also included an H2O2-sensitive fluorescent probe as described below. Pipettes had resistances of 3–6 MΩ. Recordings were obtained using an Axopatch 200B amplifier, low pass filtered at 2 kHz, and digitized by a Digidata 1322A connected to a personal computer running Clampex 9 (Molecular Devices, Sunnyvale, CA, USA). In the present experiments, we used visualized whole-cell current clamp recordings to allow for control of the intracellular and extracellular environments in neurons while monitoring how H2O2 affects the spontaneous activity of these cells. Previous studies found no differences in the firing rate and regularity of firing between whole-cell and perforated-patch recordings in SNr GABAergic neurons (Atherton and Bevan, 2005).
The SN contains both DAergic and GABAergic neurons which can be distinguished by their electrophysiological characteristics. When compared to DAergic neurons, nigral GABAergic neurons recorded in guinea-pig slices in vitro have a higher spontaneous firing rate, narrower action potential, shorter duration after hyperpolarization (AHP), and a less pronounced sag in response to hyperpolarizing current pulses (Hainsworth et al., 1991; Yung et al., 1991; Hajós and Greenfield, 1994), characteristics that are indistinguishable from those observed in rat SNr GABAergic neurons (Matsuda et al., 1987; Nakanishi et al., 1987; Grace and Onn, 1989; Lacey et al., 1989; Richards et al., 1997; Gulácsi et al., 2003; Lee and Tepper, 2007a,b). Data presented are from neurons determined to be GABAergic based on these characteristics.
Fluorescent imaging of H2O2
Fluorescent imaging of H2O2 was carried out using methods similar to those described previously (Avshalumov et al., 2005, 2007, 2008; Bao et al., 2005). The H2O2-sensitive indicator 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCF-DA, Invitrogen, Carlsbad, CA, USA) was loaded into individual neurons via the pipette backfill solution. For these experiments, stock solutions of CM-H2DCF-DA were made in ethanol with 10% v/v KOH (8 N); the final concentration of indicator in the pipette solution was 8 μM. Following electrophysiological identification of SNr GABAergic neurons, cells were held for 20 min before imaging to allow the indicator to infiltrate the recorded cell (Avshalumov et al., 2005). After diacetate cleavage, the parent molecule H2DCF becomes fluorescent DCF when oxidized by H2O2 or other reactive oxygen species. Excitation wavelength (488 nm) was controlled by a DeltaRam monochromator (Photon Technology International, Birmingham, NJ, USA) and emission at 535 nm detected using an IC-200 CCD camera (Photon Technology International). Images were acquired at 1 Hz with 30 ms exposure and eight frame averaging using ImageMaster 5.0 (Photon Technology International).
Drugs and chemicals
All components of physiological solutions, as well as H2O2, mercaptosuccinate (MCS), flufenamic acid (FFA), and glibenclamide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tetrodotoxin (TTX) citrate and 2-aminoethoxydiphenyl borate (2-APB) were purchased from Tocris Bioscience (Ellisville, MO, USA). Catalase (bovine liver) was purchased from Calbiochem (San Diego, CA, USA). Solutions of H2O2, MCS, and catalase were made fresh daily. Solutions of FFA, 2-APB, and glibenclamide were prepared in DMSO (Sigma-Aldrich) before dilution in aCSF; final concentrations of DMSO did not exceed 0.05%, which was also present in control aCSF for studies with these agents. All other agents were added directly to aCSF; application of all agents to slices via the superfusing aCSF did not exceed 20 min.
Data analysis
Electrophysiological data were analyzed using Clampfit 9 (Molecular Devices). Spontaneous firing rates were determined from 60 s of spontaneous activity with zero holding current under control conditions and during the period of maximal effect following drug or enzyme application. Maximal effects were observed within 10 min of application. Neurons that did not exhibit a steady baseline firing rate under control conditions were excluded from analysis. Regularity of firing was assessed from the coefficient of variation (CV) which was calculated as the standard deviation of the interspike interval divided by the mean interspike interval (Atherton and Bevan, 2005). Action potential parameters were measured from spike threshold, which was determined manually. Membrane potential between action potentials was measured from a region just after repolarization of the AHP and just prior to depolarization preceding the next action potential. Voltages were corrected for the liquid junction potential which was estimated to be 13 mV using JPCalc (Barry, 1994).
Fluorescence imaging data were analyzed using ImageMaster 5.0 (Photon Technology International) to determine the fluorescence intensity (FI) for a region of interest in each frame drawn around the cell body. Background fluorescence was measured from an area within the same field of view but outside of the region of interest and subtracted from the region of interest. The resulting FI was normalized and data are presented as [(intensity − basal)/(basal)] × 100%.
All data are presented as mean ± SEM. Statistical evaluation of the data was conducted using paired t-tests or repeated measures ANOVA followed by pairwise contrasts to assess significance between groups using SAS (SAS Institute, Cary, NC, USA). Differences were considered significant with p < 0.05.
Results
H2O2 increases the spontaneous firing rate of guinea-pig SNr GABAergic neurons
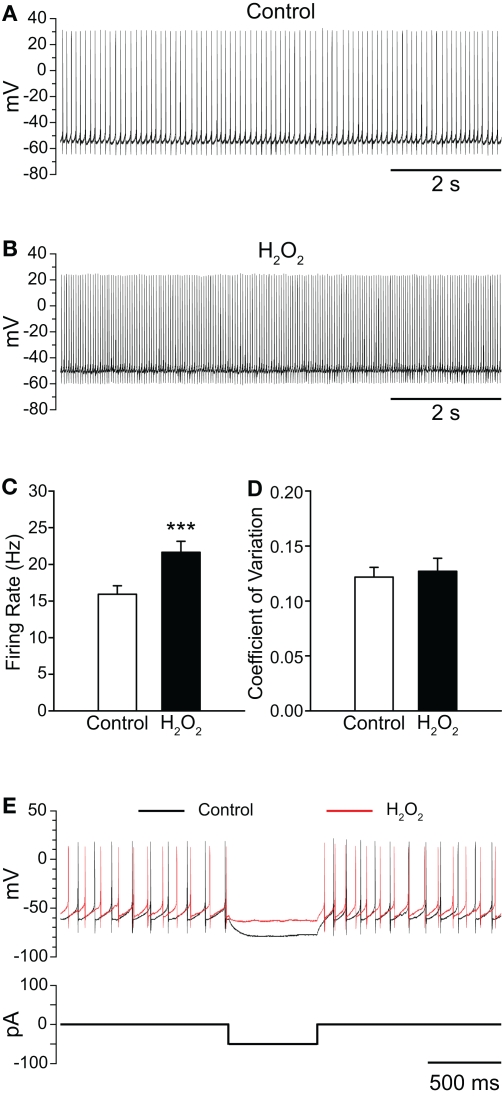
To assess the sensitivity of SNr GABAergic neurons to H2O2, we first examined the effect of exogenous H2O2 (1.5 mM, Chen et al., 2001; Avshalumov et al., 2005). In contrast to the suppression of firing seen in a large proportion of SNc DAergic neurons (Avshalumov et al., 2005), exogenous H2O2 augmented the spontaneous firing rate of SNr GABAergic neurons from 15.9 ± 1.2 Hz in control conditions to 21.6 ± 1.5 Hz in H2O2 (Figures 1A–C; n = 23; t = 6.53; p < 0.001). Firing rate increased in all SNr neurons tested, with an average increase of 39 ± 6%. The H2O2-induced increase reached a maximum after an average latency of 4.2 ± 0.5 min following H2O2 entry into the recording chamber. Previous studies demonstrated an absence of oxidative damage in brain slices with this concentration of H2O2 under similar conditions (Chen et al., 2001). Importantly, the effect of H2O2 on SNr neurons was reversible, providing evidence for the absence of toxicity in the present studies (control 14.2 ± 2.1 Hz; H2O2 18.4 ± 3.2 Hz; washout 14.0 ± 2.3 Hz; n = 5; F(2,8) = 13.68; p < 0.01; control vs. washout p > 0.05).
Figure 1.
Exogenous H2O2 increases the firing rate of SNr GABAergic neurons from guinea pig recorded in vitro. (A) Whole-cell current clamp recording from a SNr GABAergic neuron under control conditions and (B) following application of H2O2 (1.5 mM). (C) H2O2 caused an increase in firing rate without affecting the regularity of firing as measured by the coefficient of variation in (D). (E) The voltage deflection caused by a hyperpolarizing current pulse under control conditions (black trace) was attenuated in the presence of H2O2 (red trace) indicating that H2O2 decreased input resistance, consistent with ion-channel opening. (***p < 0.001).
The increase in firing rate elicited by H2O2 elevation was not accompanied by a change in the regularity of firing, as measured by the CV of interspike intervals, which was 0.122 ± 0.009 in control conditions and 0.127 ± 0.012 in the presence of H2O2 (Figure 1D; p > 0.05). Additionally, when a hyperpolarizing current pulse was delivered to SNr neurons from rest, the voltage deflection observed in the presence of H2O2 was strongly attenuated relative to that elicited under control conditions (Figure 1E), indicating decreased input resistance, consistent with ion-channel opening in the presence of H2O2.
Elevation of H2O2 also caused slight, but significant changes to several action potential parameters, including depolarization of action potential threshold from −48.0 ± 0.9 to −46.7 ± 0.9 mV in H2O2 (n = 23; t = 3.08; p < 0.01) and attenuation of spike amplitude from 67.1 ± 1.8 to 61.7 ± 1.7 mV (n = 23; t = −4.32; p < 0.001). The amplitude of spike AHP was also attenuated from −28.2 ± 0.6 to −26.2 ± 0.8 mV in the presence of H2O2 (n = 23; t = 4.55; p < 0.001). Lastly, membrane potential measured between action potentials was depolarized from −60.2 ± 0.9 to −58.2 ± 1.0 mV in the presence of H2O2 (n = 23; t = 4.39; p < 0.001).
SNr GABAergic neurons are excited by elevated levels of endogenous H2O2
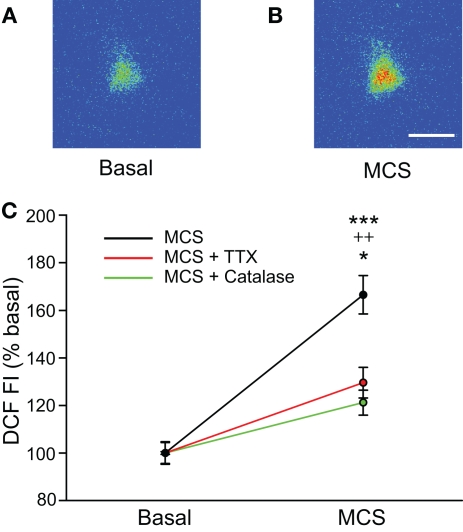
The use of exogenous H2O2 established that this potential modulator can affect the spontaneous firing rate of SNr GABAergic neurons. We next examined whether elevation of endogenously produced H2O2 also alters the activity of these cells. For these experiments, basal levels of H2O2 were enhanced by inhibiting glutathione (GSH) peroxidase, an H2O2 metabolizing enzyme, with MCS (1 mM; Avshalumov et al., 2005). The ability of MCS to enhance endogenous H2O2 levels was verified by monitoring H2O2-sensitive DCF fluorescence. Application of MCS led to an increase in DCF FI in guinea-pig SNr GABAergic neurons that reached a plateau 8.3 ± 0.7 min after MCS entered the recording chamber, with an average increase to 166 ± 8% of basal FI (Figures 2A–C; n = 12; t = 11.45; p < 0.001). It should be noted that oxidation of H2DCF to fluorescent DCF is irreversible, precluding washout measurements. The increase in DCF FI induced by MCS was strongly attenuated when applied simultaneously with the H2O2 metabolizing enzyme, catalase (500 U/mL; Avshalumov et al., 2003; n = 7; F(1, 17) = 27.01; p < 0.001 two-way repeated measures ANOVA), confirming that the DCF signal was largely H2O2 dependent (Figure 2C).
Figure 2.
Glutathione (GSH) peroxidase inhibition increases levels of endogenously produced H2O2 in SNr GABAergic neurons. (A) Pseudocolored photomicrograph of basal DCF fluorescence in a SNr GABAergic neuron. (B) DCF fluorescence intensity (FI) increased following inhibition of GSH peroxidase with mercaptosuccinate (MCS; 1 mM). Scale bar = 20 μm. (C) Plot of the DCF FI increase caused by MCS alone (black), by MCS in the presence of catalase (500 U/mL; green), and by MCS in the presence of TTX (2 μM; red). The MCS-induced increase in DCF FI was strongly attenuated when MCS was applied in the presence of catalase as well as when spontaneous activity was silenced with TTX when measured at the same duration of MCS exposure. These data indicate that GSH peroxidase inhibition by MCS increases intracellular H2O2 concentration in SNr neurons and that spontaneous activity contributes to endogenous H2O2 production. (***p < 0.001 basal vs. MCS; ++p < 0.01 MCS vs. MCS + catalase; *p < 0.05 MCS vs. MCS + TTX).
We hypothesized that tonic H2O2 generation in SNr GABAergic neurons was activity dependent, given the spontaneous activity of these cells in slice preparations (Richards et al., 1997; Gulácsi et al., 2003; Atherton and Bevan, 2005; Lee and Tepper, 2007a,b). To test this, we examined the effect of MCS on DCF FI after blocking spontaneous activity with TTX (2 μM). Under these conditions, the increase in DCF FI measured at the time of maximal increase in DCF FI in MCS alone was significantly attenuated compared to that seen with normal activity (Figure 2C; n = 5; F(1, 15) = 15.85; p < 0.01 two-way repeated measures ANOVA). The attenuated increase in DCF FI in TTX was still significant compared to basal DCF FI (t = 13.38; p < 0.001), likely reflecting the small amount of H2O2 produced during basal metabolism. These data indicate that the MCS-induced increase in DCF FI in SNr GABAergic neurons largely reflects amplification of activity-dependent H2O2 levels.
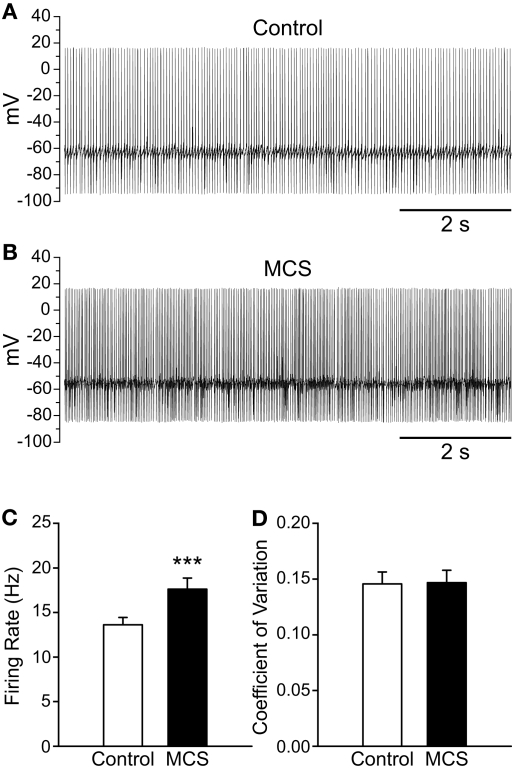
As with exogenous H2O2, elevation of endogenous H2O2 by MCS caused a significant increase in the spontaneous firing rate of guinea-pig SNr GABAergic neurons from 13.6 ± 0.8 to 17.6 ± 1.2 Hz (Figures 3A–C; n = 29; t = 6.00; p < 0.001), with an average increase of 30 ± 4%. Maximal effects were seen 7.8 ± 0.6 min after MCS application. This was slightly longer than that seen with exogenous H2O2, presumably reflecting the time required for enzyme inhibition and endogenous H2O2 accumulation. Most neurons (28/29) responded to MCS with an increase in firing rate, though one exhibited a decrease. The increase was reversible upon washout of MCS with aCSF (control 12.3 ± 1.2 Hz; MCS 14.6 ± 1.6 Hz; washout 11.8 ± 1.3 Hz; n = 5; F(2, 8) = 9.16; p < 0.01; control vs. washout p > 0.05), again indicating that the effect of H2O2 on cell firing is not a consequence of irreversible oxidative damage. The regularity of firing was unaffected by MCS, as reflected in the CV which was 0.146 ± 0.011 under control conditions and 0.147 ± 0.011 in MCS (Figure 3D; p > 0.05).
Figure 3.
Amplifying endogenous H2O2 levels increases SNr GABAergic neuron firing rate. (A) Spontaneous firing from a SNr GABAergic neuron under control conditions and (B) after inhibition of GSH peroxidase with mercaptosuccinate (MCS; 1 mM). (C) Amplifying endogenous H2O2 with MCS caused a significant increase in the firing rate of SNr GABAergic neurons. (D) The regularity of firing as measured by the coefficient of variation was unaffected by increasing endogenous H2O2. (***p < 0.001).
Several small changes in action potential parameters were seen when endogenous H2O2 levels were enhanced with MCS, including depolarization of action potential threshold from −48.5 ± 1.6 to −44.6 ± 1.7 mV in MCS (n = 21; t = 4.62; p < 0.001) and attenuation of spike amplitude from 75.1 ± 1.7 to 70.4 ± 1.6 mV (n = 21; t = −6.83; p < 0.001). Action potential AHP was attenuated from −27.8 ± 1.1 to −25.3 ± 1.1 mV in MCS (n = 21; t = 5.46; p < 0.001). Finally, the membrane potential measured between action potentials was depolarized in the presence of MCS from −58.8 ± 1.5 to −54.0 ± 1.5 mV (n = 21; t = 7.90; p < 0.001).
H2O2 produced during spontaneous activity maintains firing rate and regularity of firing
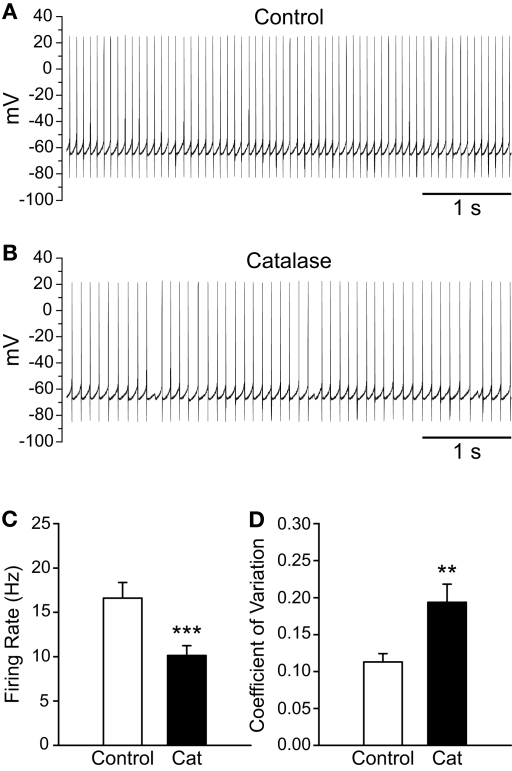
To determine whether basal levels of endogenous H2O2 generated in SNr GABAergic neurons during spontaneous activity also influence firing rate, we depleted endogenous H2O2 using catalase (500 U/mL; Avshalumov et al., 2003). Catalase caused a ∼40% decrease in the spontaneous firing rate of SNr GABAergic neurons from 16.6 ± 1.8 to 10.1 ± 1.1 Hz (Figures 4A–C; n = 11; t = −6.33; p < 0.001). Additionally, the precision of action potential discharge was decreased, as indicated by an increase in the CV from 0.113 ± 0.011 under control conditions to 0.194 ± 0.024 after catalase (Figure 4D; n = 11; t = 4.06; p < 0.01). These data show that basal H2O2 levels modulate the rate and regularity of spontaneous activity in SNr GABAergic neurons.
Figure 4.
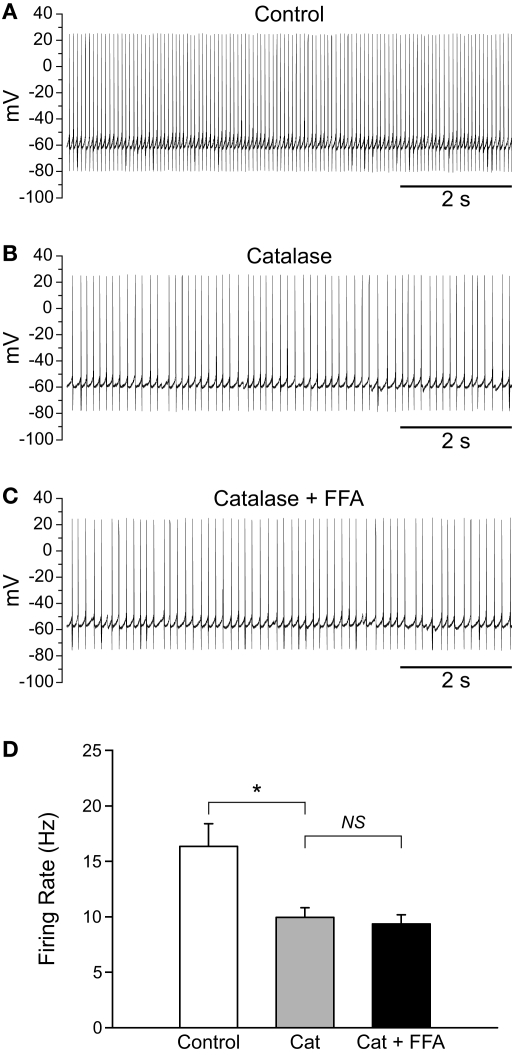
Depletion of endogenous H2O2 with catalase slows the firing rate of SNr GABAergic neurons and decreases the regularity of their spontaneous activity. (A) Spontaneous activity of a SNr GABAergic neuron under control conditions and (B) after depletion of endogenous H2O2 with catalase (500 U/mL). (C) Catalase (Cat) caused a decrease in the spontaneous firing rate of SNr GABAergic neurons. (D) In addition, spontaneous activity became more irregular in catalase, reflected in an increase in the coefficient of variation. (**p < 0.01; ***p < 0.001).
H2O2-induced increases in SNr GABAergic neuron firing rate are reversed by FFA
Having shown that H2O2 elevation increases the firing rate of SNr GABAergic neurons and that H2O2 depletion decreases it, we next sought to determine whether putative TRP channels had a role in these effects. We first tested FFA (20–40 μM; Bao et al., 2005; Lee and Tepper, 2007b; Zhou et al., 2008), which can block a number of TRP channel subtypes, including H2O2-activated TRPM2 channels (Hill et al., 2004; Clapham, 2007). The concentrations of FFA found to be effective in the present experiments are lower than those associated with non-specific effects on ion channels other than TRP channels (Takahira et al., 2005; Wang et al., 2006; Gardam et al., 2008; Yau et al., 2010). Consistent with a role for H2O2-sensitive TRP channels, FFA reversed the increase in firing rate caused by either exogenous or endogenous H2O2 elevation. In these experiments, exogenous H2O2 caused an increase in firing rate from 15.3 ± 1.3 to 21.5 ± 1.9 Hz, which was reversed by FFA (Figures 5A–D; n = 11; F(2, 20) = 30.85; p < 0.001; all pairwise contrasts p < 0.01). In fact, when TRP channels were blocked, firing rate during H2O2 exposure fell below control to 7.3 ± 2.4 Hz (Figure 5D). The suppression of firing rate below control levels could reflect the blockade of a tonic depolarizing current mediated by TRP channels (Zhou et al., 2008) and/or the unmasking of an additional effect of H2O2 on channels mediating a hyperpolarizing conductance. We explored these possibilities in separate experiments described in the following section.
Figure 5.
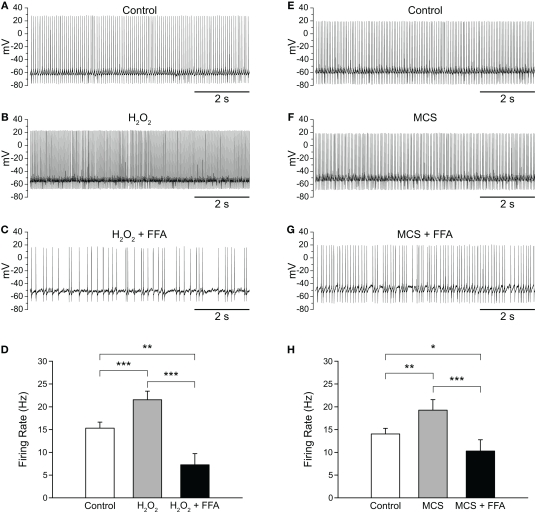
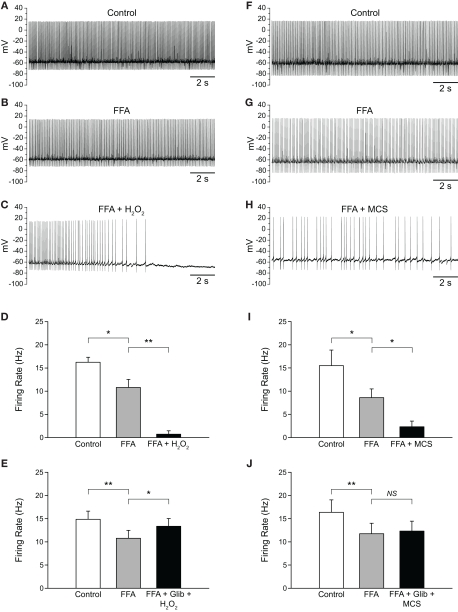
Flufenamic acid (FFA) reverses H2O2-induced increases in firing rate. (A) Spontaneous activity of a SNr GABAergic neuron under control conditions, (B) following H2O2 (1.5 mM) application, and (C) with FFA (20 μM) applied in the continued presence of H2O2. (D) The H2O2-induced increase in firing rate was reversed by FFA and the resulting firing rate suppressed below control. (E) Activity of another SNr GABAergic neuron under control conditions, (F) following amplification of endogenous H2O2 with MCS (1 mM), and (G) in FFA (20 μM) in the continued presence of MCS. (H) Increases in firing rate induced by amplified endogenous H2O2 were similarly reversed and suppressed below control levels by FFA. (*p < 0.05; **p < 0.01; ***p < 0.001).
As with exogenous H2O2, MCS-enhanced endogenous H2O2 levels caused an increase in firing rate from 14.0 ± 1.3 to 19.2 ± 2.3 Hz, which was reversed by FFA to 10.3 ± 2.5 Hz (Figures 5E–H; n = 11; F(2, 20) = 18.35; p < 0.001; all pairwise contrasts p < 0.05). Again, in the presence of FFA, MCS caused a suppression of firing rate below control (Figure 5H). We then tested the efficacy of another TRP channel blocker, 2-APB (Xu et al., 2005; Clapham, 2007; Togashi et al., 2008; not illustrated). Results with 2-APB (100 μM) were similar with H2O2 or MCS, so that data were pooled for analysis. As with FFA, 2-APB reversed the increase in firing rate seen with H2O2 or MCS (control 14.1 ± 2.1 Hz; H2O2 or MCS 17.9 ± 2.5 Hz; H2O2 or MCS + 2-APB 11.4 ± 2.8 Hz; n = 6; F(2, 10) = 23.67; p < 0.001; control vs. H2O2 or MCS p < 0.01; H2O2 or MCS vs. H2O2 or MCS + 2-APB p < 0.01).
Previous experiments have shown that tonic activation of TRP channels maintains basal firing rate, as well as the regularity of firing, in SNr GABAergic neurons (Zhou et al., 2008), with consequences of TRP channel blockade that appear much like the consequences of H2O2 depletion by catalase reported here (Figure 4). To determine whether endogenous H2O2 contributes to the tonic activation of putative TRP channels in SNr GABAergic neurons, we tested whether the decrease in firing rate caused by FFA (Zhou et al., 2008) would persist following catalase-induced H2O2 depletion. Catalase alone caused a decrease in firing rate from 16.4 ± 2.0 to 10.0 ± 0.9 Hz (Figures 6A,B; n = 4; F(2, 6) = 22.93; p < 0.01; control vs. catalase p < 0.05). However, when FFA (20 μM) was applied in the continued presence of catalase, the effect of FFA on firing rate was abolished, resulting in no change in firing rate from that observed with catalase alone (Figures 6C,D; 9.4 ± 0.8 Hz; p > 0.05). These data indicate that basal H2O2 is an important factor underlying the tonic activation of TRP channels in SNr neurons.
Figure 6.
FFA-sensitive channels are tonically activated by H2O2 in SNr GABAergic neurons. (A) Spontaneous firing of a SNr GABAergic neuron under control conditions, (B) following depletion of basal H2O2 with catalase (500 U/mL), and (C) in FFA (20 μM) in the continued presence of catalase. (D) Catalase (Cat) caused a significant decrease in firing rate which was unchanged when FFA was added in the continued presence of catalase. (NS not significant; *p < 0.05).
H2O2 suppresses firing via KATP channel activation in the presence of FFA
As described in the previous section, blocking TRP channels with FFA in the presence of elevated H2O2 not only reversed the excitatory effect seen, but led to a decrease in firing rate below control. Initially, we assumed that this decrease below control reflected blockade of TRP channel contributions to the tonic activity of SNr GABAergic neurons reported previously (Zhou et al., 2008). To test this assumption, we first applied FFA, then applied H2O2 or MCS in the continued presence of FFA. Application of FFA (20 μM) caused a decrease in firing rate from 16.2 ± 1.1 to 10.8 ± 1.7 Hz (p < 0.05) and an increase in the CV from 0.092 ± 0.007 to 0.154 ± 0.022 (n = 5; t = 3.48; p < 0.05), consistent with tonic excitation and maintenance of firing regularity mediated by TRP channels (Zhou et al., 2008). Addition of exogenous H2O2 in the continued presence of FFA caused a further decrease in firing rate to 0.7 ± 0.7 Hz (Figures 7A–D; n = 5; F(2, 8) = 82.49; p < 0.001; FFA vs. FFA + H2O2 p < 0.01). In 4/5 neurons, H2O2 caused a complete suppression of firing when applied in the presence of FFA. The same pattern was seen when endogenous H2O2 levels were elevated while TRP channels were blocked. Again, FFA alone caused a decrease in firing rate from 15.5 ± 3.4 to 8.6 ± 1.9 Hz (p < 0.05). Addition of MCS caused a further decrease in firing rate to 2.3 ± 1.2 Hz (Figures 7F–I; n = 7; F(2, 12) = 10.30; p < 0.01; FFA vs. FFA + MCS p < 0.05).
Figure 7.
H2O2 can alter SNr GABAergic neuron activity via both TRP and KATP channels. (A) Spontaneous firing of a SNr GABAergic neuron under control conditions, (B) with TRP channels blocked by FFA (20 μM), and (C) with H2O2 (1.5 mM) in the continued presence of FFA. (D) Following blockade of TRP channels, exogenous H2O2 suppressed SNr neuron firing. In some cases [as in (C)] a marked hyperpolarization was seen that was sufficient to silence the neuron. (E) This suppression of firing was prevented by the KATP channel blocker glibenclamide (Glib; 3 μM). (F) Recording from another SNr GABAergic neuron under control conditions, (G) with FFA (20 μM), and (H) with MCS (1 mM) in the continued presence of FFA. (I) Amplifying endogenous H2O2 levels with MCS caused a suppression of firing rate when TRP channels were blocked with FFA. (J) The MCS-induced suppression of neuronal activity was also prevented by glibenclamide. (NS not significant; *p < 0.05; **p < 0.01).
The finding that elevated H2O2 caused a further decrease in firing rate when TRP channels were blocked led us to investigate whether this effect was due to unopposed activation of KATP channels. To test this hypothesis we applied FFA followed by H2O2 or MCS along with a KATP-channel blocker, glibenclamide (3 μM). Glibenclamide was co-applied with H2O2 or MCS to minimize total recording time. As before, FFA (20–40 μM) caused a decrease in firing rate from 14.9 ± 1.7 to 10.8 ± 1.7 Hz (p < 0.01). However, in contrast to the suppression of activity when H2O2 alone was applied in the presence of FFA, co-application of H2O2 plus glibenclamide in the continued presence of FFA produced a slight increase in firing rate to 13.4 ± 1.7 Hz (Figure 7E; n = 8; F(2, 14) = 8.33; p < 0.01; FFA vs. FFA + glibenclamide ⩲ H2O2 p < 0.05). Similarly, in experiments with MCS plus glibenclamide, FFA caused a decrease in firing rate from 16.4 ± 2.7 Hz under control conditions to 11.8 ± 2.2 Hz (p < 0.01), which did not change when MCS was applied with glibenclamide in the continued presence of FFA (12.3 ± 2.2 Hz; Figure 7J; n = 7; F(2, 12) = 13.82; p < 0.001; FFA vs. FFA + glibenclamide ⩲ MCS p > 0.05). Thus, the suppression of SNr neuron firing caused by H2O2 when TRP channels are blocked is mediated by KATP-channel activation.
Next, we tested whether KATP channel activation attenuated the increase in firing rate caused by H2O2 when TRP channels were functioning. In these experiments, glibenclamide (3μM ) alone did not alter the firing rate of SNr GABAergic neurons (control 15.6 ± 0.8 Hz; glibenclamide 14.9 ± 0.9 Hz; n = 15; p > 0.05); in the presence of glibenclamide, exogenous H2O2 caused an increase in firing rate of 52 ± 17% (n = 6) and MCS caused an increase of 46 ± 13% (n = 9). Although the increases in firing rate caused by H2O2 or MCS in the presence of glibenclamide tended to be greater than those in the absence of KATP channel blockade (see preceding sections), these increases were not significantly greater than with H2O2 or MCS alone (p > 0.05 two-way repeated measures ANOVA in both cases). Therefore, it would appear that H2O2-induced activation of KATP channels only modestly attenuates the excitation caused by TRP channel activation. Overall, these data indicate that the primary effect of H2O2 elevation is to increase the activity of guinea-pig SNr GABAergic neurons by activating one or more FFA-sensitive channel.
H2O2 suppresses firing in GABAergic neurons recorded in mouse SNr
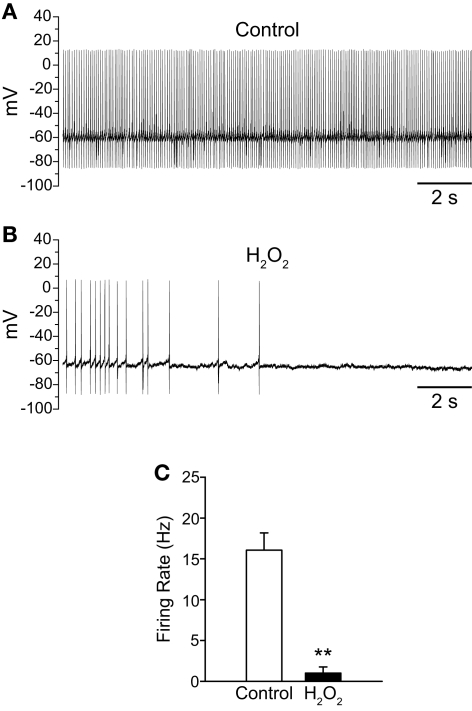
To provide mechanistic insight into the regulation of TRP channels by H2O2 we investigated the effects of H2O2 on SNr neurons in mouse midbrain slices where a specific subtype of TRP channel, namely TRPC3, is selectively expressed (Zhou et al., 2008). In contrast to our results in guinea-pig SNr, exogenous H2O2 (150 μM to 1.5 mM) inhibited the firing of these neurons (Figures 8A,B). Mouse SNr GABAergic neurons exhibited a spontaneous firing rate of 16.1 ± 2.1 Hz under control conditions which fell to 1.0 ± 0.7 Hz in the presence of exogenous H2O2 (Figure 8C; n = 6; t = −6.23; p < 0.01). This inhibition of firing was surprisingly strong and resulted in 4/6 neurons falling silent. In a subset of neurons where washout was assessed, H2O2 caused complete silencing of firing from an average control firing rate of 14.9 ± 2.4 Hz (n = 3; p < 0.05). The firing rate returned to 7.2 ± 1.2 Hz following washout in control aCSF, which was not significantly different from the control firing rate in this sample (F(2,4) = 17.43; p < 0.05; control vs.washout p > 0.05).
Figure 8.
Exogenous H2O2 suppresses the firing of SNr GABAergic neurons in mouse slices. (A) Whole-cell current clamp recording from a mouse SNr GABAergic neuron recorded in vitro under control conditions. (B) The same neuron recorded in the presence of exogenous H2O2 (750 μM) exhibited decreased firing rate and eventual hyperpolarization leading to cessation of spontaneous firing. (C) H2O2 caused a significant decrease in firing rate in SNr GABAergic neurons from mouse. (**p < 0.01).
Discussion
Here we report regulation of SNr GABAergic neuron activity by the emerging neuromodulator H2O2. Elevation of endogenous H2O2 increased the spontaneous firing rate of SNr GABAergic neurons recorded in vitro from guinea-pig slices whereas depletion of H2O2 decreased firing rate and regularity. We also found a minor inhibitory role for H2O2-sensitive KATP channels that was magnified in the presence of TRP-channel blockers. Further, we found that the firing of SNr GABAergic neurons recorded in vitro from slices obtained from mouse was suppressed by H2O2, indicating that the mode of H2O2 regulation of these neurons may be species specific. Overall, our results provide the first evidence that H2O2 fine-tunes the firing rate and regularity of basal ganglia output neurons through TRP channels, which appear to be the primary targets of H2O2-dependent modulation in guinea-pig SNr GABAergic neurons.
H2O2 signaling via ion-channel activation
A key source of cellular H2O2 production is the mitochondrial electron transport chain, in which H2O2 is formed from O2 during the process of oxidative phosphorylation to produce ATP (Boveris and Chance, 1973; Peuchen et al., 1997; Liu et al., 2002). Mitochondria are the primary source of H2O2 for rapid neuronal signaling via ion-channel activation (Bao et al., 2009), although other sources of H2O2 contribute to slower signaling processes, including downstream effects of growth factors (Rhee et al., 2005; Miller et al., 2007). As shown here, the metabolic demand of neurons in the SNr during spontaneous activity governs H2O2 generation, as seen in the marked attenuation of MCS-enhanced DCF FI in SNr GABAergic neurons when neuronal activity was silenced by TTX relative to that seen during spontaneous firing. These findings are consistent with the previously reported link between neuronal activity and mitochondrial metabolism (Kann et al., 2003).
The present findings complement earlier studies of the effects of H2O2 on neuronal excitability, in which both inhibitory and excitatory effects have been observed. For example, an H2O2-dependent hyperpolarization of CA1 pyramidal neurons has been reported that is mediated by an unidentified K+ channel(s) (Seutin et al., 1995). Similarly, a predominantly inhibitory effect of H2O2 has been reported for guinea-pig and rat SNc DAergic neurons and guinea-pig striatal DAergic axons that is mediated by KATP channels (Avshalumov and Rice, 2003; Avshalumov et al., 2003, 2005; Freestone et al., 2009). However, there is also evidence for excitatory effects of H2O2 in guinea-pig and rat GABAergic striatal MSNs, mediated by an FFA-sensitive channel (Smith et al., 2003; Bao et al., 2005). Moreover, rat SNr GABAergic neurons have been shown to exhibit both inward and outward currents in response to rotenone, with the majority of neurons exhibiting a presumed TRP-channel mediated inward current (Freestone et al., 2009).
The studies of neuronal TRP channel activation by H2O2 noted above used exogenous peroxide or rotenone, a mitochondrial inhibitor, to elevate H2O2 levels and activate a FFA-sensitive conductance (Smith et al., 2003; Bao et al., 2005; Freestone et al., 2009). We show here not only that milder H2O2 elevation by GSH peroxidase inhibition activates TRP channels in SNr GABAergic neurons, but also that the activity of these cells is regulated by tonically produced H2O2. The predominantly excitatory effect of H2O2 on guinea-pig SNr GABAergic neurons, despite the presence of functional H2O2-sensitive KATP channels, may reflect the relative density of TRP channels to KATP channels in these cells. Overall, these results suggest that differential responsiveness of basal ganglia neurons to H2O2 may be based largely on the ratio of TRP to KATP channels.
Evidence for presumed TRP channel involvement in H2O2-dependent regulation of SNr GABAergic neurons comes from several complementary results reported here. First, two distinct agents that block TRP channels, FFA and 2-APB, similarly reversed the increase in firing rate induced by H2O2. Second, although blocking tonically active TRP channels alone which decreases the firing rate of SNr GABAergic neurons (Zhou et al., 2008; results here) could contribute to these results, we also showed that when TRP channels were first blocked by FFA, resulting in a decrease in firing rate, application of H2O2 with FFA causes a further suppression of firing rate. If H2O2 and FFA were acting at separate conductances, it is unlikely that a suppression of firing rate would have been observed; rather an increase in firing rate would be expected. Third, application of FFA following a decrease in firing rate induced by catalase did not result in a further decrease in firing rate. If FFA were acting on a non-H2O2-sensitive channel, a further change in firing rate would have been expected. It should be noted that in all experiments, we limited off-target actions of FFA by using low concentrations: concentrations used were similar or lower than those used previously to examine the role of TRP channels in modulating SNr neuron firing (Zhou et al., 2008), and lower than concentrations shown to cause non-specific effects (Takahira et al., 2005; Wang et al., 2006; Gardam et al., 2008; Yau et al., 2010). Given this strong and consistent pharmacological evidence, we are pursuing additional approaches to identify the precise TRP-channel subtype(s) mediating H2O2-induced increases in firing rate in these neurons, with the caveat that we cannot rule out the possibility that H2O2 is acting on another channel class with similar pharmacological properties.
Mechanisms of H2O2-dependent ion-channel activation are not completely understood. There is evidence for direct action at some TRP channels, namely TRPM2 (Wehage et al., 2002; Eisfeld and Lückhoff, 2007), and KATP channels (Ichinari et al., 1996; Tokube et al., 1998). However, recent studies argue against direct TRP channel activation by H2O2 (Toth and Csanady, 2010) and suggest that activation may be mediated by H2O2-dependent elevation of an intracellular signaling molecule (Kolisek et al., 2005; Perraud et al., 2005; Lange et al., 2008; Hecquet and Malik, 2009). Intracellular calcium can also be elevated by H2O2 (Freestone et al., 2009). This could lead to activation of a calcium-activated conductance such as that mediated by the TRPC3 channel (Zitt et al., 1997), which is reported to be the sole TRP channel in neonatal mouse SNr neurons (Zhou et al., 2008). Our finding that young adult mouse SNr neurons are inhibited rather than excited by H2O2 suggests that the nature of H2O2-dependent modulation of SNr neurons may be species- and/or developmentally determined, possibly reflecting different complements of H2O2-sensitive channels.
Implications of modulation of SNr neuron activity by endogenous H2O2
Basal activity-dependent H2O2 generation in SNr GABAergic neurons contributes to the maintenance of tonic firing rate in these cells, with a decrease in firing rate and regularity after H2O2 depletion by catalase that is similar to the effect of blocking TRP channels reported previously (Zhou et al., 2008). These results suggest that TRP-channels are tonically active, at least in part through a mechanism involving H2O2. However, these channels are not maximally active at rest as evidenced by the ability of H2O2, as reported here, as well as other neuromodulators, including dopamine, to increase the firing rate of these neurons via further TRP-channel activation (Zhou et al., 2009). The decrease in firing rate and increase in CV observed when TRP channels are blocked by FFA (Zhou et al., 2008; results here) or when tonic H2O2-dependent activation is lost in the presence of catalase reflects the removal of a tonic depolarizing influence on these neurons. Indeed, a slight hyperpolarization of these neurons by direct current injection causes a similar decrease in firing rate and decrease in regularity of firing (Zhou et al., 2006). Further, the slight changes in spike parameters observed in the presence of H2O2 or MCS are consistent with those observed in response to injection of a slight depolarizing current in SNr GABAergic neurons (Lee et al., unpublished observations).
The ability of H2O2 to modulate the firing rate of SNr neurons raises the possibility that H2O2 might influence network interactions in the SNr. As a neutral, membrane-permeable molecule, H2O2 is not confined to the cell in which it is produced, but rather leaves cells by diffusing through the lipid membrane or through membrane aquaporins (Bienert et al., 2007). Thus, increased activity in one SNr neuron might lead to increased H2O2 production and subsequent excitation of neighboring cells, resulting in feed-forward excitation. Such local effects could be even more far reaching through circuit interactions. For example, much of the inhibitory input to SNc DAergic neurons is from axon collaterals of SNr GABAergic neurons (Tepper and Lee, 2007; Lee and Tepper, 2009). Previous studies have shown that H2O2 elevation inhibits somatodendritic release of DA in the SNc (Chen et al., 2002); one contributing factor could be increased inhibitory input to those neurons from H2O2-enhanced excitation of SNr GABAergic neurons.
In addition to these possible physiological functions of endogenous H2O2, aberrant H2O2 signaling could contribute to the pathological changes in firing of SNr GABAergic neurons that is seen in Parkinson's disease (PD). In human PD and in PD animal models, increased SNr output causes abnormal inhibition of thalamocortical neurons (Albin et al., 1989; DeLong, 1990; MacLeod et al., 1990; Wichmann and DeLong, 1996, 2006; Murer et al., 1997; Bergman et al., 1998; Hurtado et al., 1999; Hutchison et al., 2004). Linking these observations to the present findings, PD is associated with impaired activity of mitochondrial complex I and increased oxidative stress possibly including elevated H2O2 production in the SN (Parker et al., 1989; Schapira et al., 1990; Greenamyre et al., 2001; Turnbull et al., 2001; Dauer and Przedborski, 2003; Dawson and Dawson, 2003; Lin and Beal, 2006). Most studies of the contribution of H2O2 and other reactive oxygen species to the pathogenesis of PD have focused on the contribution of these molecules to DAergic neurodegeneration through oxidative damage (Jenner and Olanow, 1998; Zhang et al., 2000). The present findings suggest a more dynamic role for H2O2 as a contributing factor to the pathological changes in the activity of SNr GABAergic output neurons in PD by increasing the excitability of these cells via TRP-channel activation.
Conclusion
Overall, the present results from SNr GABAergic neurons build on a growing body of evidence supporting a role for H2O2 as a neuromodulator. The primary effect of H2O2 on guinea-pig SNr GABAergic neurons is to maintain and regulate their firing rate through presumed TRP-channel activation. An inhibitory effect of H2O2 resulting from KATP-channel activation predominates when TRP-channel activity is blocked in guinea-pig SNr, or under control conditions in mouse SNr. However, KATP-channel blockade alone has no effect on tonic firing rate in guinea-pig SNr GABAergic neurons, in contrast to the tonic, inhibitory effect of H2O2 acting via KATP channels in SNc DAergic neurons in the same species (Avshalumov et al., 2005). Activation of inhibitory KATP channels during H2O2 elevation is also less effective in SNr GABAergic neurons than in striatal MSNs, in which the net depolarizing effect of elevated H2O2 or other reactive oxygen species is attenuated significantly by concurrent activation of H2O2-sensitive KATP channels (Calabresi et al., 1999; Bao et al., 2005). Together with the present data, these findings indicate that H2O2 is an important signaling molecule throughout the basal ganglia, with effects determined by the relative responsiveness of H2O2-sensitive excitatory TRP channels and inhibitory KATP channels that defines the specificity of signaling by this diffusible messenger.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Jyoti C. Patel for helpful comments on this manuscript. This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke grants NS063656 (Christian R. Lee) and NS036362 (Margaret E. Rice), the Richard H. Chartrand Foundation (Paul Witkovsky), and the Edmond J. Safra Philanthropic Foundation(Margaret E. Rice).
References
- Albin R. L., Young A. B., Penney J. B. (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 10.1016/0166-2236(89)90074-X [DOI] [PubMed] [Google Scholar]
- Atherton J. F., Bevan M. D. (2005). Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J. Neurosci. 25, 8272–8281 10.1523/JNEUROSCI.1475-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov M. V., Bao L., Patel J. C., Rice M. E. (2007). H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxid. Redox Signal. 9, 219–231 10.1089/ars.2007.9.219 [DOI] [PubMed] [Google Scholar]
- Avshalumov M. V., Chen B. T., Koós T., Tepper J. M., Rice M. E. (2005). Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J. Neurosci. 25, 4222–4231 10.1523/JNEUROSCI.4701-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov M. V., Chen B. T., Marshall S. P., Peña D. M., Rice M. E. (2003). Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J. Neurosci. 23, 2744–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov M. V., Patel J. C., Rice M. E. (2008). AMPA receptor-dependent H2O2 generation in striatal medium spiny neurons, but not dopamine axons: one source of a retrograde signal that can inhibit dopamine release. J. Neurophysiol. 100, 1590–1601 10.1152/jn.90548.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov M. V., Rice M. E. (2003). Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc. Natl. Acad. Sci. U.S.A. 100, 11729–11734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Avshalumov M. V., Patel J. C., Lee C. R., Miller E. W., Chang C. J., Rice M. E. (2009). Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J. Neurosci. 29, 9002–9010 10.1523/JNEUROSCI.1706-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Avshalumov M. V., Rice M. E. (2005). Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J. Neurosci. 25, 10029–10040 10.1523/JNEUROSCI.2652-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. H. (1994). JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods 51, 107–116 [DOI] [PubMed] [Google Scholar]
- Beckstead R. M., Frankfurter A. (1982). The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience 7, 2377–2388 10.1016/0306-4522(82)90202-0 [DOI] [PubMed] [Google Scholar]
- Bergman H., Raz A., Feingold A., Nini A., Nelken I., Hansel D., Ben-Pazi H., Reches A. (1998). Physiology of MPTP tremor. Mov. Disord. 13, 29–34 10.1002/mds.870131305 [DOI] [PubMed] [Google Scholar]
- Bienert G. P., Møller A. L., Kristiansen K. A., Schulz A., Møller I. M., Schjoerring J. K., Jahn T. P. (2007). Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. (1973). The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 134, 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Marfia G. A., Centonze D., Pisani A., Bernardi G. (1999). Sodium influx plays a major role in the membrane depolarization induced by oxygen and glucose deprivation in rat striatal spiny neurons. Stroke 30, 171–179 [DOI] [PubMed] [Google Scholar]
- Cebrián C., Parent A., Prensa L. (2005). Patterns of axonal branching of neurons of the substantia nigra pars reticulata and pars lateralis in the rat. J. Comp. Neurol. 492, 349–369 [DOI] [PubMed] [Google Scholar]
- Chen B. T., Avshalumov M. V., Rice M. E. (2001). H2O2 is a novel, endogenous modulator of synaptic dopamine release. J. Neurophysiol. 85, 2468–2476 [DOI] [PubMed] [Google Scholar]
- Chen B. T., Avshalumov M. V., Rice M. E. (2002). Modulation of somatodendritic dopamine release by endogenous H2O2: susceptibility in substantia nigra but resistance in VTA. J. Neurophysiol. 87, 1155–1158 [DOI] [PubMed] [Google Scholar]
- Clapham D. E. (2007). SnapShot: mammalian TRP channels. Cell 129, 220. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Julius D., Montell C., Schultz G. (2005). International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 57, 427–450 [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Montell C., Schultz G., Julius D. (2003). International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol. Rev. 55, 591–596 [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. (2003). Parkinson's disease: mechanisms and models. Neuron 39, 889–909 10.1016/S0896-6273(03)00568-3 [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L. (2003). Molecular pathways of neurodegeneration in Parkinson's disease. Science 302, 819–822 10.1126/science.1087753 [DOI] [PubMed] [Google Scholar]
- DeLong M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285 10.1016/0166-2236(90)90110-V [DOI] [PubMed] [Google Scholar]
- Deniau J. M., Chevalier G. (1992). The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience 46, 361–377 10.1016/0306-4522(92)90058-A [DOI] [PubMed] [Google Scholar]
- Deniau J. M., Hammond C., Riszk A., Feger J. (1978). Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp. Brain Res. 32, 409–422 [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell A. A., Rawson N. E., Levin B. E. (1998). Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 814, 41–54 10.1016/S0006-8993(98)00956-1 [DOI] [PubMed] [Google Scholar]
- Eisfeld J., Lückhoff A. (2007). TRPM2. Handb. Exp. Pharmacol. 179, 237–252 [DOI] [PubMed] [Google Scholar]
- Freestone P. S., Chung K. K., Guatteo E., Mercuri N. B., Nicholson L. F., Lipski J. (2009). Acute action of rotenone on nigral dopaminergic neurons – involvement of reactive oxygen species and disruption of Ca2+homeostasis. Eur. J. Neurosci. 30, 1849–1859 [DOI] [PubMed] [Google Scholar]
- Gardam K. E., Geiger J. E., Hickey C. M., Hung A. Y., Magoski N. S. (2008). Flufenamic acid affects multiple currents and causes intracellular Ca2+ release in Aplysia bag cell neurons. J. Neurophysiol. 100, 38–49 [DOI] [PubMed] [Google Scholar]
- Grace A. A., Onn S. P. (1989). Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J. Neurosci. 9, 3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre J. T., Sherer T. B., Betarbet R., Panov A. V. (2001). Complex I and Parkinson's disease. IUBMB Life 52, 135–141 10.1080/15216540152845939 [DOI] [PubMed] [Google Scholar]
- Gulácsi A., Lee C. R., Sík A., Viitanen T., Kaila K., Tepper J. M., Freund T. F. (2003). Cell type-specific differences in chloride-regulatory mechanisms and GABAA receptor-mediated inhibition in rat substantia nigra. J. Neurosci. 23, 8237–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P. G., Aghajanian G. K. (1978). Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 150, 69–84 10.1016/0006-8993(78)90654-6 [DOI] [PubMed] [Google Scholar]
- Hainsworth A. H., Röper J., Kapoor R., Ashcroft F. M. (1991). Identification and electrophysiology of isolated pars compacta neurons from guinea-pig substantia nigra. Neuroscience 43, 81–93 10.1016/0306-4522(91)90419-O [DOI] [PubMed] [Google Scholar]
- Hajós M., Greenfield S. A. (1994). Synaptic connections between pars compacta and pars reticulata neurons: electrophysiological evidence for functional modules within the substantia nigra. Brain Res. 660, 216–224 10.1016/0006-8993(94)91292-0 [DOI] [PubMed] [Google Scholar]
- Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., Shimizu N., Kurose H., Okada Y., Imoto K., Mori Y. (2002). LTRPC2 Ca2+ -permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 9, 163–173 [DOI] [PubMed] [Google Scholar]
- Hecquet C. M., Malik A. B. (2009). Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb. Haemost. 101, 619–625 [PMC free article] [PubMed] [Google Scholar]
- Herson P. S., Ashford M. L. (1997). Activation of a novel non-selective cation channel by alloxan and H2O2 in the rat insulin-secreting cell line CRI-G1. J. Physiol. 501, 59–66 10.1111/j.1469-7793.1997.059bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K., Benham C. D., McNulty S., Randall A. D. (2004). Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 47, 450–460 10.1016/j.neuropharm.2004.04.014 [DOI] [PubMed] [Google Scholar]
- Hurtado J. M., Gray C. M., Tamas L. B., Sigvardt K. A. (1999). Dynamics of tremor-related oscillations in the human globus pallidus: a single case study. Proc. Natl. Acad. Sci. U.S.A. 96, 1674–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison W. D., Dostrovsky J. O., Walters J. R., Courtemanche R., Boraud T., Goldberg J., Brown P. (2004). Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J. Neurosci. 24, 9240–9243 10.1523/JNEUROSCI.3366-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Sandoval O., Carrillo-Reid L., Galarraga E., Tapia D., Mendoza E., Gomora J. C., Aceves J., Bargas J. (2007). Bursting in substantia nigra pars reticulata neurons in vitro: possible relevance for Parkinson disease. J. Neurophysiol. 98, 2311–2323 10.1152/jn.00620.2007 [DOI] [PubMed] [Google Scholar]
- Ichinari K., Kakei M., Matsuoka T., Nakashima H., Tanaka H. (1996). Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: its potentiation by MgADP. J. Mol. Cell. Cardiol. 28, 1867–1877 [DOI] [PubMed] [Google Scholar]
- Jenner P., Olanow C. W. (1998). Understanding cell death in Parkinson's disease. Ann. Neurol. 44, S72–S84 [DOI] [PubMed] [Google Scholar]
- Kann O., Schuchmann S., Buchheim K., Heinemann U. (2003). Coupling of neuronal activity and mitochondrial metabolism as revealed by NAD(P)H fluorescence signals in organotypic hippocampal slice cultures of the rat. Neuroscience 119, 87–100 10.1016/S0306-4522(03)00026-5 [DOI] [PubMed] [Google Scholar]
- Kolisek M., Beck A., Fleig A., Penner R. (2005). Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol. Cell 18, 61–69 [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. (1989). Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J. Neurosci. 9, 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I., Penner R., Fleig A., Beck A. (2008). Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium 44, 604–615 10.1016/j.ceca.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. R., Tepper J. M. (2007a). Morphological and physiological properties of parvalbumin- and calretinin-containing gamma-aminobutyric acidergic neurons in the substantia nigra. J. Comp. Neurol. 500, 958–972 10.1002/cne.21220 [DOI] [PubMed] [Google Scholar]
- Lee C. R., Tepper J. M. (2007b). A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J. Neurosci. 27, 6531–6541 10.1523/JNEUROSCI.1678-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. R., Tepper J. M. (2009). Basal ganglia control of substantia nigra dopaminergic neurons. J. Neural. Transm. Suppl. 73, 71–90 [DOI] [PubMed] [Google Scholar]
- Lin M. T., Beal M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- Liu Y., Fiskum G., Schubert D. (2002). Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 80, 780–787 10.1046/j.0022-3042.2002.00744.x [DOI] [PubMed] [Google Scholar]
- MacLeod N. K., Ryman A., Arbuthnott G. W. (1990). Electrophysiological properties of nigrothalamic neurons after 6-hydroxydopamine lesions in the rat. Neuroscience 38, 447–456 10.1016/0306-4522(90)90041-2 [DOI] [PubMed] [Google Scholar]
- Mana S., Chevalier G. (2001). The fine organization of nigro-collicular channels with additional observations of their relationships with acetylcholinesterase in the rat. Neuroscience 106, 357–374 10.1016/S0306-4522(01)00283-4 [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Fujimura K., Yoshida S. (1987). Two types of neurons in the substantia nigra pars compacta studied in a slice preparation. Neurosci. Res. 5, 172–179 [DOI] [PubMed] [Google Scholar]
- Miller E. W., Tulyathan O., Isacoff E. Y., Chang C. J. (2007). Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 3, 263–267 [DOI] [PubMed] [Google Scholar]
- Murer M. G., Riquelme L. A., Tseng K. Y., Pazo J. H. (1997). Substantia nigra pars reticulata single unit activity in normal and 6OHDA-lesioned rats: effects of intrastriatal apomorphine and subthalamic lesions. Synapse 27, 278–293 [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Kita H., Kitai S. T. (1987). Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 437, 45–55 10.1016/0006-8993(87)91525-3 [DOI] [PubMed] [Google Scholar]
- Nambu A. (2007). Globus pallidus internal segment. Prog. Brain Res. 160, 135–150 10.1016/S0079-6123(06)60008-3 [DOI] [PubMed] [Google Scholar]
- Parker W. D., Boyson S. J., Parks J. K. (1989). Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 26, 719–723 [DOI] [PubMed] [Google Scholar]
- Perraud A. L., Takanishi C. L., Shen B., Kang S., Smith M. K., Schmitz C., Knowles H. M., Ferraris D., Li W., Zhang J., Stoddard B. L., Scharenberg A. M. (2005). Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 280, 6138–6148 [DOI] [PubMed] [Google Scholar]
- Peuchen S., Bolaños J. P., Heales S. J., Almeida A., Duchen M. R., Clark J. B. (1997). Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog. Neurobiol. 52, 261–281 [DOI] [PubMed] [Google Scholar]
- Redgrave P., Marrow L., Dean P. (1992). Topographical organization of the nigrotectal projection in rat: evidence for segregated channels. Neuroscience 50, 571–595 10.1016/0306-4522(92)90448-B [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Kang S. W., Jeong W., Chang T. S., Yang K. S., Woo H. A. (2005). Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 17, 183–189 [DOI] [PubMed] [Google Scholar]
- Richards C. D., Shiroyama T., Kitai S. T. (1997). Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience 80, 545–557 10.1016/S0306-4522(97)00093-6 [DOI] [PubMed] [Google Scholar]
- Rick C. E., Lacey M. G. (1994). Rat substantia nigra pars reticulata neurons are tonically inhibited via GABAA, but not GABAB, receptors in vitro. Brain Res. 659, 133–137 10.1016/0006-8993(94)90872-9 [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. (1990). Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 54, 823–827 10.1111/j.1471-4159.1990.tb02325.x [DOI] [PubMed] [Google Scholar]
- Schwanstecher C., Panten U. (1993). Tolbutamide- and diazoxide-sensitive K+ channel in neurons of substantia nigra pars reticulata. Naunyn Schmiedebergs Arch. Pharmacol. 348, 113–117 [DOI] [PubMed] [Google Scholar]
- Seutin V., Scuvée-Moreau J., Massotte L., Dresse A. (1995). Hydrogen peroxide hyperpolarizes rat CA1 pyramidal neurons by inducing an increase in potassium conductance. Brain Res. 683, 275–278 10.1016/0006-8993(95)00436-T [DOI] [PubMed] [Google Scholar]
- Shen K. Z., Johnson S. W. (2006). Subthalamic stimulation evokes complex EPSCs in the rat substantia nigra pars reticulata in vitro. J. Physiol. 573, 697–709 10.1113/jphysiol.2006.110031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Herson P. S., Lee K., Pinnock R. D., Ashford M. L. (2003). Hydrogen-peroxide-induced toxicity of rat striatal neurones involves activation of a non-selective cation channel. J. Physiol. 547, 417–425 10.1113/jphysiol.2002.034561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford I. M., Lacey M. G. (1996). Electrophysiological investigation of adenosine trisphosphate-sensitive potassium channels in the rat substantia nigra pars reticulata. Neuroscience 74, 499–509 10.1016/0306-4522(96)00151-0 [DOI] [PubMed] [Google Scholar]
- Takahira M., Sakurai M., Sakurada N., Sugiyama K. (2005). Fenamates and diltiazem modulate lipid-sensitive mechano-gated 2P domain K+ channels. Pflugers Arch. 451, 474–478 10.1007/s00424-005-1492-5 [DOI] [PubMed] [Google Scholar]
- Takakusaki K., Habaguchi T., Ohtinata-Sugimoto J., Saitoh K., Sakamoto T. (2003). Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 119, 293–308 10.1016/S0306-4522(03)00095-2 [DOI] [PubMed] [Google Scholar]
- Tepper J. M., Lee C. R. (2007). GABAergic control of substantia nigra dopaminergic neurons. Prog. Brain Res. 160, 189–208 10.1016/S0079-6123(06)60011-3 [DOI] [PubMed] [Google Scholar]
- Togashi K., Inada H., Tominaga M. (2008). Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB). Br. J. Pharmacol. 153, 1324–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokube K., Kiyosue T., Arita M. (1998). Effects of hydroxyl radicals on KATP channels in guinea-pig ventricular myocytes. Pflugers Arch. 437, 155–157 10.1007/s004240050760 [DOI] [PubMed] [Google Scholar]
- Toth B., Csanady L. (2010). Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J. Biol. Chem. 285, 30091–30102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull S., Tabner B. J., El-Agnaf O. M., Moore S., Davies Y., Allsop D. (2001). alpha-Synuclein implicated in Parkinson's disease catalyses the formation of hydrogen peroxide in vitro. Free Radic. Biol. Med. 30, 1163–1170 [DOI] [PubMed] [Google Scholar]
- Wang D., Grillner S., Wallén P. (2006). Effects of flufenamic acid on fictive locomotion, plateau potentials, calcium channels and NMDA receptors in the lamprey spinal cord. Neuropharmacology 51, 1038–1046 10.1016/j.neuropharm.2006.06.012 [DOI] [PubMed] [Google Scholar]
- Wehage E., Eisfeld J., Heiner I., Jüngling E., Zitt C., Lückhoff A. (2002). Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J. Biol. Chem. 277, 23150–23156 [DOI] [PubMed] [Google Scholar]
- Wichmann T., DeLong M. R. (1996). Functional and pathophysiological models of the basal ganglia. Curr. Opin. Neurobiol. 6, 751–758 [DOI] [PubMed] [Google Scholar]
- Wichmann T., DeLong M. R. (2006). Basal ganglia discharge abnormalities in Parkinson's disease. J. Neural. Transm. Suppl. 70, 21–25 [DOI] [PubMed] [Google Scholar]
- Wilson C. J., Young S. J., Groves P. M. (1977). Statistical properties of neuronal spike trains in the substantia nigra: cell types and their interactions. Brain Res. 136, 243–260 10.1016/0006-8993(77)90801-0 [DOI] [PubMed] [Google Scholar]
- Xu S. Z., Zeng F., Boulay G., Grimm C., Harteneck C., Beech D. J. (2005). Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br. J. Pharmacol. 145, 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau H. J., Baranauskas G., Martina M. (2010). Flufenamic acid decreases neuronal excitability through modulation of voltage-gated sodium channel gating. J. Physiol. 588, 3869–3882 10.1113/jphysiol.2010.193037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung W. H., Häusser M. A., Jack J. J. (1991). Electrophysiology of dopaminergic and non-dopaminergic neurons of the guinea-pig substantia nigra pars compacta in vitro. J. Physiol. 436, 643–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Dawson V. L., Dawson T. M. (2000). Oxidative stress and genetics in the pathogenesis of Parkinson's disease. Neurobiol. Dis. 7, 240–250 [DOI] [PubMed] [Google Scholar]
- Zhou F. W., Jin Y., Matta S. G., Xu M., Zhou F. M. (2009). An ultra-short dopamine pathway regulates basal ganglia output. J. Neurosci. 29, 10424–10435 10.1523/JNEUROSCI.4402-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F. W., Matta S. G., Zhou F. M. (2008). Constitutively active TRPC3 channels regulate basal ganglia output neurons. J. Neurosci. 28, 473–482 10.1523/JNEUROSCI.3978-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F. W., Xu J. J., Zhao Y., LeDoux M. S., Zhou F. M. (2006). Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J. Neurophysiol. 96, 1581–1591 10.1152/jn.00148.2006 [DOI] [PubMed] [Google Scholar]
- Zitt C., Obukhov A. G., Strübing C., Zobel A., Kalkbrenner F., Lückhoff A., Schultz G. (1997). Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J. Cell Biol. 138, 1333–1341 10.1083/jcb.138.6.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]