Introduction
α particle emitters are of increasing interest as the radionuclide attached to monoclonal antibodies or other targeting mechanisms for applications in cell-directed therapy of cancer. R particles are more effective than β− particles for cell-killing and promise a more effective treatment of cancer than other forms of radiation. This is because α particles have high initial energy (4–8 MeV), short path lengths (40–80 μm, or several cell diameters), and consequently greater energy dissipation per unit length.1 Cell-directed immunotherapy can help improve irradiation of tumor cells while sparing normal tissues.2 The success of this approach will require effective chemistry for attaching the radionuclide to the antibody.3 Therefore, a concerted effort has been directed toward the design of chelating agents capable of holding the desired α-emitting radionuclide, both selectively and with high stability, to the antibody. This stability must be maintained in the body under physiological conditions and challenged by metal cations (at much higher concentration) that might otherwise compete for binding with the chelate. Bifunctional chelating agents such as tetraaza macrocycles have been used for this purpose to specifically bind the β emitters 90Y and 67Cu to antibodies.4 One of the R-emitting radionuclides considered suitable for radioimmunotherapy of cancer is the 11.4 d half-life 223Ra, which decays through a rapid chain of daughter products to 207Pb, emitting four R particles, two β particles, and several γ rays, with a combined energy of about 28 MeV.1b
Although 223Ra has desirable decay properties for radioimmunotherapy, bifunctional radium-selective ligands together with effective linkers to the protein antibody have not been reported. A few radium-complexing agents reported in the literature either lack selectivity or do not have sufficient binding stability to serve as cancer-therapeutic agents, and none have linkers attached.5
It is known that the 1,3-alkoxycalix[4]arene-crown-6 cavity has a high selectivity for Cs+ over K+.6 Since Ra2+ has an ionic radius (r = 1.62 Å) that is similar to that of Cs+ (r = 1.67 Å),7 the size of this crown cavity would presumably be suitable for Ra2+. However, neutral calixarene-crowns usually have relatively weak coordination with alkaline earth metal ions. Attaching sidearm functional groups to macrocyclic hosts can enhance their binding ability with alkaline earth metal ions. For example, proton-ionizable crowns with carboxylate sidearms have been shown to exhibit higher stability constants with alkaline earth metal ions relative to the nonionizable counterparts.5c We report here the binding characteristics of two new ionizable calixarene-crowns, p-tert-butylcalix[4]arene-crown-6-dicarboxylic acid (3) and p-tert-butylcalix[4]arene-crown-6-dihydroxamic acid (4), which show high selectivity for Ra2+ over light alkaline earth metal ions. The two proton-ionizable groups and the rigid calixarene-crown cavity apparently provide a strong and selective binding environment for Ra2+. This type of ionizable calixarene–crown appears promising for complexing 223Ra and linking the complex to monoclonal antibodies or other cell-specific proteins for radioimmunotherapy of cancer.
Experimental Section
General Procedures and Techniques
All reactions were carried out in a nitrogen atmosphere. All reagents were of reagent grade and were used without further purification. Tetrahydrofuran (THF) was freshly distilled from sodium benzophenone ketyl; N,N′-dimethylformamide (DMF) was distilled from KOH pellets and kept over molecular sieves (4 Å). 1H NMR (200 MHz) spectra were recorded with a Bruker AC200 in the absence of internal standard. J values are given in Hertz. FAB mass spectra were obtained with a Finnigan MAT90 mass spectrometer using m-nitrobenzyl alcohol (NBA) as a matrix. For reasons of clarity and to reduce space, the name calix[4]arene was used instead of the original IUPAC name: pentacyclo[19.3.1.13,7.19,13.115,19]octacosa-1(25),3,5,7(28),9,11,13(27),15,17,19(26),21,23-dodecane. Compound 18 was prepared according to a literature procedure, and 29 was prepared according to a modified literature procedure. We used Cs2-CO3 instead of KtBuO as base and template for the synthesis of p-tert-butylcalix[4]arene-crown-6 with a yield similar to that reported but easier to purify. Standard workup means that the organic layers were finally washed with water, dried over sodium sulfate (Na2SO4), filtered, and concentrated in vacuo.
p-tert-Butylcalix[4]arene-crown-6-dicarboxylic Acid (3)
To a solution of p-tert-butylcalix[4]arene-crown-6 (2) (1.70 g, 2 mmol) in THF–DMF (9:1 v/v) (50 cm3) was added sodium hydride (0.25 g, 10 mmol), and the mixture was heated under reflux for 0.5 h. Subsequently ethyl bromoacetate (1.34 g, 8 mmol) was added. The reaction mixture was heated under reflux for 5 h, and after cooling, the mixture was cautiously poured into ice-cold HCl (6 M, 20 cm3). The solvent was evaporated under reduced pressure, and the residue was partitioned between 100 cm3 of chloroform and 100 cm3 of 0.5 N HCl. The organic layer was followed by standard workup. The crude reaction product was triturated with 80% ethanol (20 cm3) to give the diester (1.49 g, 73%). The diester thus obtained (1.02 g, 1 mmol) was dissolved in THF (10 cm3), tetramethylammonium hydroxide (Me4NOH) (25% solution in methanol, 3 cm3, 7 mmol), and water (10 cm3) were added, and the mixture was heated under reflux for 24 h. The cooled mixture was concentrated to dryness and treated with 10% hydrochloric acid (10 cm3) to form a precipitate which was filtered off and redissolved in chloroform, followed by standard workup to afford p-tert-butylcalix[4]arene-crown-6-dicarboxylic acid (3) as a colorless solid (0.79 g, 82%). IR (KBr, cm−1): 3200–3600 (OH), 1750 (C=O). 1H NMR (CDCl3, 200 MHz): δ 0.70–1.50 (m, 36H, C(CH3)3), 3.00–4.10 (m, 24H, OCH2CH2O and ArCH2Ar), 4.30–4.70 (m, 4H, ArCH2Ar), 5.27 (s, 4H, OCH2), 6.50–7.20 (m, 8H, ArH), 8.95 (br s, 2H, COOH). Anal. Calcd for C58H78O12: C, 72.05; H, 8.07. Found: C, 71.92; H, 8.17. Mass spectrum: m/e 967.2 (M+, calcd 967.2).
p-tert-Butylcalix[4]arene-crown-6-dihydroxamic Acid (4)
Compound 3 (0.97 g, 1 mmol) and oxalyl chloride (5 cm3, 57 mmol) in carbon tetrachloride (50 cm3) were refluxed for 5 h. After being cooled, the solution was concentrated under a stream of nitrogen. The residual acid chloride (confirmed by IR spectroscopy: absence of νO−H) was dissolved in THF (50 cm3). The solution was added dropwise to a THF solution containing O-benzylhydroxylamine hydrochloride (2 g, 12.5 mmol) and pyridine (1 g, 12.7 mmol). The solution was heated at 35 °C for 24 h. After being cooled, the precipitate was removed by filtration, and the filtrate was concentrated to dryness under reduced pressure. The residue was dissolved in chloroform (100 cm3) and followed by standard workup. The residue left was triturated with CHCl3–hexanes (1:5 v/v) to give the pure hydroxamic acid precursor (0.76 g, 65%). The benzyl group was deprotected by catalytic hydrogenation with palladium on activated charcoal at room temperature under atmospheric pressure in acetic acid–methanol (1:3 v/v) (0.60 g, 92%). IR (KBr, cm−1): 2400–3600 (OH and NH), 1665 (CdO). 1H NMR (CDCl3, 200 MHz): δ 0.84 (s, 18H, C(CH3)3), 1.34 (s, 18H, C(CH3)3), 3.23 (d, 4H, Jax,eq) 12.8 Hz, ArCH2Ar), 3.40–4.00 (m, 20H, OCH2CH2O), 4.48 (d, 4H, Jax,eq = 12.8 Hz, ArCH2Ar), 5.10 (s, 4H, OCH2), 6.55 (s, 4H, ArH), 7.14 (s, 4H, ArH), 8.05 (s, 2H, NH). Anal. Calcd for C58H80N2O12: C, 69.85; H, 8.09; N, 2.81. Found: C, 69.97; H, 8.18; N, 2.75. Mass spectrum: m/e 997.2 (M+, calcd 997.2).
General Procedure for the Preparation of Barium Complexes
To a desired diacid ligand (0.1 mmol) dissolved in CH3CN (50 cm3) was added Ba(OH)2 (0.17 g, 1 mmol), and the mixture was refluxed for 2 h and concentrated to dryness. Chloroform (50 cm3) was added and filtered to remove an extra amount of Ba(OH)2. The filtrate was followed by standard workup to give the barium complex.
Barium Complex with 3
1H NMR (CDCl3, 200 MHz): δ 0.92 (s, 18H, C(CH3)3), 1.26 (s, 18H, C(CH3)3), 3.27 (d, 4H, Jax,eq = 12.3 Hz, ArCH2Ar), 3.50–4.30 (m, 20H, OCH2CH2O), 4.38 (d, 4H, Jax,eq = 12.3 Hz, ArCH2Ar), 4.58 (br s, 4H, OCH2), 6.84 (s, 4H, ArH), 7.18 (s, 4H, ArH). Anal. Calcd for C58H76O12Ba: C, 63.20; H, 6.90; Ba, 12.45. Found: C, 63.02; H, 6.97; Ba, 12.29. Mass spectrum: m/e 1102.5 (M+, calcd 1102.5).
Barium Complex with 4
1H NMR (CDCl3, 200 MHz): δ 0.93 (s, 18H, C(CH3)3), 1.27 (s, 18H, C(CH3)3), 3.28 (d, 4H, Jax,eq = 12.3 Hz, ArCH2Ar), 3.50–4.30 (m, 20H, OCH2CH2O), 4.42 (d, 4H, Jax,eq = 12.3 Hz, ArCH2Ar), 4.57 (br s, 4H, OCH2), 6.85 (s, 4H, ArH), 7.18 (s, 4H, ArH), 7.96 (s, 2H, NH). Anal. Calcd for C58H78N2O12Ba: C, 61.51; H, 6.94; N, 2.47; Ba, 12.12. Found: C, 61.29; H, 7.02; N, 2.39; Ba, 12.03. Mass spectrum: m/e 1132.5 (M+, calcd 1132.5).
Solvent Extraction Study
A standard Ra2+ solution (75 μg of 223Ra in 3 N hydrochloric acid solution) was obtained from the Pacific Northwest National Laboratory. For solvent extraction studies, the solutions were prepared using deionized water obtained from a Milli-Q reagent water system. Measurements of pH were made with an Orion Research Model 701A pH meter with an Orion 91-03 glass semimicroelectrode. Radium concentrations were measured by γ spectrometry using a high-resolution Ge(Li) detector (EG&G Ortec) with a multi-channel analyzer.
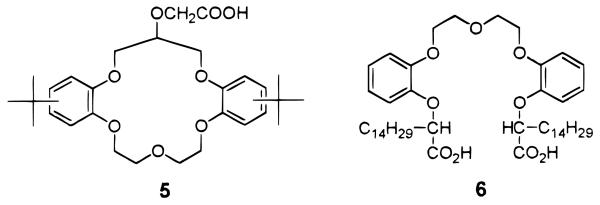
For solvent extraction experiments, 1.5 cm3 of a chloroform solution containing a desired ligand, 1.5 cm3 of the aqueous phase containing radium (0.031 mg/L), and buffer solution were placed in stoppered glass vials. Samples were shaken vigorously for a period of 30 min, which was shown to give constant values of distribution coefficients (D). After phase equilibration, 1-octanol (0.5%) was added to suppress the formation of an emulsion. Aliquots of 1.0 cm3 of each phase were then taken by pipets and placed into 5 cm3 polyethylene vials for radioactivity measurements. The area of the peak corresponding to 223-Ra (269 keV) was taken as the measure of its concentration. The typical standard deviation of the radioactivity measurements was less than 3%. The concentration of other alkaline earth metal ions such as Ba2+,Sr2+, Ca2+, and Mg2+ was detected by the inductively coupled plasma spectrometer (ICP-AES) (IRIS Model, Thermo Jarrell Ash Co., Franklin, MA). Solvent extraction experiments were also performed with a crown ether monocarboxylic acid ligand, 5,5e known to have affinity for Ra2+, and an acyclic crown ether dicarboxylic acid, 6,10 for comparison (Figure 2).
Figure 2.
Structures of the crown ether monocarboxylic acid 5 and the acyclic crown ether dicarboxylic acid 6.
Results and Discussion
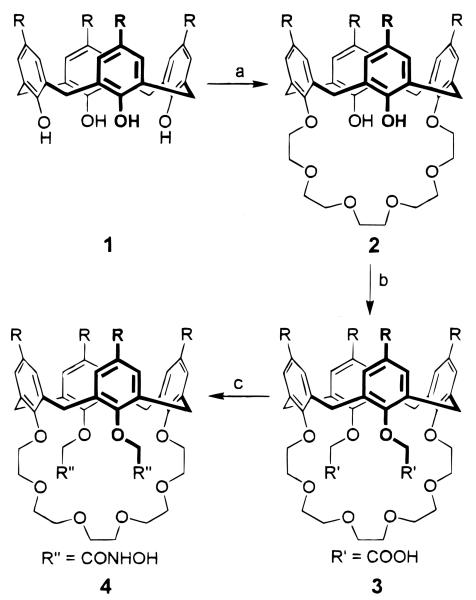
Compounds 3 and 4 were synthesized according to the reaction route shown in Figure 1. The 1H NMR spectra of the free ligands indicated a mixture of conformations for ligand 3, and a “conic” structure for ligand 4. Treatment of the ionizable calixarene-crowns with a suspension of Ba(OH)2 in acetonitrile resulted in a 1:1 complex between the ligand and the Ba2+ ion. For the barium complex with ligand 3, the two carboxylic acid protons disappeared, and the conformation was fixed in a conic structure as observed with 1H NMR. The Ba2+ complex of 4 showed little conformational change during the complexation.
Figure 1.
Preparation of the proton-ionizable calixarene-crown ethers p-tert-butylcalix[4]arene-crown-6-dicarboxylic acid (3) and p-tert-butylcalix[4]arene-crown-6-dihydroxamic acid (4). Reagents: (a) Ts(OCH2CH2)5OTs, Cs2CO3, CH3CN, reflux 24 h, 32%; (b) (i) NaH, BrCH2COOEt, THF, reflux 5 h, 73%; (ii) Et4NOH, THF–H2O, reflux 24 h, 82%; (c) (i) (COCl)2; (ii) O-benzylhydroxamine, pyridine, 35 °C, 24 h, 65%; (iii) H2/Pd–C, HOAc–MeOH, 92%.
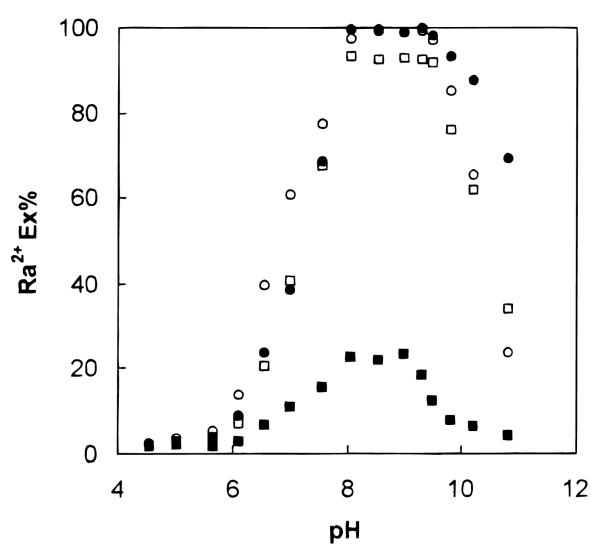
Figure 3 shows the percent extraction (Ex%) of 223Ra with different ligands plotted against the pH of the aqueous phase. The percentage of extracted Ra2+ became appreciable for both ligands 3 and 4 at about pH 4.5, reached a maximum at about pH 8.0, and declined sharply at pH > 9.5. At higher pH values, radium existed as the Ra(OH)+ species, which was probably too large to enter the calixarene-crown cavity. Ligand 6, an acyclic polyether with two carboxylic acid groups, also exhibited high extraction efficiency for Ra2+ in the pH range studied. Ligand 5, a crown ether monoacetic acid, showed limited extraction of Ra2+.
Figure 3.
Two-phase solvent extraction of Ra2+ with different ligands. The extraction conditions are given in the text. The pH of the aqueous phase was adjusted with succinic acid–NH4OH for pH 4–6, Tris–HCl for pH 7–9, and Tris–Me4NOH for pH over 10. (O) 3, (●) 4, (■) 5, and (□) 6.
The selectivity of ligands 3 and 4 for Ra2+ versus other alkaline earth metal ions was evaluated by competition experiments between water containing five alkaline earth metal ions (5 cm3, [Ra2+] = 0.03 mg/L, [Mg2+] = [Ca2+] = [Sr2+] = [Ba2+] = 0.20 mM) and chloroform containing a specific ligand (5 cm3, [ionophore] = 1.0 mM). The competition results at pH 8.9 are given in Table 1. In the competition experiments, Ra2+ was measured by γ spectroscopy and the other alkaline earth metal ions in the aqueous phase were determined by ICP-AES. As shown in Table 1, ligands 3 and 4 were able to extract Ra2+ almost quantitatively. The extractability of the alkaline earth metal ions followed the order Ra2+ > Ba2+ > Sr2+ > Ca2+ ⪢ Mg2+. Nearly zero amount of Mg2+ was extracted in the competition experiments with either ligand 3 or ligand 4. Ligand 4 with two hydroxamic acid groups showed a slightly higher selectivity for Ra2+ than its carboxylic acid counterpart, ligand 3. In terms of the distribution coefficient D (metal content in the organic phase/aqueous phase), the values for Ra2+, Ba2+, Sr2+, Ca2+, and Mg2+ were found to be about 1000, 120, 3.4, 0.3, and 0.001, respectively. Both ligands 5 and 6 showed no selectivity for Ra2+ versus the other alkaline earth metal ions. The reliability of ICP-AES in measuring extraction efficiency was confirmed by using a 133Ba tracer technique. For this purpose, the above-mentioned competition experiment was modified by adding a trace amount of 133Ba (Isotopes Products Laboratories, Burbank, CA), and the concentrations of barium in the CHCl3 and aqueous phases were measured by counting the areas of the peak at 356 keV. The D value thus obtained was about 100 for ligand 4, which was comparable to the value obtained from ICP-AES.
Table 1.
Percent Extraction of Alkaline Earth Metal Ions from Water to CHCl3 at pH 8.90 and 25 °C
| percent extraction |
|||||
|---|---|---|---|---|---|
| ionophore | Mg2+ | Ca2+ | Sr2+ | Ba2+ | Ra2+ |
| 3 | <0.1 | 15.6 | 73.5 | 98.7 | >99.9 |
| 4 | <0.1 | 12.7 | 78.3 | 99.2 | >99.9 |
| 5 | 12.4 | 17.8 | 13.8 | 12.5 | 13.4 |
| 6 | 93.8 | 92.5 | 90.3 | 87.0 | 89.0 |
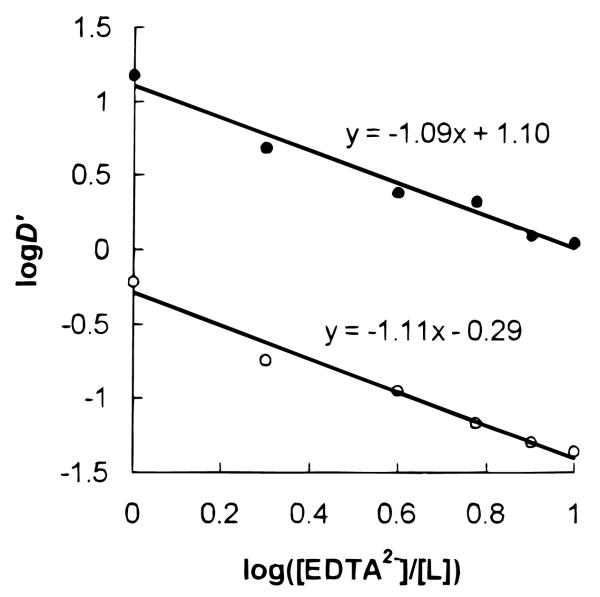
Because of its short half-life and high radioactivity, it was not possible for us to obtain stability constants of the 223Ra complexes using common spectroscopic or potentiometric titration methods. A competition method was used to obtain the relative extraction constants of 223Ra by ligands 3 and 4 with respect to EDTA at pH 8.5.11 Using an EDTA solution, the new distribution ratio D′ was assumed to be [RaL]org/ [RaEDTA]aq. Assuming that the solubility of RaEDTA in the CHCl3 phase and RaL in the aqueous phase was negligible, the following simplified equations were obtained:
A linear relationship was observed between log D′ and log([EDTA2−]/[L2−]) for both ligands (Figure 4), with a slope close to unity, suggesting that the above assumptions were reasonable.
Figure 4.
log D′ vs log([EDTA2−]/[L]) at pH 8.50 for ligands 3 (○) and 4 (●).
From the intercept we obtained the extraction constants of the Ra2+ complexes with the calixarene-crown ligands relative to that of EDTA. Ligand 3 had a value of K2 = 0.51K1, and ligand 4 had a value of K2 = 12.6K1, where K1 is the extraction constant of Ra2+ with EDTA at pH 8.5. Ligand 4 had an extraction constant that appeared to be about an order of magnitude higher than that of ligand 3. This could be explained from the following two reasons: (1) the stronger hydroxamic acid group12 was more effective in radium binding relative to the carboxylic acid group in the ionizable calixarene-crowns; (2) from the proton NMR study of the Ba2+ complexes, ligand 4 showed less conformational change during the complexation than ligand 3, thus minimizing the unfavorable entropy and enthalpy change during the complexation.
Finally, we investigated the kinetic stability of the radium complexes of ligands 3 and 4 in the presence of serum-abundant metal ions including Na+, K+, Mg2+, Ca2+, and Zn2+. Radium was first extracted into the CHCl3 phase at pH 8.5. The organic phase was then back-extracted with a pH 7.4 buffer containing 10−2 M each of Na+, K+, Mg2+, Ca2+, and Zn2+. After the organic phase was shaken for 24 h, <5% of 223Ra was removed. The 223Ra coordinated with ligands 3 and 4 showed high kinetic stability in the presence of other metal ions at relatively high concentrations and at a near neutral pH.
Conclusion
For the first time, we were able to design ionophores derived from calix[4]arene to have selectivity for Ra2+ over the lighter alkaline earth metal ions and high kinetic stability for 223Ra complex in the presence of serum-abundant metal ions. Calix[4]arene-crown-6 offers a more rigid cavity than common crown ethers to fit the size of radium. Two ionizable groups neutralize the charge of the complex and act as lariat arms to prevent radium from escaping from the cavity. The extraction stability constant of carboxylate derivative 3 with Ra2+ falls into the same order of magnitude as that of EDTA. The hydroxamate derivative 4 with Ra2+ is 1 order of magnitude higher than that of 3. Although the thermodynamic stability constants of these two ligands with Ra2+ are not extremely high, the kinetic stability is more attractive. Once radium was included in the pseudo-three-dimensional cage, proton- and cation-promoted pathways show little effect on the loss of radium from the complexes. Further work to make water–soluble bifunctional chelating agents, which have ionizable calixarene-crown moieties at the lower rim as radium ionophore and an isothiocyanate group at the upper rim for linkage to monoclonal antibodies, is now in progress.
Acknowledgment
This work was supported by the Pacific Northwest National Laboratory (PNNL) and the U.S. Department of Energy under Contract No. DE–AC06–76RLO 1830.
References
- 1.(a) Hall EJ. Radiobiology for the Radiologist. 3rd ed. J.B. Lippincott Co.; Philadelphia: 1988. [Google Scholar]; (b) Wilbur DS. Antibody Immunocon. Radiopharm. 1991;4:85–97. [Google Scholar]; (c) Geerlings MW. Int. J. Biol. Markers. 1993;8:180–186. doi: 10.1177/172460089300800308. [DOI] [PubMed] [Google Scholar]
- 2.(a) Order SE, Klein JL, Leichner PK, Fincke J, Lollo C, Carlo DJ. Int. J. Radiot. Oncol., Biol., Phys. 1986;12:277–281. doi: 10.1016/0360-3016(86)90110-0. [DOI] [PubMed] [Google Scholar]; (b) DeNardo SJ, DeNardo GL, Deshpande SV, Adams GP, Macey DJ, Meares CF. NATO ASI Ser., Ser. A. 1988;152:111–122. Radiolabeled Monoclonal Antibodies Imaging Ther. [Google Scholar]; (c) DeNardo SJ, Jungerman JA, DeNardo GL, Lagunas-Solar MC, Cole WC, Meares CF. DOE Symp. Ser. 1985;56:401–414. Dev. Role Short-Lived Radionuclides Nucl. Med. [Google Scholar]; (d) Meares CF, Wensel TG. Acc. Chem. Res. 1984;17:202–209. [Google Scholar]
- 3.(a) Cram DJ. Science. 1983;219:1177–1183. doi: 10.1126/science.219.4589.1177. [DOI] [PubMed] [Google Scholar]; (b) Pedersen CJ. Science. 1988;241:536–540. doi: 10.1126/science.241.4865.536. [DOI] [PubMed] [Google Scholar]; (c) Gansow OA, Atcher RW, Link DC, Friedman AM, Seevers RH, Anderson W, Scheinberg DA, Stand M. ACS Symp. Ser. 1984;241:215–222. Radionuclide Gener. [Google Scholar]; (d) Moi MK, Meares CF, McCall MJ, Cole WC, DeNardo SJ. Anal. Biochem. 1985;148:249–253. doi: 10.1016/0003-2697(85)90653-0. [DOI] [PubMed] [Google Scholar]; (e) Roberts JC, Adams YE, Tomalia D, Mercer-Smith JA, Lavallee DK. Bioconjugate Chem. 1990;1:305–308. doi: 10.1021/bc00005a001. [DOI] [PubMed] [Google Scholar]; (f) Ruser G, Ritter W, Maecke HR. Ibid. 1990;1:345–349. doi: 10.1021/bc00005a008. [DOI] [PubMed] [Google Scholar]; (g) Koppel GA. Ibid. 1990;1:13–23. doi: 10.1021/bc00001a002. [DOI] [PubMed] [Google Scholar]; (h) Parker D. Chem. Soc. Rev. 1990;19:271–291. [Google Scholar]; (i) Goldenberg DM. Immunobiol. Proteins Pep. 1991;6:107. [Google Scholar]
- 4.(a) Desphande SV, DeNardo SJ, Meares CF, McCall MJ, Adams GP, DeNardo GL. Nucl. Med. Biol. 1989;16:587–597. doi: 10.1016/0883-2897(89)90075-5. [DOI] [PubMed] [Google Scholar]; (b) Moi MK, Meares CF. J. Am. Chem. Soc. 1988;110:6266–6267. doi: 10.1021/ja00226a063. [DOI] [PubMed] [Google Scholar]; (c) Chen LH, Chung CS. Inorg. Chem. 1988;27:1880–1883. [Google Scholar]; (d) Schlom J, Siler K, Milenic DE, Eggensperger D, Colcher D, Miller LS, Houchens D, Cheng RC, Kaplan D, Goeckeler W. Cancer Res. 1991;51:2889–2896. [PubMed] [Google Scholar]; (e) Craig AS, Helps Ian M., Jankowski KJ, Parker D, Beeley NRA, Boyce BA, Eaton MAW, Millican AT, Millar K. J. Chem. Soc., Chem. Commun. 1989:794–796. [Google Scholar]; (f) Sharkey RM, Kaltovich FA, Shih LB, Fand I, Govelitz G, Goldenberg DM. Cancer Res. 1988;48:3270–3275. [PubMed] [Google Scholar]; (h) Kruper WJ, Jr., Rudolf PR, Langhoff CA. J. Org. Chem. 1993;58:3869–3876. [Google Scholar]
- 5.(a) McDowell WJ, Case GN, Bartsch RA, Czech BP. Solvent Extr. Ion Exch. 1986;4:411–419. [Google Scholar]; (b) McDowell WJ, Arndsten BA, Case GN. Ibid. 1989;7:377–393. [Google Scholar]; (c) Rollat A, Sabot JL, Burgard M, Delloye T. Eur. Pat. Appl. EP 188,394. Chem. Abstr. 1987;106:35491v. 1986.; (d) Ehrenfeld U. U. S. Pat. 5,116,614 1992; (e) Bekemishev MK, Elshani S, Wai CM. Anal. Chem. 1994;66:3521–3524. [Google Scholar]; (f) Case GN, McDowell WJ. Radioact. Radiochem. 1990;1:60–69. 58. [Google Scholar]; (h) Dietz ML, Chiarizia R, Horwitz EP, Bartsch RA, Talanov V. Anal. Chem. 1997;69:3028–3037. doi: 10.1021/ac9700437. [DOI] [PubMed] [Google Scholar]
- 6.(a) Dijkstra PJ, Brunink JAJ, Bugge K-E, Reinhoudt DN, Harkema S, Ungaro R, Ugnozzoli F, Ghidini E. J. Am. Chem. Soc. 1989;111:7567–7575. [Google Scholar]; (b) Ungaro R, Casnati A, Ugozzoli F, Pochini A, Dozol J-F, Hill C, Rouquette H. Angew. Chem., Int. Ed. Engl. 1994;34:1506–1509. [Google Scholar]; (c) Casnati A, Pochini A, Ungaro R, Ugozzoli F, Arnaud F, Fanni S, Schwing M-J, Egberink RJM, de Jong F, Reinhoudt DN. J. Am. Chem. Soc. 1995;117:2767–2777. [Google Scholar]; (d) Bochi C, Careri M, Casnati A, Mori G. Anal. Chem. 1995;67:4234–4238. [Google Scholar]
- 7.Marcus Y. Ion Properties. Marcel Dekker; New York: 1997. [Google Scholar]
- 8.Gutsche CD, Iqbal M. Org. Synth. 1989;68:234. [Google Scholar]
- 9.Ghidini E, Ugozzoli F, Ungaro R, Harkema S, El-Fadl AA, Reinhoudt DN. J. Am. Chem. Soc. 1990;112:6979–6985. [Google Scholar]
- 10.(a) Charewicz WA, Heo GS, Bartsch RA. Anal. Chem. 1982;54:2094–2097. [Google Scholar]; (b) Bartsch RA, Heo GS, Kang SL, Liu Y, Strzelbicki J. J. Org. Chem. 1982;47:457–460. [Google Scholar]
- 11.Chen X, Ji M, Fisher DR, Wai CM. Chem. Commun. (Cambridge) 1998:377–378. [Google Scholar]
- 12.(a) Jencks WP, Rengenstein J. In: Handbook of Biochemistry and Molecular Biology. Fasman GD, editor. CRC Press; Cleveland, OH: 1976. [Google Scholar]; (b) Nagasaki T, Shinkai S. J. Chem. Soc., Perkin Trans. 1991;2:1063–1066. [Google Scholar]