Abstract
While patients with advanced prostate cancer initially respond favorably to androgen ablation therapy, most experience a relapse of the disease within 1–2 years. Although hormone-refractory disease is unresponsive to androgen-deprivation, androgen receptor (AR)-regulated signaling pathways remain active and are necessary for cancer progression. Thus, both AR itself and the processes downstream of the receptor remain viable targets for therapeutic intervention. Microarray analysis of multiple clinical cohorts showed that the serine/threonine kinase Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) is both highly expressed in the prostate and further elevated in prostate cancers. Using cellular models of prostate cancer, we have determined that androgens 1) directly increase the expression of a CaMKKβ splice variant and 2) increase functional CaMKKβ protein levels as determined by the phosphorylation of both CaMKI and AMP-activated protein kinase (AMPK), two of CaMKKβ’s primary substrates. Importantly, inhibition of the CaMKKβ-AMPK, but not CaMKI, signaling axis in prostate cancer cells by pharmacological inhibitors or siRNA-mediated knockdown blocks androgen-mediated migration and invasion. Conversely, overexpression of CaMKKβ alone leads to both increased AMPK phosphorylation and cell migration. Given the key roles of CaMKKβ and AMPK in the biology of prostate cancer cells, we propose that these enzymes are potential therapeutic targets in prostate cancer.
Keywords: androgen receptor, prostate cancer, Ca2+/calmodulin-dependent protein kinase kinase β, AMP-activated protein kinase, migration
Introduction
Prostate cancer is the most common malignancy in men and is second only to lung cancer in terms of cancer mortalities (1). If diagnosed early, most localized prostate tumors are successfully treated by surgery alone. However, as with many cancers, the treatment of the advanced disease state requires a systemic approach to inhibit the growth and spread of secondary metastases. Prostate cancers express the androgen receptor (AR) and rely on androgens for growth and survival (2). Subsequently, androgen ablation therapies are the standard of care for late-stage disease. While 80% of patients with prostate cancer respond favorably to initial androgen ablation therapy, most patients experience a relapse of the disease within 1–2 years (2). Despite the unresponsiveness of the hormone-refractory disease to androgen-deprivation therapy, AR-regulated signaling pathways remain active and are necessary for cancer progression (3). Consequently, AR and the processes downstream of the receptor remain viable targets for therapeutic intervention.
Several approaches are currently used to target the AR signaling axis in prostate cancer. Current therapies focus on decreasing the levels of circulating androgens and/or competitively blocking the AR transcriptional complex. Specifically, gonadotropin-releasing hormone (GnRH) agonists are used to suppress the testicular production of testosterone whereas antiandrogens, such as bicalutamide, function by competitively inhibiting the interaction of androgens with AR. The initial response to either form of androgen deprivation is very high. However, the rapid onset of resistance to these interventions has highlighted the need for novel strategies to target the hormone-independent activities of AR. In this regard, our group and others have shown that the targeting of specific signaling pathways downstream of AR represents a potential new modality for the treatment of prostate cancer (4–7).
Most of the studies on the role of androgens in prostate cancer have focused on defining the mechanisms underlying the mitotic actions of this hormone (8). However, there is a growing body of evidence that AR signaling also influences tumor cell migration and invasion. Of note, different clinical trials of goserelin (a GnRH analog) in prostate cancer patients demonstrate reduced incidences of distant metastases (9, 10). Furthermore, it has recently been reported that MDV3100, a second generation AR-antagonist, decreases the number of circulating tumor cells in approximately half of the treated castration-resistant patients (11). Cumulatively, these data suggest that androgen ablation therapy not only inhibits the growth of the primary tumor, but also reduces progression to metastatic disease. The onus is now on researchers to identify what specific cellular processes regulated by AR contribute to the pathogenesis of prostate cancer and ultimately, whether they represent realistic therapeutic targets.
To identify potential new points of intervention in AR-driven prostate cancer, we focused on candidate target proteins that are (a) expressed in the prostate, (b) regulated by AR, (c) track with disease outcome and (d) likely to be druggable. We also included in our criteria the requirement that the target be expressed in various cellular models of prostate cancer. Using these criteria, the Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) was identified as a protein of interest. Subsequently, we performed a comprehensive analysis of its role in prostate cancer and demonstrate that CaMKKβ is likely to be a useful target for the treatment of this disease.
Materials and Methods
A description of the chemicals, antibodies, plasmids and stable cell lines used in this study can be found in the Supplementary Materials.
Cell culture and RNA
The LNCaP and VCaP human prostate carcinoma cell lines were obtained from ATCC and maintained as recommended. All experiments were performed with cells of passage less than 25. These cells were authenticated by morphological inspection and mycoplasma testing by the ATCC. Furthermore, their response to androgens was authenticated using growth and reporter gene assays. RNA from placenta, skeletal muscle, cerebellum, whole brain and normal prostate was from Clontech (Mountain View, CA). RNA from glioblastoma cell lines was a generous gift from Valerie Curtis (Duke University, Durham, NC).
RNA isolation, cDNA preparation, and quantitative and standard reverse transcription (RT)-PCR
RNA isolation, cDNA preparation and quantitative RT-PCR (qPCR) were performed as previously described using 36B4 as a control (12). Standard RT-PCR was performed using the Advantage GC 2 Polymerase Mix and PCR Kit (Clontech). All qPCR and RT-PCR primers used in this study are listed in Supplementary Table 1.
Western blot analysis
Western blots were performed as previously described (12) with the exception that a modified radioimmunoprecipitation assay (RIPA) buffer [50 mM Tris (pH 8.0), 200 mM NaCl, 1.5 mM MgCl2, 1% Triton X-100, 1 mM EGTA, 10% glycerol, 50 mM NaF, 2 mM Na3VO4 and protease inhibitors] was used. Results shown are representative blots. For each sample, protein levels were determined by densitometry using the ImageJ software (NIH) and normalizing to indicated controls.
Small interfering RNA (siRNA) transfection of human prostate cells
Stealth siRNA (Invitrogen) transfections were performed as previously described (5). The sequences of all siRNAs used in this study are listed in Supplementary Table 1.
Chromatin immunoprecipitation (ChIP)
ChIP was performed as previously described (4). All primers used for ChIP qPCR analysis are listed in Supplementary Table 1.
Transient transfections and reporter gene assays
Transient transfections and reporter gene assays were performed as previously described (4).
Cell proliferation assay
Proliferation assays were performed as previously described (12) by measuring the cellular DNA content using the FluoReporter Blue Fluorometric double-stranded DNA Quantitation Kit (Invitrogen) as per the manufacturer’s protocol.
Migration and invasion assays
Boyden dual chamber migration assays were performed as previously described (4). Invasion assays were performed the same as migration assays except that inserts were layered with 100 μl of Matrigel extracellular matrix (BD Biosciences) prior to reseeding of cells.
Statistical analysis
Data were analyzed using one-way ANOVA and post hoc Dunnett’s test with GraphPad Prism, Version 4 (GraphPad Software, Inc.). Unless otherwise noted, significance was determined at the P < 0.05 level.
Results
Androgens increase CaMKKβ mRNA and protein levels in an AR-dependent manner
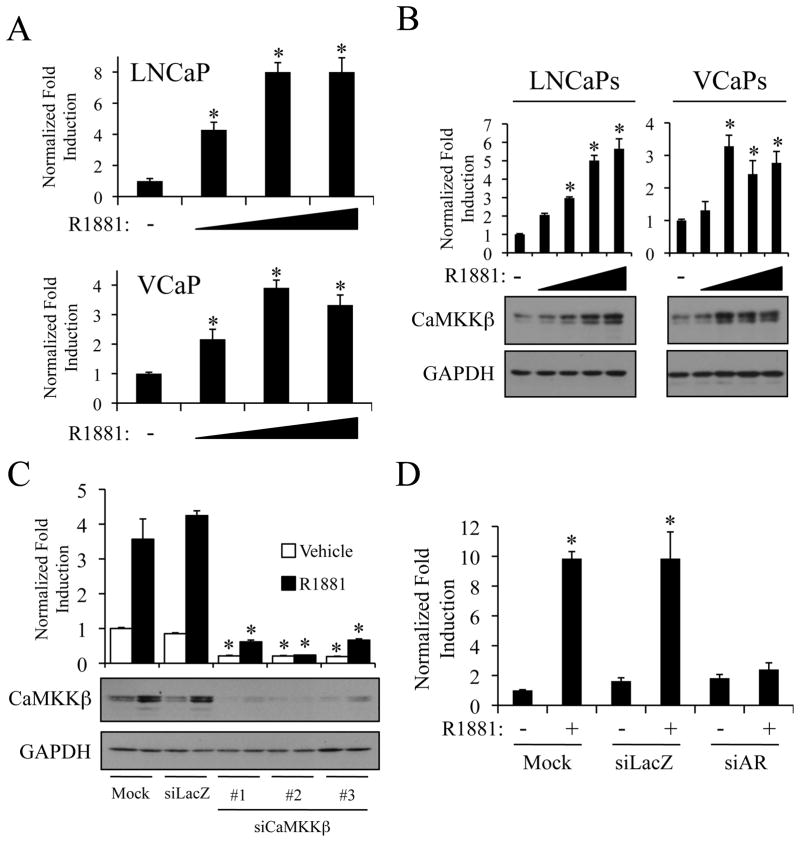
In an effort to identify novel prostate cancer therapeutics, we have focused on defining key regulators downstream of AR action that contribute to prostate pathobiology and that may be amenable to pharmacological exploitation. As a first step in this process, we analyzed the expression level of mRNAs encoding targetable signaling molecules using microarray data derived from androgen-treated LNCaP prostate cancer cells (13). These studies suggested that one such candidate, CaMKKβ, was upregulated by androgens. To confirm the significance of this observation, CaMKKβ mRNA levels were analyzed by qPCR following treatment with the synthetic androgen R1881. In both LNCaP and VCaP prostate cancer cell lines, CaMKKβ mRNA levels increased in a dose-dependent manner (Fig. 1A). Further, western immunoblot analysis revealed a corresponding dose-dependent increase in CaMKKβ protein levels in both cell lines (Fig. 1B). The specificity of the antibodies used in this study was verified using three different siRNAs targeting CaMKKβ mRNA (Fig. 1C). In addition, analogous immunoblot results were obtained using a second antibody (clone 1A11) directed against CaMKKβ (Supplementary Fig. S1). Finally, androgen-mediated induction, but not the basal expression, of CaMKKβ mRNA was abrogated in cells in which AR expression was inhibited using a validated siRNA (4) directed against the AR mRNA (Fig. 1D). Taken together, these data demonstrate that androgens, acting through AR, increase both CaMKKβ mRNA and protein levels in multiple cellular models of prostate cancer.
Figure 1.
Androgens increase CaMKKβ levels in an AR-dependent manner. LNCaP or VCaP cells were treated for 24 h with vehicle or increasing concentrations of the synthetic androgen R1881 (A-0.1, 1, and 10 nM; B-0.01, 0.1, 1, and 10 nM). A, after treatment, cells were lysed, and RNA was isolated and reversed transcribed. The expression of CaMKKβ was assessed using qPCR. B, after treatment, cells were subjected to western blot analysis and subsequent densitometry (top). CaMKKβ protein levels were normalized to GAPDH loading control. A and B, results are expressed as fold induction over vehicle-treated cells + SE (n = 3). *, significant changes from vehicle-treated cells. C, LNCaP cells were transiently transfected with mock or Stealth siRNAs targeting a negative control (siLacZ) or CaMKKβ(#1–3). Two days later, cells were treated for 24 h +/− 10 nM R1881. Whole-cell extracts were subjected to western blot analysis and densitometry (top) as described in B. *, significant changes from mock-transfected cells. D, LNCaP cells were transfected as described in C with mock or Stealth siRNAs targeting LacZ or AR and treated for 24 h. The expression of CaMKKβ was assessed as in A using qPCR.
Functionally active splice variants of CaMKKβ are expressed in response to androgens in the prostate
Given that AR increases CaMKKβ levels in multiple cellular models of prostate cancer, we next determined if its expression correlated with the development of prostate cancer in human samples. Analysis of the clinically annotated prostate cancer data sets accessible through Oncomine revealed that CaMKKβ expression increases with grade (14–17) (Supplementary Figs. S2A+B). Interestingly, this analysis also revealed that CaMKKβ was consistently overexpressed in prostate tumors, but not other malignancies (Supplementary Fig. S2C) (18). Importantly, ~80% of metastatic prostate cancers from noncastrated patients overexpress CaMKKβ, whereas fewer than 15% of castrated patients demonstrate elevated levels of CaMKKβ, indicating AR regulation of CaMKKβ in an in vivo clinical setting (19)
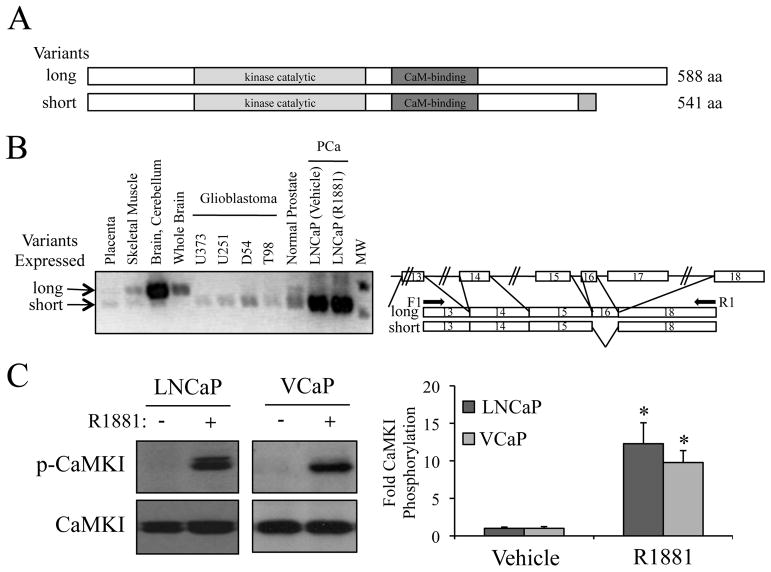
The full-length CaMKKβ protein is encoded by an mRNA composed of 18 exons. Interestingly, the majority of commercially available CaMKKβ antibodies target the C-terminus of the protein that is absent in some functionally active splice variants (20). Thus, given that the expression of CaMKKβ in the prostate has not been reported previously, we hypothesized that the prostate, and prostate cancers, may express a functionally important splice variant(s) of CaMKKβ that was not recognized by the most commonly used antibodies. To test this hypothesis, we performed RT-PCR analysis using primers spanning various exon boundaries to examine the splice variant repertoire in the normal prostate and in prostate cancer cells. In this manner, it was demonstrated that unlike in brain, which expresses a longer variant, both normal prostate and prostate cancer cells predominantly express shorter variants of CaMKKβ (Figs. 2A and B and Supplementary Fig. S3). The variants found are equivalent to the previously described CaMKKβ splice variants 2 and 7 that lack exon 16 (of note, splice variants 2 and 7 make identical protein products) (20). Interestingly, these shorter variants were also found in brain tumors (Fig. 2B). A complete analysis of the additional variants expressed in the prostate/prostate cancer is described in Supplementary Figure S3. Importantly, phosphorylation of the classical CaMKKβ target CaMKI was observed in both androgen-treated LNCaP and VCaP cells (Fig. 2C), indicating that the CaMKKβ variant expressed in prostate cancer cells is functionally active.
Figure 2.
The prostate expresses a different functional splice variant of CaMKKβ compared to brain A, schematic of CaMKKβ splice variants. B, RT-PCR using primers spanning specific exons (indicated in right schematic) was performed on cDNA generated from various tissues and cell lines. C, LNCaP or VCaP cells were treated for 24 h +/− 10 nM R1881. Cell lysates were then subjected to western blot analysis and subsequent densitometry (right). Phospho-CaMKI (p-CaMKI) protein levels were normalized to total CaMKI. Results are expressed as fold CaMKI phosphorylation over vehicle-treated cells + SE (n = 3). *, significant changes from vehicle-treated cells.
CaMKKβ is necessary and sufficient for AR-mediated prostate cancer cell migration and invasion
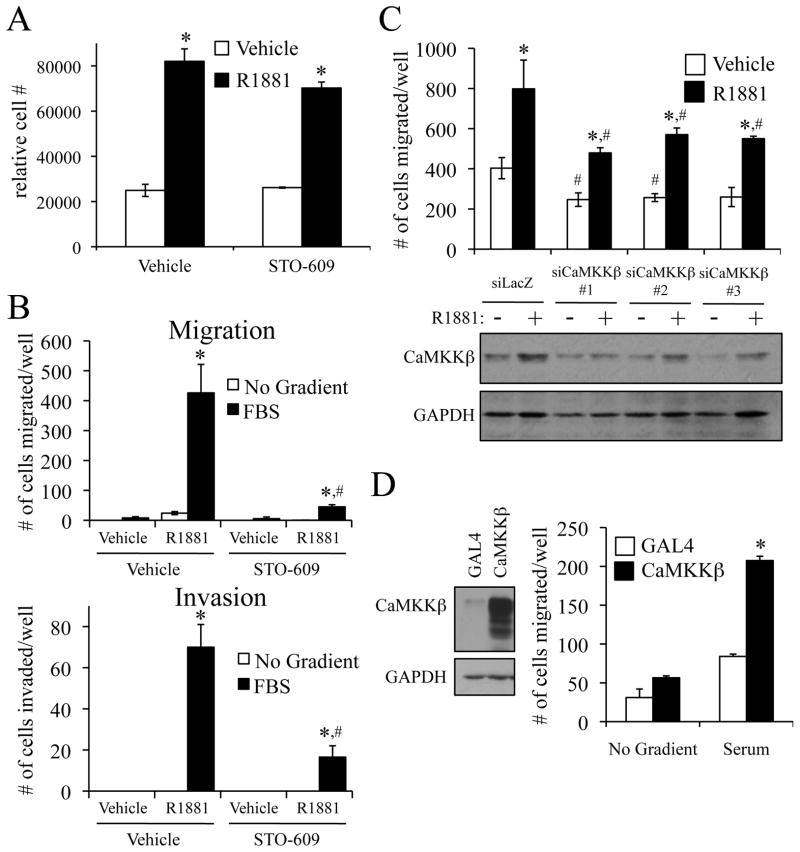
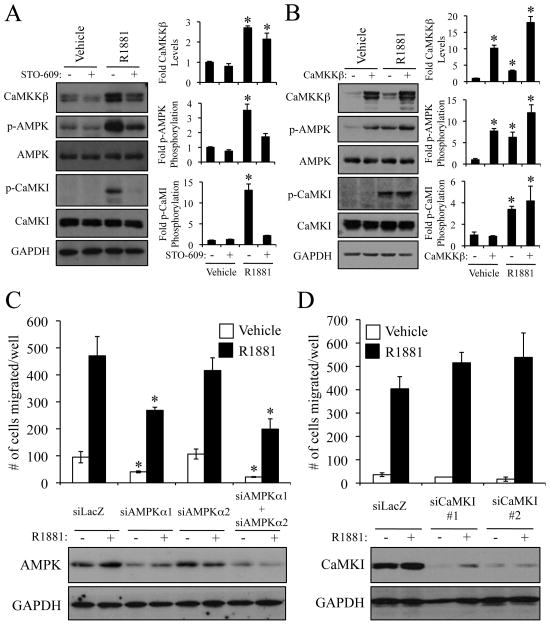
Given that the expression of CaMKKβ is upregulated by androgens and is elevated in prostate cancer, we next wanted to assess its potential role(s) in processes of pathological importance in this disease. As a first step, we evaluated the ability of the CaMKK antagonist STO-609 to inhibit the androgen-mediated cellular growth of prostate cancer cells. However, at a concentration that suppressed CaMKKβ activity (Supplementary Fig. S4A), this drug had no significant effect on LNCaP and VCaP cell number over the seven-day period of this assay (Fig. 3A and Supplementary Fig. S4B).
Figure 3.
CaMKKβ is required and sufficient for the androgen-mediated migration and invasion of prostate cancer cells. A, LNCaP cells were plated in 96-well plates and grown for 3 d. Cells were treated +/− 1 nM R1881 and +/− 30 μM STO-609 on d 3, d 5, and d 7. On d 10, cells were lysed and the relative number of cells was measured with the fluorescent DNA binding dye FluoReporter Blue. Each sample was performed in triplicate, and results from a representative experiment are shown. Results are expressed as relative cell number ± SE (n = 2). *, significant changes from vehicle (no R1881)-treated cells. B, LNCaP cells were pretreated for 1 h +/− 30 μM STO-609 prior to overnight treatment +/− 10 nM R1881. Cells were then dissociated and reseeded into the top chamber for a Boyden migration or Matrigel extracellular matrix invasion assay. Fresh medium with the corresponding treatments was added to the top and bottom chambers while either no chemoattractant or 5% FBS (serum) was added to the bottom chamber. After 16 h, migrated cells were fixed, stained and counted in three different microscopic fields and added together. The results are expressed as mean ± SE (n = 3). *, significant changes from vehicle (no R1881)-treated cells. #, significant changes from vehicle (no STO-609)-treated cells. C top, LNCaP cells were transfected with indicated siRNAs. Two days after transfection, cells were treated +/− 10 nM R1881 and subjected to a Boyden migration assay as described in B. *, significant changes from vehicle-treated cells. #, significant changes from control (siLacZ)-transfected cells. C bottom, western blot to demonstrate CaMKKβ knockdown Quantification of these blots is presented in Supplementary Fig. S4D. D right, LNCaP cells stably expressing either GAL4 (control) or CaMKKβ were subjected to a migration assay as described in B using +/− 5% FBS as chemoattractant. The results are expressed as mean + SE (n = 3). *, significant changes from LNCaP-GAL4 cells. D left, western blot confirming CaMKKβ expression. Quantification of these blots is presented in Supplementary Fig. S4E.
In addition to proliferation, androgens increase the migration of prostate cancer cells (4, 21). Since CaMKKβ has recently been implicated in cell migration during neuronal development (22, 23), we next asked whether CaMKKβ is important for AR-meditated prostate cancer cell migration and/or invasion. Using Boyden dual chamber migration assays, treatment with the CaMKK antagonist STO-609 blocked the androgen-mediated migration of both LNCaP (Fig. 3B, top) and VCaP prostate cancer cells (Supplementary Fig. S4C). Importantly, STO-609 also inhibited androgen-mediated invasion of LNCaP cells through a Matrigel extracellular matrix (Fig. 3B, bottom). Furthermore, knockdown of CaMKKβ suppressed, while its overexpression increased, both basal and androgen-stimulated cell migration (Figs. 3C, 3D and Supplementary Figs. S4D, S4E). These findings highlight a heretofore-unrecognized role for CaMKKβ in prostate cancer cell migration and invasion.
Definition of the molecular mechanism for AR-mediated CaMKKβ mRNA expression
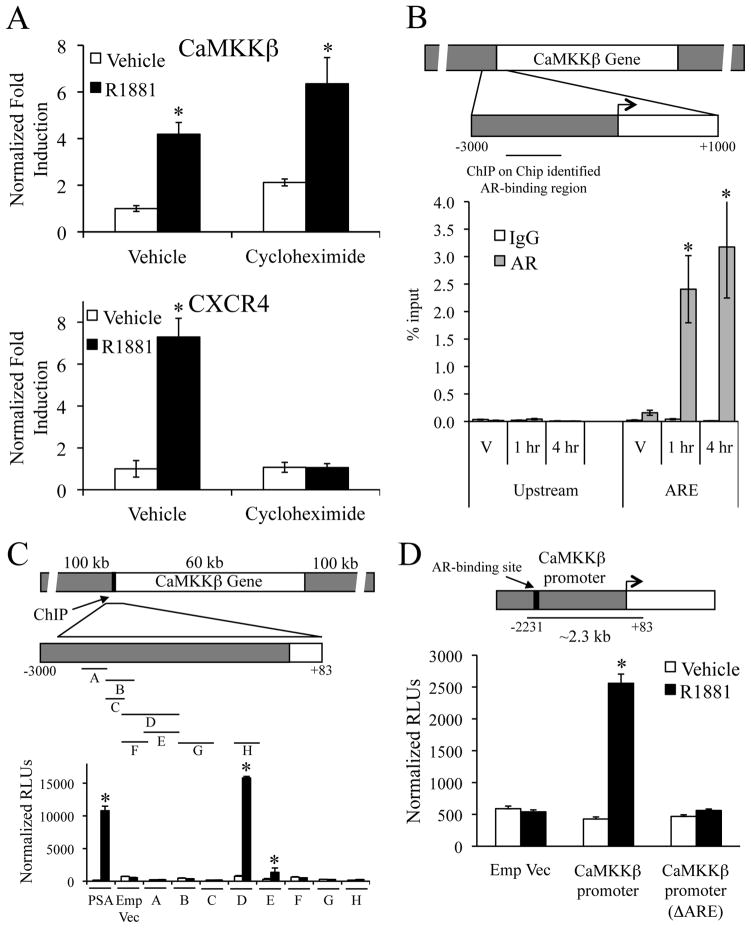
Using a knockdown/replacement strategy, it was demonstrated that expression of wild-type AR, but not a transcriptionally inactive DNA binding mutant (C562S), was able to complement the knockdown of endogenously expressed AR in an LNCaP cell migration assay (Supplementary Fig. S5). Further, at a concentration that inhibits the expression of secondary androgen target genes (ex. CXCR4 (4)), cycloheximide treatment did not block the R1881-mediated increase in CaMKKβ mRNA levels (Fig. 4A). Together, these data indicate that CaMKKβ is a primary AR target gene.
Figure 4.
Identification of the ARE that regulates CaMKKβ expression. A, LNCaP cells were pretreated for 1 h with vehicle or 1 μg/ml cycloheximide followed by vehicle or 10 nM R1881 for 24 h. CaMKKβ or CXCR4 mRNA levels were quantitated using qPCR. Results are expressed as fold induction over vehicle (no R1881)-treated cells ± SE (n = 3). *, significant changes from vehicle-treated cells. B, LNCaP cells were treated with vehicle (V) or 10 nM R1881 for 1 or 4 h. Cross-linked chromatin was immunoprecipitated with indicated antibodies. The precipitated DNA was amplified using primers spanning a region identified using ChIP on Chip data as a potential AR-binding site (indicated in top schematic) or a distal upstream region (negative control). The results are presented as percent input ± SE (n = 3). *, significant changes from IgG controls. C, various enhancer luciferase reporter constructs (depicted in top model) were transfected into LNCaP cells and treated overnight +/−10 nM R1881. After treatment, cells were harvested and assayed for luciferase activity. Luciferase values were normalized to β-galactosidase control. Data are the mean relative light units (RLUs) + SEM for one representative experiment performed in triplicate (n = 3). *, significant changes from vehicle-treated cells. D, CaMKKβ promoter constructs (depicted in top model) were transfected into LNCaP cells and then treated overnight with vehicle or 10 nM R1881. After treatment, cells were harvested and assayed for luciferase activity as in C. Emp Vec, empty vector.
By mining our previously published ChIP on Chip data (24), we identified a putative AR binding region located ~2.3 kb upstream of the CaMKKβ transcriptional start site (Fig. 4B, top). No other AR binding was detected within the CaMKKβ gene or within 100 kb in either direction of the gene. The validity of this AR-binding site was confirmed using ChIP assays, which showed that AR was recruited to this region of the promoter within one hour following R1881 treatment (Fig. 4B, bottom). Given these data, we focused on characterizing the functionality of the putative ARE identified. To this end, we cloned overlapping regions of CaMKKβ’s 5′ upstream region and tested their ability to confer androgen responsiveness to an enhancerless luciferase reporter gene. In this manner, we determined that a construct incorporating a fragment, −2231 to −1632 (D), and an overlapping fragment, −2019 to −1632 (E), contained an AR-dependent enhancer (Fig. 4C). Both fragments D and E demonstrated androgen responsiveness in a dose-dependent manner that was suppressed by the antiandrogen Casodex (Supplementary Fig. S6A). Similar results were obtained in VCaP cells (Supplementary Fig. S6B). Deletion analysis further narrowed down the androgen-responsive region to a 79 bp stretch of DNA that included a sequence, GTAACAtgaTGTAAA, that resembled the consensus androgen response element (ARE) AGAACAnnnTGTTCT (Supplementary Fig. S6C). Importantly, deletion of the 15 bp ARE in the full-length CaMKKβ promoter construct (−2231 to +83) completely abolished the androgen responsiveness (Fig. 4D). Thus, in the context of prostate cancer cells, CaMKKβ is a direct target of AR.
Androgens promote prostate cancer cell migration through an AR-CaMKKβ-AMPK signaling axis
CaMKI, CaMKIV and, more recently, AMPK have been shown to be downstream targets of CaMKKβ (25). Since CaMKIV is not expressed in the prostate (data not shown), we tested whether AR-CaMKKβ signaling led to increased CaMKI and/or AMPK signaling. Western blot analysis revealed that androgens increased the phosphorylation of both CaMKI and AMPK at their CaMKKβ activation loop target sites (T177 and T172 respectively) in both LNCaP and VCaP cells, an effect that was reversed by pretreatment with STO-609 (Fig. 5A and Supplementary Fig. S7A). Interestingly, we found that overexpression of CaMKKβ alone was sufficient to increase the phosphorylation/activity of AMPK, but not CaMKI (Fig. 5B). These findings indicated that AMPK, rather than CaMKI, could be regulating cell migration because CaMKKβ overexpression alone was also sufficient to increase migration (Fig. 3D). To verify this, we used our most efficacious siRNAs (Supplementary Fig. S7B) to knockdown both isoforms of the catalytic subunit of AMPK (Fig. 5C, bottom and Supplementary Fig. S7C) or CaMKI (Fig. 5D, bottom and Supplementary Fig. S7D). In this manner, it was demonstrated that loss of AMPK, but not CaMKI, resulted in decreased prostate cancer cell migration (Figs. 5C and D). Likewise, siRNA-mediated knockdown of AMPK decreased both basal and CaMKKβ-driven migration, indicating that either the residual AMPK activity left after siRNA transfection is sufficient to promote migration or an additional downstream target, unknown at this time, exists for CaMKKβ (Supplementary Fig. S8). In support of the results observed upon CaMKKβ mRNA knockdown, cotreatment of cells with the AMPK antagonist compound C, at a concentration that inhibited its kinase activity, completely abolished androgen-mediated cell migration (Supplementary Figs. S9A and B). However, in addition to inhibiting AMPK, we have determined that compound C also exhibits indirect inhibitory actions on AR-mediated transcription, a finding that makes it difficult to use the drug alone to implicate AMPK as the sole target of CaMKKβ (Supplementary Fig. S9C). Nevertheless, treatment of LNCaP cells with the AMP mimetic AICAR alone was sufficient to increase cell migration (Supplementary Figs. S9A and D). These data highlight a central role for AMPK in prostate cancer cell migration. Definition of the mechanism(s) by which AMPK interfaces with the cellular processes responsible for migration and invasion is currently under investigation.
Figure 5.
Androgen-mediated migration occurs through a CaMKKβ-AMPK-dependent pathway. A, LNCaP cells were pretreated for 1 h +/− 30 μM STO-609 prior to overnight treatment +/− 10 nM R1881. Cell lysates were then subjected to western blot analysis and subsequent densitometry (right). CaMKKβ levels were normalized to GAPDH. Phospho-CaMKI (p-CaMKI) levels were normalized to total CaMKI. Phospho-AMPK (p-AMPK) levels were normalized to total AMPK. Results are expressed as fold induction/phosphorylation over double vehicle-treated cells + SE (n = 3). *, significant changes from vehicle-treated cells. B, LNCaP cells stably expressing either GAL4 or CaMKKβ were treated overnight +/− 10 nM R1881. Cell lysates were then subjected as in A to western blot analysis and densitometry (right). Results are expressed as fold induction/phosphorylation over LNCaP-GAL4 vehicle-treated cells + SE (n = 3). *, significant changes from LNCaP-GAL4 vehicle-treated cells. C and D, LNCaP cells were transfected with indicated siRNAs, treated and subjected to a migration assay (top) or western blot analysis (bottom) as in Fig. 3C. *, significant changes from control (siLacZ)-transfected cells. Quantification of the blots is presented in Supplementary Fig. S7.
Discussion
The androgen-signaling axis constitutes the primary and most successful therapeutic target in prostate cancer (26). Regardless, the mechanism(s) by which AR impacts processes of pathological importance and the signaling pathways it modulates to accomplish these activities remain largely unknown. It is of significance, therefore, that we demonstrate that the CaMKKβ-AMPK signaling pathway is downstream of AR and mediates the effects of androgens on prostate cancer cell migration and invasion. Importantly, both CaMKKβ and AMPK are druggable targets that potentially can be exploited to generate new prostate cancer therapeutics.
CaMKKβ is highly expressed in the brain, where it functions to regulate axonal outgrowth, dendritic maturation, and the formation of dendritic spines and synapses (27). These processes are regulated by the CaMKKβ-initiated phosphorylation and activation of CaMKI and CaMKIV, two of its known primary substrates. Recently, AMPK has been identified as a third substrate of CaMKKβ (28–30). AMPK coordinates energy balance, fatty acid oxidation, autophagy and CO2 sensing in both neuronal and nonneuronal tissues. It is composed of an α catalytic subunit and β and γ regulatory subunits. Our data demonstrate that CaMKKβ-induced prostate cancer cell migration requires AMPK and, more specifically, the α1 catalytic kinase subunit of AMPK (Fig. 5). These findings are not completely surprising as 1) the α1 subunit, but not the α2 subunit, has a predominately cytoplasmic cellular localization and thus, would be the more likely target for the cytoplasmically-localized CaMKKβ protein (31) and perhaps more importantly 2) the α2 subunit is not highly expressed in the prostate (32) or in prostate cancer cells (data not shown). Regardless, our data demonstrate an additional role for the CaMKKβ-AMPK signaling axis in prostate cancer cell migration.
The role of AMPK in prostate cancer pathogenesis has been controversial. Studies have shown that AMPK is frequently activated in human prostate cancers and inhibition of its activity, using the antagonist compound C, has inhibitory effects on cell growth (33). Conversely, several laboratories, including our own (data not shown), have demonstrated that AICAR and the antidiabetic drugs metformin and rosiglitazone, activators of AMPK, also inhibit prostate cancer cell growth (34–36). These discrepancies could be attributed to the pleiotropic effects of the various small molecule modulators. For example, AMPK-activators, such as AICAR, function by mimicking cellular stress and therefore, may potentiate other stress responses and activate all cellular AMPK. Hence, small molecule AMPK activators may block cell growth through a variety of indirect mechanisms. Nonetheless, it is possible that the role of AMPK as a master regulator of metabolism includes sensing cellular starvation, halting cellular growth and the subsequent induction of cell motility, thus allowing cells to migrate towards more nutrient-rich environments.
AMPK signaling has been implicated in angiogenesis and specifically in endothelial cell migration (37). At this time, however, it is unclear how AMPK controls prostate cancer cell migration. In both neuronal and endothelial cells, CaMKKβ and/or AMPK have been demonstrated to potentiate the activity of Rac1 (23, 38, 39), a master regulator of cellular migration (40). Thus, the CaMKKβ-AMPK signaling pathway may augment prostate cell migration and invasion through activation of Rac1. Indeed, preliminary data in our laboratory suggests androgens increase Rac1 activity (data not shown). Additionally, elevated Rac1 activity has been shown to increase the aggressiveness of prostate cancer cells (41, 42). Thus, Rac1 may function as a conduit for cellular signaling pathways such as CaMKKβ-AMPK to control aspects of prostate cancer pathogenesis.
The enzymatic activity of CaMKKβ is regulated by Ca2+/calmodulin. Recently, augmented calcium intake has been correlated with increased prostate cancer incidence (43). Further, calcium influx promotes the migration and metastasis of both prostate and breast cancers (44, 45). The data presented here may provide a mechanistic link between calcium uptake and cell migration. Our studies also show that overexpression of CaMKKβ alone was sufficient to increase AMPK activity and cellular migration. This suggests that the basal levels of calcium present in the prostate cancer cells were sufficient to result in CaMKKβ activation (Figs. 3 and 5). Hence, the observation that the levels of CaMKKβ alone dictate cellular processes (Fig. 3) underscores the importance of AR’s regulation of CaMKKβ expression (Figs. 1 and 4).
While various upstream signaling pathways have been shown to regulate the activity of CaMKKβ, to our knowledge, this is the first demonstrated regulation of CaMKKβ expression by any signaling pathway. This strongly implicates a role for genomic androgen signaling in cellular migration. Other laboratories have suggested that androgens, through rapid nongenomic mechanisms, alter cytoskeletal reorganization and, in this manner, may impact migration (46, 47). In our hands, only prostate cancer cells expressing a wild-type AR, but not an AR containing a DNA-binding domain mutation that abrogated its transcriptional activity, could convey androgen-mediated cell migration (Supplementary Fig. S5). Additionally, androgens did not promote significant levels of cell migration a) within six hours of hormone treatment or b) in the presence of the transcriptional inhibitor actinomycin D (data not shown), indicating that androgen-mediated migration is not rapid and likely requires genomic actions of AR. Thus, while androgens may increase cellular migration in part through nongenomic signaling, this work underscores the importance of the genomic actions of androgens in this process.
This study advocates the inhibition of the AR-CaMKKβ-AMPK pathway as a novel therapeutic approach for the treatment of prostate cancer. In particular, CaMKKβ represents a practical target for future drug development because of its restricted expression and the demonstrated ability of small molecules to block its activity (ex. STO-609). Additionally, CaMKKβ −/− mice display no overt developmental prostate abnormalities and do not exhibit fertility problems (data not shown). In subsequent studies, it will be interesting to cross these knockout animals with various prostate cancer mouse models to determine if CaMKKβ is required for their pathogenesis. Given what is currently known about CaMKKβ biology and considering the results of the studies reported here, we believe that in regards to prostate cancer therapeutics, an ideal inhibitor of this enzyme should exhibit selectivity for CaMKKβ over the related and more ubiquitous CaMKKα isoform and should not be able to cross the blood-brain barrier. This would isolate the actions of the drug and prevent it from interfering with CaMKKβ-regulated processes in the brain. Taken together, the data presented here indicate that a next-generation CaMKKβ antagonist displaying the above-described pharmacological properties is likely to find utility as a treatment for prostate cancer.
Supplementary Material
Footnotes
Disclosure of potential conflicts of interest: None
Financial support: Supported by NIH grants K01 DK084205 (D.E. Frigo), R01 GM033976 (A.R. Means) and R01 CA139818 (D.P. McDonnell).
References
- 1.Cancer Facts and Figures. American Cancer Society; 2007. [Google Scholar]
- 2.Isaacs JT, Isaacs WB. Androgen receptor outwits prostate cancer drugs. Nat Med. 2004;10:26–7. doi: 10.1038/nm0104-26. [DOI] [PubMed] [Google Scholar]
- 3.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nature Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 4.Frigo DE, Sherk AB, Wittmann BM, et al. Induction of Kruppel-like factor 5 expression by androgens results in increased CXCR4-dependent migration of prostate cancer cells in vitro. Mol Endocrinol. 2009 doi: 10.1210/me.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherk AB, Frigo DE, Schnackenberg CG, et al. Development of a small molecule serum and glucocorticoid-regulated kinase 1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008;68:1–9. doi: 10.1158/0008-5472.CAN-08-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–92. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 7.Migita T, Ruiz S, Fornari A, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101:519–32. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawton CA, Winter K, Murray K, et al. Updated results of the phase III radiation therapy oncology group (RTOG) trial 85–31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int J Radiation Oncology Biol Phys. 2001;49:937–46. doi: 10.1016/s0360-3016(00)01516-9. [DOI] [PubMed] [Google Scholar]
- 10.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. The Lancet. 2002;360:103–8. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frigo DE, McDonnell DP. Differential effects of prostate cancer therapeutics on neuroendocrine transdifferentiation. Mol Cancer Ther. 2008;7:659–69. doi: 10.1158/1535-7163.MCT-07-0480. [DOI] [PubMed] [Google Scholar]
- 13.Kazmin D, Prytkova T, Cook CE, et al. Linking ligand-induced alterations in androgen receptor structure to differential gene expression: a first step in the rational design of selective androgen receptor modulators. Mol Endocrinol. 2006;20:1201–17. doi: 10.1210/me.2005-0309. [DOI] [PubMed] [Google Scholar]
- 14.Lapointe J, Li C, Higgins JP, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–6. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varambally S, Yu J, Laxman B, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Welsh JB, Sapinoso LM, Su AI, et al. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–8. [PubMed] [Google Scholar]
- 17.Yu YP, Landsittel D, Jing L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–9. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 18.Su AI, Welsh JB, Sapinoso LM, et al. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res. 2001;61:7388–93. [PubMed] [Google Scholar]
- 19.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu LS, Chen GD, Lee LS, Chi CW, Cheng JF, Chen JY. Human Ca2+/calmodulin-dependent protein kinase kinase beta gene encodes multiple isoforms that display distinct kinase activity. J Biol Chem. 2001;276:31113–23. doi: 10.1074/jbc.M011720200. [DOI] [PubMed] [Google Scholar]
- 21.Liao X, Thrasher JB, Pelling J, Holzbeierlein J, Sang QX, Li B. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology. 2003;144:1656–63. doi: 10.1210/en.2002-0157. [DOI] [PubMed] [Google Scholar]
- 22.Kokubo M, Nishio M, Ribar TJ, Anderson KA, West AE, Means AR. BDNF-mediated cerebellar granule cell development is impaired in mice null for CaMKK2 or CaMKIV. J Neurosci. 2009;29:8901–13. doi: 10.1523/JNEUROSCI.0040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saneyoshi T, Wayman G, Fortin D, et al. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Means AR. The Year in Basic Science: calmodulin kinase cascades. Mol Endocrinol. 2008;22:2759–65. doi: 10.1210/me.2008-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15:3251–5. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 27.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–31. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 31.Salt I, Celler JW, Hawley SA, et al. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334 ( Pt 1):177–87. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berglund L, Bjorling E, Oksvold P, et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7:2019–27. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Park HU, Suy S, Danner M, et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8:733–41. doi: 10.1158/1535-7163.MCT-08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Huang W, Tao R, et al. Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene. 2009;28:1993–2002. doi: 10.1038/onc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–86. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 36.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–7. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 37.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–6. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 38.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–64. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 39.Kou R, Sartoretto J, Michel T. Regulation of Rac1 by simvastatin in endothelial cells: differential roles of AMP-activated protein kinase and calmodulin-dependent kinase kinase-beta. J Biol Chem. 2009;284:14734–43. doi: 10.1074/jbc.M808664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 41.Knight-Krajewski S, Welsh CF, Liu Y, et al. Deregulation of the Rho GTPase, Rac1, suppresses cyclin-dependent kinase inhibitor p21(CIP1) levels in androgen-independent human prostate cancer cells. Oncogene. 2004;23:5513–22. doi: 10.1038/sj.onc.1207708. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi T, Inoue T, Shimizu Y, et al. Activation of Rac1 is closely related to androgen-independent cell proliferation of prostate cancer cells both in vitro and in vivo. Mol Endocrinol. 2010;24:722–34. doi: 10.1210/me.2009-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler LM, Wong AS, Koh WP, Wang R, Yuan JM, Yu MC. Calcium intake increases risk of prostate cancer among Singapore Chinese. Cancer Res. 2010;70:4941–8. doi: 10.1158/0008-5472.CAN-09-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–34. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Monet M, Lehen’kyi V, Gackiere F, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–35. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- 46.Kampa M, Papakonstanti EA, Alexaki VI, Hatzoglou A, Stournaras C, Castanas E. The opioid agonist ethylketocyclazocine reverts the rapid, non-genomic effects of membrane testosterone receptors in the human prostate LNCaP cell line. Exp Cell Res. 2004;294:434–45. doi: 10.1016/j.yexcr.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol. 2003;17:870–81. doi: 10.1210/me.2002-0253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.