Abstract
Polymeric materials have been applied in therapeutic applications, such as drug delivery and tissue regeneration, for decades owing to their biocompatibility and suitable mechanical properties. In addition, select polymer–drug conjugates have been used as bioactive pharmaceuticals owing to their increased drug efficacy, solubility, and target specificity compared with small-molecule drugs. Increased synthetic control of polymer properties has permitted the production of polymer assemblies for the targeted and controlled delivery of drugs, and polymeric sequestrants take advantage of their lack of solubility for the sequestration of target molecules in vivo. In more recent studies reviewed in greater detail here, the properties of polymers that distinguish them from small-molecule drugs, such as their high molecular weight and their ability to display multiple pendant moieties, have been specifically exploited for activating cellular targets or inhibiting the binding of pathogens. The elucidation of relevant structure–function relationships in investigations of this kind has relied on the combination of living polymerization methods with chemical conjugation methods, and protein engineering methods have shown increasing potential in the manipulation of architectural features of such polymer therapeutics. Garnering a detailed understanding of the various mechanisms by which multivalent polymers engage biological targets is certain to expand the role of polymers as therapeutics, by enabling highly specific activities of designed polymers in the biological environment.
Introduction
Polymeric materials have been used for many decades in biomedical applications such as drug delivery, implants, contact lenses, vascular grafts, dental materials, and select artificial organs. Their useful and tunable mechanical properties have offered broad utility in the structural support or replacement of tissues or in controlled retention and release of drugs.1–3 The development of polymers as bioactive pharmaceuticals in their own right has only more recently been exploited.4 The introduction of the polymer–anticancer drug concept by Ringsdorf in 1975 marked the beginning of an era of fruitful research in this topic. Long thought to be too heterogeneous with respect to molecular weight (polydispersity), composition, and structure to be useful therapeutically, polymers are now known to offer many specific advantages critical to treating human disease and have recently entered into medical practice.3 Indeed, the early studies of Duncan, Kopecek, and Ringsdorf in the late 1970s resulted in the first polymer–drug conjugates to be used as medical treatment.5
In the treatment of disease and injury, therapeutic molecules, regardless of their composition and physical form, must fulfill several basic requirements, including biocompatibility, stability under physiological conditions, specificity for the target, desired mechanical properties, and minimal adverse effects. The immunogenic responses and side effects of many drugs, especially protein drugs, are exacerbated by their hydrophobicity; therefore, drug toxicity can be reduced by increasing the drug solubility by conjugation of a hydrophilic polymer scaffold to the drug in question.6 With conjugation to a polymer, drugs can also be protected from degradation, resulting in improved efficacy due to increased drug circulation times. In addition to the environmental protection afforded by polymers, the tunable and responsive properties of many polymeric scaffolds have also permitted improved routes for targeted drug delivery. The controlled release of drugs from polymer–drug conjugates, by variations in pH, temperature, enzyme concentration, or attachment of targeting ligands, can increase drug efficacy by increasing local drug concentration at the desired site of therapeutic need.7–9 In a different therapeutic approach, toxic small molecules can be eliminated selectively from the body via their sequestration in polymeric scaffolds.10 Finally, polymers themselves can also offer special opportunities over small-molecule drugs in the manipulation of multivalent binding events, due not only to their display of multiple pendant ligands but also to the potential to vary polymer structure (and therefore biological activity) via living polymerization methods.4,11
In this article, an overview is provided on a variety of polymer-based therapeutics, including noncovalent and covalent polymeric delivery vehicles, polymer sequestrants, polymerized drugs, and, in greater detail owing to recent advances in this emerging area, polymeric theraupeutics. Various polymerization methods and new approaches that have been applied to improve the control of polymer structure for the design and understanding of polymers in therapeutic applications are also discussed. Enhanced understanding of the mechanisms by which polymers elicit specific biological outcomes has propelled progress in this field. This significant progress in the development of polymer therapeutics–in both synthetic approaches and characterization methods–suggests continued exciting therapeutic applications for macromolecules in the future.
Polymeric Delivery Vehicles
A wide range of polymeric drug delivery vehicles have been developed for the delivery of both small molecular and biomacromolecular drugs. Polymeric delivery vehicles for biomacromolecular drugs have provided particular advantages in protecting molecules such as DNA, RNA, and proteins from degradation and inactivation in vivo. Therefore, polymer matrices, assemblies, and complexes, with properties that can be modulated by stimuli such as pH, temperature, and net charge, have been designed and used to control delivery of therapeutic drugs with increased efficacy and optimized doses.
Polymer Matrices
Drugs entrapped in polymer matrices (Figure 1A) can be released via passive diffusion of the drug from a static polymer scaffold (e.g., through the pores in the polymer matrix or between polymer chains). Enhanced diffusion can be attained with swelling of the polymer matrix (via changes in pH, ionic strength, temperature, enzymatic conversion, application of electric or magnetic field), providing a mechanism for stimuli-responsive release. Alternatively, drug release can be initiated by polymer degradation (proteolytic or hydrolytic), thus eliminating the need to remove the scaffold after drug release. A variety of hydrophilic and biocompatible hydrogels based on natural and synthetic polymers have been used for encapsulation of drugs for drug delivery or cells for repairing tissues and organs; indeed, their use dates back decades. For example, poly(hydroxyethyl methacrylic) acid (HEMA) hydrogels were introduced by Wichterle and Lim in 1960,12 and calcium alginate microcapsules were introduced by Lim and Sun in 1980.13 These hydrogels were suitable for drug delivery as they hydrolyze with difficulty and are compatible in many biological environments. They withstand heat sterilization, and their mechanical properties and water content can be adjusted to meet use requirements. Since then, an enormous amount of work has been done in the development of various improved hydrogel-based polymer matrices.14 In particular, poly(ethylene glycol) (PEG)-based hydrogels have shown excellent properties for use as biomaterials because of their biodegradation and biocompatibility15 and have been regularly employed as matrices for drug delivery and cell encapsulation as well as conjugated to biomacromolecules to improve their half-lives in vivo.16 Park and co-workers have reported superporous hydrogels and composites from a wide range of polymers including poly-(acrylic acid) (PAA) and PNIPAAm.17 In recent studies, Oh et al. have synthesized biodegradable nanogels by uniformly cross-linking, with disulfide linkages, POEOMA (poly(oligo(ethylene oxide) monomethyl ether methacrylate)) carrying pendant oligo-(ethylene oxide). The polymers were produced via ATRP methods in inverse miniemulsions, and the high ethylene oxide content prevents protein adsorption to the nanogels. Cytotoxicity assays revealed that the resulting nanogels were nontoxic to cells. The nanogels can be degraded to soluble polymers in the presence of reducing agents such as the water-soluble biocompatible glutathione tripeptide, suggesting their potential use as delivery matrices that target specific cells. Thus, in the future, porous hydrogels of controlled structure and chemical composition should be useful for optimal release of drugs under various therapeutically relevant conditions.18
Figure 1.

Schematics of polymeric delivery vehicles: (A) polymer matrix; (B) polymer assembly; (C) polymer complex.
Polymer Assemblies
Small molecule drugs can be physically entrapped or covalently attached to polymer micelles and/or to core–shell nanoparticles to form polymer assemblies (Figure 1B).19 These assemblies have attracted great interest for their potential application as carriers in drug delivery as they demonstrate high drug-loading capability, biodegradability, prolonged circulation time, slow plasma clearance, and controllable drug-release profiles. The entrapment/attachment is achieved generally via passive diffusion or in situ loading at the inner hydrophobic core of the micelles/particles during assembly/particle formation.20 Increased therapeutic efficacy of drug, selective delivery of the drug to the target cell, and reduced toxic side effects on other organs have been a major challenge and area of research in the design of these polymer assemblies.21,22 In one example, Morelli and co-workers have developed a polymer assembly system by assembling nanocarriers that target cholecystokinin receptors that are overexpressed by cancerous cells. The nanocarriers were produced by aggregating two amphiphilic monomers. One contained the bioactive peptide CCK8 (a C-terminal sequence of cholecystokinin hormone which provides the binding sequence for cholecystokinin receptors) linked by a polydisperse poly(ethylene glycol) to two C18 hydrophobic tails ((C18)2PEG2000CCK8). The other contained an anionic DTPAGlu, a derivative of DTPA (diethylenetriamine pentaacetic acid, a chelating agent which provides stable radio-labeled indium-111 complexes) also linked to C18 hydrophobic tails ((C18)2DTPAGlu). The presence of the bioactive peptide, exposed on the external surface of the aggregate, permitted selective targeting of nanocarriers to the cell receptors, with primarily selective intracellular release.23 To test the main site of release, Forster resonance energy transfer (FRET) imaging and spectroscopy was used to monitor the release of hydrophobic probes from dual-labeled micelles containing fluorescently labeled copolymers and hydrophobic probes. This study suggested that the cellular uptake of hydrophobic core molecules, preloaded in polymeric micelles, was mediated by membrane pathway which provides a temporal residence for hydrophobic probes release before their delivery to targeted intracellular destinations.24
Polymer Complexes
Polymer–nucleic acid complexes have been explored as nonviral vectors in gene therapy, although they are less efficient than viral gene delivery systems. As schematically shown in Figure 1C, cationic polymers form condensed complex nanoparticles with negatively charged DNA, due to electrostatic forces, and protect the DNA from degradation during cellular uptake. Their simple processing and capacity to carry large genes have motivated their design as therapeutic DNA carriers.25–30 To overcome a range of extra and intracellular transport barriers, Hammond and co-workers have developed a family of linear–dendritic hybrid polymers consisting of linear PEG and dendritic polyamidoamine (PAMAM) to bind DNA. This polymer hybrid has the essential functionality for targeting tissue, minimizing nonspecific interactions, and buffering in the endosome.27 The nonviral vectors often suffer from high toxicity, low specificity, and/or the instability of the resulting complexes in serum. To address these issues, Reineke and co-workers have designed polycations which are biocompatible, target-specific, and efficient delivery vehicles. They synthesized a series of trehalose-based copolymers, formed via 3 + 2 Cu(I)-catalyzed azide–alkyne cycloaddition (“click”) reactions, that carried variations in amine stoichiometry. These polymers contained between one and four secondary amines and/or 1,2,3-triazole groups in their repeat units with varying degrees of polymerization. The polymers bind plasmid DNA with high affinity and can compact genetic material into polyplexes that are serum stable. As a result, these trehalose-based systems were generally found to yield efficient cellular delivery in the presence of serum. Their findings suggested that increasing the amine density in the polymer facilitates effective plasmid DNA compaction, serum stabilization, high cellular uptake, and gene expression.31,32 Previous studies have shown the incorporation of poly(ethylene glycol) chains in polycationic vectors improves their potential for in vivo gene delivery by favorably stabilizing the polyplexes, polycation, and DNA complexes against precipitation in conditions containing salt and serum.33 The use of PEGs and heparin-binding peptides in the complexation of nucleic acids may also offer unique opportunities for controlling and improving DNA delivery. Polyplexes containing heparin-binding peptides may increase cell–surface adhesion and subsequent uptake, compared to nonspecific polycationic vectors, via sequence-specific association.34
Polymeric Sequestrants
Owing to the lack of their adsorption through intestinal walls, coupled with the ability to control their electrostatic charge and hydrophobicity, polymeric sequestrants in the form of hydrogels and resins have been widely used to remove ions, bile acids, fats, and toxins from the body, as illustrated in Figure 2. One such polymeric sequesterant is an insoluble, anionic polystyrene-based resin, commercially known as Kayexalate (sodium polystyrene), which has been approved for the treatment of hyperkalemia since 1975, to sequester excess potassium ions in the gastrointestinal (GI) tract. An additional commercial product, Renagel (sevelamer hydrochloride, based on polyallylamine), can bind with phosphates and thus lower the serum phosphorus level in patients with chronic kidney disease.35,36 Another example of a polymeric sequestrant based on hydroxamic acid removes excess iron from the body; the presence of excess iron (hemochromatosis) can lead to toxic effects due to the catalytic transformation of molecular oxygen to hydroxyl radicals. Nonabsorbed and biocompatible polymeric sequestrants that can remove excess dietary iron from the GI tract with high affinity and selectivity offer an attractive method to treat hemochromatosis. A cross-linked hydrogel containing hydroxamic acid has shown good binding properties with dietary iron at both low and neutral pH values (3.5–7.0) and has been well tolerated by test animals, suggesting its potential clinical use in the future.10
Figure 2.

Schematic illustrating polymer sequestrants.
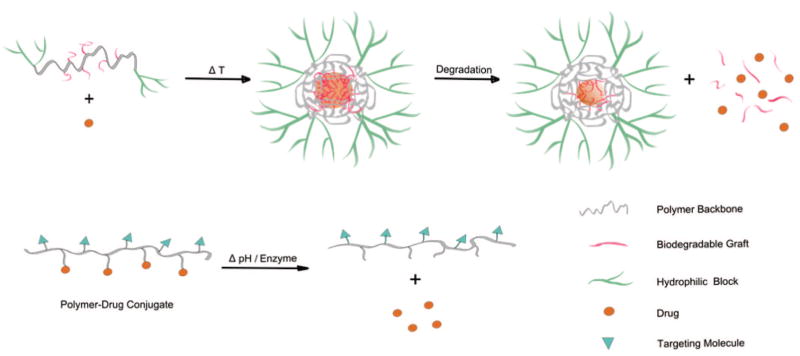
Polymer–Drug Conjugates
The concept of polymer–drug conjugates was first introduced by Ringsdorf in 1975,37 and Duncan and co-workers further exploited the biological rationale and the mechanism of polymer–drug designs to pioneer a field that has remained of great scientific and therapeutic interest.38,39 As mentioned above and as shown in Figure 3, polymer–drug conjugates can improve drug solubility, circulation time (through the properties of the polymer carrier), and drug targeting (via the use of appropriate linkers that can respond to changes in physiological conditions such as temperature, pH, and the presence of enzymes) and have emerged as an attractive approach in polymer therapeutics. In this type of polymer conjugate, multiple copies of bioactive agents, ranging from small molecule drugs to larger compounds like oligosaccharides and peptides, are attached to a polymer scaffold. For example, the use of high molecular weight PEGs resolves the solubility issues of neutral small prodrug species after forming conjugates.40
Figure 3.
Schematics illustrating temperature-, pH-, and enzyme-sensitive drug delivery systems.
Protein–Polymer and Peptide–Polymer Conjugates
Abu-chowski et al. in 1977 first introduced the conjugation of poly(ethylene glycol) (PEG) to protein drugs,41 and it is now very well accepted that PEG extends circulation time and efficiency of many drugs. In the 1980s, Hoffman and co-workers conjugated temperature-responsive polymers such as polyNIPAAm to proteins.42 PolyNIPAAm–monoclonal antibody conjugates were produced to develop a new thermally induced phase-separation immunoassay.43 Since then, the combination of biological macromolecules with polymers to achieve polymer–protein bioconjugates has been of particular interest for applications in nanobiotechnology.44–47 For example, Klok and co-workers demonstrated the preparation of thin polymer layers composed of poly(2-hydroxyethyl methacrylate) (PHEMA) or poly(poly(ethylene glycol) methacrylate) (PPEGMA), to decrease nonspecific adsorption of proteins. The polymers can be functionalized with peptide ligands and thus can be used as coatings to promote endothelialization of blood contacting biomaterials.45
In recent studies, Francis and co-workers have introduced a robust and versatile modular carrier system for drugs via modification of the exterior and interior surfaces of genome-free viral capsids. The interior surface of the capsid was decorated with drug molecules, and via orthogonal coupling strategies the exterior surface was modified with multiple copies of PEG-linked ligands that target specific cell receptors. This carrier design provides resistance to antibody binding that would neutralize the carriers before they reach their destinations.48
Temperature-Sensitive Conjugates
Polymers that can respond in a biological environment via changes in temperature have been a focus of significant research for decades. Poly(N-isopropylacrylamide) (polyNIPAM)-based polymers have been widely investigated for drug delivery applications owing to their thermo-responsive behavior. PolyNIPAM copolymerized with the hydrophobic and biodegradable polymer poly(L-lactic acid), along with a hydrophilic poly(L-lysine) dendron, shows an altered lower critical solution temperature (LCST) relative to that of polyNIPAM; the LCST can be tailored to near body temperature. Poly(L-lysine) (PLL) enhances delivery of therapeutic agents into cells or across biological barriers due to its bulky branched structure and its electrostatic interaction with the polyanionic phospholipids of cell membranes, suggesting the potential application of this copolymer in biomedical fields.8 Elastin-like polypeptides (ELP), another well-known thermo-responsive polymer comprising repeated pentapeptide units, have been widely used in drug delivery owing to their biodegradable, biocompatible, and nonimmunogenic properties.49,50 They have the ability to spontaneously entrap drugs by undergoing an LCST-like inverse phase transition that results in aggregation of the polypeptide at temperatures above their transition temperature.51 An advantage to the application of ELPs for the delivery of protein drugs is that the two can be conjugated at the genetic level via recombinant methods.52 ELP drug delivery systems have been designed to aggregate upon intra-articular injection at 37 °C and slowly degrade and clear from the joints over time, suggesting a potent approach to treat localized joint disease of a variety of etiologies. In vivo studies in a rat model were conducted to compare the half-life of soluble and insoluble (aggregates) of the polypeptides after intra-articular injection, by varying the transition temperature of the ELPs. The soluble polypeptides had a half-life of less than 4 h while the aggregated ELPs had a half-life of more than 85 h, suggesting that aggregating ELPs concentrate in the joint upon injection and slowly disaggregate to release protein drugs.53
pH-Sensitive Conjugates
Hydrolysis of the link between the polymer and drug in polymer–drug conjugates can be stimulated by a change of pH to release bioactive reagents to targeted areas. In the early 1980s Shen and Ryser first utilized the concept of pH-controlled drug release via modified amino-ethyl polyacrylamide beads and poly(D-lysine) conjugated with daunomycin via cis-aconityl linkages. The cis-aconityl linkage between the drug and the polymer is pH-sensitive with a hydrolysis half-life of 3 h at pH 4 and greater than 96 h at pH 6 and above.54 Since then many pH-sensitive drug conjugates have been developed.55 Hydrazone linkages, cis-aconityl amide linkages, or other groups like trityl, acetal, and imino groups are some well-known pH-sensitive linkages used in this drug conjugates category.56,57 Conjugates of doxorubicin (Dox) with HPMA (N-(2-hydroxypropyl)methacrylamide) via pH-sensitive linkers have been developed as anticancer drugs.58–60 Moreover, HPMA copolymer conjugates carrying a combination of both endocrine (an aromatase inhibitor) and chemotherapeutic doxorubicin, HPMA–aminogluthimide–doxorubicin, have been reported to show greater cytotoxicity toward MCF-7 breast cancer cells in vitro as compared to the individuals alone.60 Ulbrich and co-workers have reported acid-sensitive HPMA–Dox copolymer conjugates containing hydrazone or cis-aconityl linkers. Their study showed that the rate of Dox release from these different conjugate systems was pH-dependent with the highest release rate obtained at pH 5, while only a very small amount of Dox release was observed at physiological pH. The cytotoxicity of various pH-sensitive conjugates was tested and compared with that of other conjugates such as the enzymatically degradable conjugate PK1 (HPMA–Dox copolymer conjugate, linked via Gly-Phe-Leu-Gly peptidyl spacer).61 The cytotoxicity of the hydrazone-based conjugates was the highest and comparable to that of the free drug, Dox. The in vivo activities of various conjugates were also compared in protective and therapeutic regimes of drug administration, and the in vivo antitumor activity of the hydrazone-based conjugates was notably better compared to free drug or the clinically tested, enzymatically degradable conjugate PK1; these studies suggest a reliable rationale for the design of pH-sensitive polymer–drug conjugates.62
Enzyme-Sensitive Conjugates
Another powerful method for targeted drug release exploits the enzymatic cleavage of linkers in polymer–drug conjugates.58,63 In an attempt to increase the rate and maximum extent of side-chain hydrolysis by lysosomal enzymes, Duncan and co-workers developed polymer–drug conjugates using N-(2-hydroxypropyl)methacrylamide copolymers and p-nitroaniline drug analogues, bearing oligopeptidyl-p-nitroanilide side chains, which are specific to certain lysosomal proteinases, yielding a potential delivery system. Degradation, by rat liver lysosomal enzymes, of the drug-carrying side chains occurred only in the presence of reduced glutathione and was inhibited by leupeptin, indicating the involvement of thiol–proteinases in the degradation and suggesting their effectiveness in targeted delivery.64 This enzyme-sensitive strategy was also explored by Langer and co-workers, with drug molecules linked to polymeric carriers via a peptide linker (Pro-Val-Gly-Leu-Ile-Gly), which is susceptible to cleavage by tumor associated matrix metalloproteinases (MMP). Methotrexate, a chemotherapeutic drug, was conjugated to the biocompatible and biodegradable dextran polymeric carrier using the above peptide sequence, and the liberation of the drug in response to the matrix metalloproteinases MMP-2 and MMP-9 was demonstrated. In vitro experiments, in tumor cell cultures, demonstrated that the extracellular release of peptidyl methotrexate from the conjugate was associated with the presence of active MMPs. Given the low activity of MMPs in blood circulation (because of their inhibition by serum proteins),65 coupled with the absence of serum protein inhibitors in the interstitial fluid of tumor tissues, MMPs are useful for such targeted delivery. These conjugates remain stable in the presence of fetal bovine serum even at high concentrations of MMPs, suggesting a potent system to treat tumors by targeted delivery of chemotherapeutics.66
Polymerized Drugs
In another type of well-investigated drug delivery system, illustrated in Figure 4, drugs can be covalently incorporated within the polymer backbone and released by hydrolysis of the backbone; this approach can eliminate the burst release that often occurs when drugs are simply entrapped in or conjugated to a polymer matrix. Drug concentrations, degradation rates, erosion rates, and mechanical properties can be controlled by tuning the composition of linkers and drug derivatives in the polymer systems, which should enable broader application of drug-based polymeric materials than free drugs.
Figure 4.

Release of small-molecule drugs from polymerized drugs via backbone hydrolysis.
Polyanhydrides have been widely used as biodegradable materials for drug controlled release, owing to the surface erosion caused by the hydrophobicity of the backbones together with the hydrolysis of the anhydride bonds.67 In contrast, polyesters undergo a slower hydrolysis and bulk erosion. Therefore, a series of salicylate-based poly(anhydride–esters) have been successfully synthesized by Uhrich and co-workers to incorporate and control the release of salicylate derivatives, which are common nonsteroidal anti-inflammatory drugs (NSAIDs) used in the treatment of diverse diseases.68–70
The degradation rates and the erosion rates are highly affected by the nature of the linker in the poly(anhydride–ester) systems, which is demonstrated by the degradation and erosion profiles of polymers with aliphatic linkers of different lengths. The poly(anhydride–ester) polymers linked by adipic acid uptake more water, show greater mass loss, and degrade and erode more rapidly compared with those linked by longer aliphatic chains, such as suberic acid and sebacic acid.71 Differences in the composition of the copolymer systems can also affect degradation and erosion as well as mechanical properties. In the comparison of three copolymers with different ratios of two monomers, carboxyphenoxydecanoate (CPD) and p-carboxyphenoxyhexane (pCPH), copolymers with a 50:50 ratio of the two monomers show slower degradation rates and improved ability to retain their mechanical properties than copolymers with 30:70 and 40:60 ratios.72 In addition, the incorporation of aromatic linkers slows polymer degradation and raises the glass transition and thermal decomposition temperatures compared to those of polymers equipped with aliphatic linkers; these variations in polymer properties enable polymer processing.69
The incorporation of different salicylate derivatives has also been employed to vary the degradation and mechanical properties of the polymeric drugs. Comparisons of poly(anhydride–esters) from salicylic acid derivatives show that those containing halogenated salicylate derivatives yield higher molecular weights and glass transition temperatures.69 Furthermore, iodinated salicylate-based poly(anhydride–esters) were found to be highly X-ray opaque, and with higher molecular weight, Young’s modulus and X-ray opacity when polymerized via melt-condensation methods compared with solution polymerization.68 Other than salicylates, various antiseptic drugs have also been incorporated into poly(anhydride–esters) and showed a controlled release over a 12 week period, with slow degradation rates as a result of polymer hydrophobicity.73 In the drug release profile of one system, the initial burst release is efficiently reduced in the first 1–3 days, and sustained release can be obtained afterward due to the controlled rate of hydrolytic cleavage of the anhydride bonds.70 The cytotoxicity and cell proliferation effects of these poly(anhydride–esters) vary with polymer structure; the released drugs containing two aromatic rings showed better cell proliferation results than those released drugs containing only one aromatic ring.70,73 Therefore, both the drugs and linkers incorporated in the polymer backbones are important for tailoring the polymers for various therapeutic targets.
Polymer Therapeutics
While the majority of work in the therapeutic use of polymeric materials focuses on the role of the polymer as a carrier, emerging studies are focusing on the use of polymers as therapeutic molecules in their own right. The surge in these types of investigations has been fueled by continued improvements in living polymer synthetic methods that permit control of polymer architecture, thus making control of biological responses via control of polymer architecture an attainable goal. Given that many extracellular biological processes of therapeutic interest–pathogen recognition, cell–cell communication, inflammation–involve multivalent binding, polymers are well-suited for their control owing to the possibility of presenting multiple ligands over relevant length scales (up to tens of nanometers). The modulation of these responses depends on receptor aggregation over large length scales and the binding of multiple receptor sites and/or subsites;11 furthermore, the nature and intensity of a given biological response can be tuned by tuning aggregate number and binding pattern.11,74 Therefore, well-defined polymers conjugated with multiple pendant ligands may be uniquely suited as therapeutics, given the range of length scales that they may access, coupled with the ability to tailor their architectures via the combination of living polymerization methods and chemical conjugation strategies. Although the clinical application of polymeric therapeutics is still in its infancy, these macromolecular systems have maintained significant research interest over the past decade or more as a result of their great potential in medicine and nanotechnology and as probes in examining mechanisms of cellular responses.4,11,75
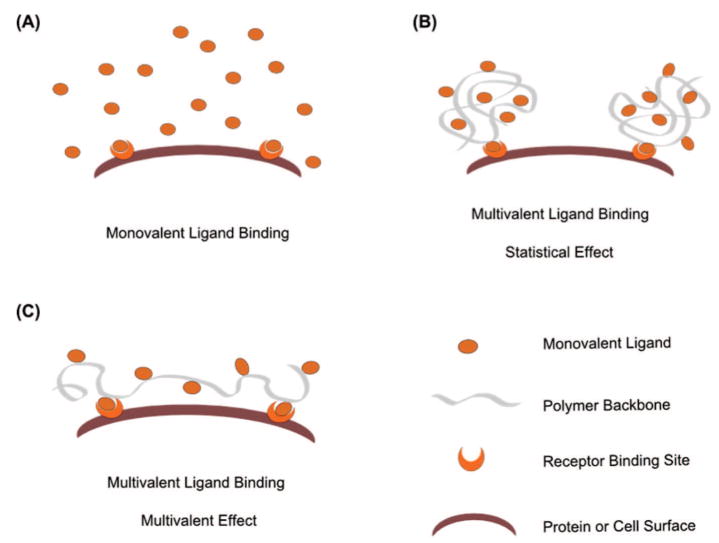
Multivalent ligands that are presented on polymeric backbones generally yield greater binding avidity and specificity than their monovalent counterparts, for multiple reasons. The increased local concentration of ligands reduces the apparent off-rate in the binding event (statistical effects, Figure 5B). The larger size of polymeric multivalent ligands increases the length scales over which ligands can span (to allow multivalent effects, Figure 5C)76 as well as decreases the rate of clearance from the body. It may also be possible to exploit the synergistic effects of presenting multiple types of ligands on the polymer backbone. A wide variety of multivalent polymeric systems have been developed aiming at inhibition of various pathogens; however, only a few of them have shown remarkable improvements in activities over the monovalent ligands, likely because of the heterogeneity of their structures. The effects on binding of valency, chain length, and molecular weight of various multivalent macromolecules have been investigated in detail, suggesting general design principles for improving the binding event.74 Multivalent ligands with high valency and high but optimized chain length have generally afforded improvements in binding avidity, with improvements also observed with changes in ligand density.
Figure 5.
Schematics of interaction between ligands and receptors: (A) interaction between monovalent ligands and receptor; (B) interaction between multivalent ligands and receptor through statistical effects; (C) interaction between multivalent ligands and receptor mainly through multivalent effects.
In polymeric systems of very well-controlled architecture, it may be possible to reduce entropic costs in the binding of multivalent ligands if the polymer structure is well-matched to the targeted receptors (Figure 6A). Given that the rotational and translational entropy costs of binding are roughly the same for interactions between receptors and either monovalent or multivalent ligands, the entropic effects on the free energy of multivalent binding are dominated mainly by contributions from conformational entropy. When the conformational entropy cost of the multivalent interaction is less than the total cost of translational and rotational entropy losses, the total entropic cost of multivalent interactions is less than that of monovalent interactions, resulting in dramatic enhancement in binding avidity.77,78 Matching the architecture of multivalent ligands to the structures of the targets should therefore yield a lower conformational entropy cost and favorable binding and has therefore been one of the principles for multivalent ligand design. A few notable examples employing this design strategy in small-molecule or assembled systems have yielded excellent results,79–82 and the potential for polymeric systems continues to improve.83–85 The possible unfavorable enthalpic contributions to the binding event, however, cannot be neglected in such approaches, and thus the chemical details of the ligand and interactions with the polymer chain must be considered.
Figure 6.
Schematics of polymer therapeutics: (A) multivalent binding between multivalent ligands and protein receptors; (B) multivalent binding between multivalent ligands and cell surface receptors.
In addition to improving the free energy of binding via control of multivalent ligand architecture, other advantages in the manipulation of receptor organization may be possible (Figure 6B). In events such as the inflammatory response, cell adhesion, or signal transduction, modulation of the biological response relies on the clustering of receptors on the cell surface rather than on individual binding events. Therefore, multivalent binding of cell–surface receptors by polymeric ligands offers opportunities to manipulate the localization of intracellular catalytic domains and to control the cascade of intracellular signaling pathways. Thus, the mechanism and the specific biological effects of polymer therapeutics will be influenced by the architecture of the scaffold on which ligands are displayed; a variety of multivalent ligands with predesigned and well-controlled architecture have been studied.11
Multivalent Ligands as Activators
Cells respond to their environment by cell signaling mediated by the formation of multiple surface–receptor complexes; therefore, distinct cell signaling pathways may be triggered through the binding of multivalent ligands of prescribed architecture (Figure 6B). Thus, varying the architecture of a multivalent ligand may alter the consequent cell response, and multivalent ligands with predesigned and well-controlled architecture may therefore serve as probes for elucidating cell signaling cascades.11 Living polymerization methods have therefore been widely applied in the production of polymeric conjugates with narrow polydispersity index, controlled molecular weight, chain length, and ligand densities. Methods including ring-opening metathesis polymerization (ROMP), atom transfer radical polymerization (ATRP), and reversible addition–fragmentation chain transfer (RAFT) have all been employed in these types of investigations.11,74
The multivalent binding between L-selectin on leukocyte surfaces and its sulfated saccharide-containing ligands on endothelial cell surfaces plays a key role in leukocyte rolling. L-Selectin shedding, in which L-selectin is proteolytically cleaved by sheddase to release the soluble extracellular fragment, can inhibit leukocyte rolling and regulate recruitment of leukocytes and may therefore be a target for the treatment of autoimmune diseases. In some early investigations of this kind by Kiessling and co-workers, polymers equipped with sulfated oligosaccharides were synthesized via ROMP methods and were found to trigger L-selectin shedding through multivalent binding and to downregulate L-selectin expression. Different degrees of L-selectin shedding were induced by these glycopolymers based on differences in the identity of the ligands. Among them, a 3′,6-disulfo Lex-containing glycopolymer showed the best inhibition of L-selectin-mediated leukocyte rolling and the highest degree of L-selectin shedding and downregulation compared with glycopolymers containing other types of sulfated saccharides.86
Because of immunological tolerance toward carbohydrates, it has been difficult to develop antiglycan antibodies by only glycan inoculation; therefore, multivalent displays of glycans on polymers have attracted increasing interest for diagnostic and therapeutic purposes. Various types of multivalent scaffolds have been used to display carbohydrates, including proteins, dendrimers, polymers, liposomes, and viruses; however, the lack of a well-defined architecture of some of these scaffolds has diminished a specific immune response. For example, seven keyhole limpet hemocyanin (KLH) protein conjugates displaying different carbohydrates were shown to elicit antibodies to all of the glycan immunogens because of the ill-defined structure of carrier protein KLH.87 Cowpea mosaic virus (CPMV), modified with glycan antigens via Cu(I)-catalyzed azide–alkyne cycloaddition protocols, has been used to display glycan antigens due to its well-controlled structure. The CPMV conjugates have been found to elicit a similarly potent antibody response as KLH conjugates after inoculation of chickens, but the CPMV conjugates elicit polyclonal antibodies with comparable specificity to a monoclonal antibody, as detected by glycan microarray methods, due to the controlled structure of the virus display platform. This system has been modified for broader applications. A fluorescence dye has been coupled on the virus scaffold for detection, and a well-defined glycopolymer polymerized via ATRP, rather than glycans, has been conjugated through end functionalization, which demonstrated an efficient strategy for coupling end-functionalized glycopolymers to protein scaffolds.88,89
Another defined multivalent polymeric system has been applied to the study of B cell signaling, which involves the clustering of B cell antigen receptors (BCR) to promote their localization to membrane microdomains and to augment intracellular Ca2+ concentration. The binding of antigens to BCR can lead to differential antibody production and immune response in mice that depends on the architecture of multivalent antigens. Synthetic multivalent antigens generated by ROMP were found to initiate immune response in vivo through the binding to BCR, suggesting the opportunitiy to use defined multivalent ligands to study the binding between antigen and BCR. Comparison between antigens of different valencies indicated that antigens with higher valency induce a higher degree of BCR clustering, greater intercellular Ca2+ concentration, and greater antibody production than those with lower valencies, while not affecting BCR internalization significantly. Such polymers with well-defined architectures may therefore be useful in applications in the treatment of autoimmune diseases, adjuvant therapy, and vaccine development.85
Multivalent Ligands as Inhibitors
Synthetic multivalent inhibitors can also be designed to mimic naturally occurring ligands and to therefore inhibit binding to cells or protein targets (illustrated in Figure 6A). Well-defined architectures are critical for structure–function studies aimed at elucidating the effects on binding of various factors, such as ligand types, linkers, chain length, molecular weight, chain conformation, and placement.
A series of amino acid copolymers have been designed for the treatment of myelin basic protein (MBP) 85-99-induced experimental autoimmune encephalomyelitis (EAE), which is a model of the autoimmune disease multiple sclerosis.90 The MBP 85-99 protein, which has been presumed to be an autoantigen, can form complexes with human HLA-DR2 protein, and the binding can sensitize T cells to cause an immune response. Copaxone (poly(Y,E,A,K)n) has been widely used for MS treatment and is thought to act through the competitive binding of copolymer/HLA-DR2 with MBP/HLA-DR2. Two other copolymers (poly(F,Y,A,K)n and poly(V,W,A,K)n) have been designed for improving the binding between copolymers and HLA-DR2; replacement of Y/E with F, V, and W is expected to provide improved fit in the binding pocket of the HLA-DR2 protein. Indeed, higher inhibition of MBP 85-99/HLA-DR2 binding by FYAK and VWAK copolymers than YEAK is obtained in in vitro inhibition assay, and better amelioration of MS symptoms is observed in in vivo experiments, consistent with the original sequence design.90
Understanding the protein–protein and protein–carbohydrate multivalent interactions in sperm–egg recognition is of fundamental importance of fertilization. The three amino acid binding sequence of fertilin β (ECD), a protein located in the equatorial region of the sperm head and involved in sperm binding to the egg plasma membrane during fertilization, has been incorporated into a series of polymeric multivalent inhibitors for the study of fertilization inhibition. Multivalent oligopeptide displays with various chain length and valency, produced via ROMP methods, displayed differential inhibition of fertilization, with the maximal inhibition obtained for a bivalent ligand with a spanning distance of 4–5 nm between ECD pharmacophores.84 The results indicate that optimal polymer structure in terms of valency and spacing are important for improved inhibition and therefore should be considered in inhibitor design.
Multivalent interactions between antigens and cell surface receptors are important for antigen or bacterial attack of host cells; accordingly, many multivalent ligands have been synthesized and utilized as antigen inhibitors. Ligand density, one of the variable architectures for multivalent ligands, has been presumed as important as demonstrated the work of Kane and co-workers. The peptide HTSTYWWLDGAP, which binds to the protective antigen heptamer ([PA63]7) of anthrax lethal toxin (LeTx) and inhibits toxin assembly, has been incorporated on side chains of an activated polymer chain poly(N-acryloyloxysuccinimide) at various densities and at controlled molecular weights. The inhibition of LeTx by these polymers showed that the inhibition increases with increasing ligand densities and then reaches a plateau.91
Multiple types of ligands can also be incorporated into polymers for binding to protein targets. Haddleton and coworkers synthesized glycopolymers via a combination of living radical polymerization and Cu(I)-catalyzed azide–alkyne cycloaddition chemistry. The “co-clicking” reaction between “clickable” polymer scaffolds with alkyne side chains, and the appropriate mixtures of mannose- and galactose-based azides yielded multivalent ligands with controlled ratios of the two saccharide ligands on poly(methacrylate) scaffolds. The binding of mannose binding lectin Con A was tested by turbidimetric assay; the fully mannose-functionalized polymer clusters Con A and the turbidity reached a plateau more quickly than the other polymers containing fewer mannose and more galactose side chains. On the other hand, mannose-functionalized polymers were not retained on an RCA-I immobilized column, where galactose-functionalized polymers bind the column strongly and only elute with galactose in the mobile phase. This co-clicking strategy was demonstrated to be an efficient way to conjugate different ligands on polymeric scaffolds for selective binding or inhibition to different lectins.92
Similar studies on multivalent binding between lectins and polymers with two different ligands incorporated have been conducted by Cloninger and co-workers.93 PAMAM dendrimers equipped with mannose and glucose with controlled ratios and densities yield different relative hemagglutination activities of Con A correlating to the composition of mannose (which shows 4 times stronger binding to Con A than glucose). As in the studies above, these studies suggest that multivalent binding avidity is influenced by the composition of monovalent ligands and further illustrates that the avidity can be easily turned or predicted by tuning the density and ratio of the two monovalent ligands.93 In earlier work of Bovin and co-workers, two ligands–siaLea and tyrosine sulfate (sTyr)–were incorporated onto polyacrylamide-based polymer backbones;94 both ligands bind to P-selectin, but at different binding sites. The synergistic effects of their coincorporation onto a polymer scaffold were suggested by the improved inhibition of P-selectin by the bisubstituted polymers (siaLea-PAA-sTyr, IC50 value of 4 μM) over that of either of two monosubstituted ligands (siaLea-PAA or sTyr-PAA, with IC50 values of 30 μM). These results together indicate a promising strategy for improving multivalent binding through synergistic effects by the incorporation of a combination of multiple kinds of ligands. In all of these studies, however, the incorporation of multiple ligands was random in nature; thus, additional improvements in avidity may be expected by optimal presentation of multiple types of ligands on controlled scaffolds.
Protein Engineering Methods
Despite the progress in the production of well-defined polymers that elicit specific responses during a multivalent binding event, the inherent heterogeneities in these macromolecules, coupled with their ill-controlled backbone conformation, have limited elucidation and manipulation of structure–function relationships; therefore, the development of additional strategies for producing well-defined macromolecules would offer important opportunities in the study and manipulation of these important binding events. Protein engineering methods, in concert with chemical coupling, provide polypeptides with monodispersity, complete sequence control, good control over conformation, and the opportunity to engineer multiple noncovalent interactions between a multivalent ligand and its receptor. The incorporation into the polypeptide of amino acids with different propensities for specific secondary structures permits the control of chain conformation (helical, random coil, or β-sheet). By incorporating amino acids of different charges, the net charge of the polypeptide backbones can be controlled for desired application; similarly, chain flexibility and hydrophilicity can be manipulated as well.
Chemical modification of peptides and polypeptides has been achieved via the chemical modification of the amine, carboxylic acid, and thiol side-chain groups of specific amino acids to yield multivalent ligands with various linkers and ligands. A Mannich-type ligation reaction between aniline-functionalized peptides and tyrosine residues has been developed recently and can be used for peptide modification.95,96 Other desirable functional groups, such as azide groups, can be incorporated into polypep-tide-based polymers via the incorporation of non-natural amino acids;97–100 therefore, various chemical approaches can be applied for further modification of recombinant polypeptides. Multivalent ligands synthesized by protein engineering methods may therefore be designed to better match the structures of protein targets. In binding to cell surface receptors, biological response might be tuned via the control of polymer architecture, thus offering advantages for these types of polymers in therapeutic application.
A series of α-helical and random-coil galactose-functionalized glycopolypeptides with controlled saccharide spacing, valency, conformation, and chain length were obtained via the combination of protein engineering and post-translational chemical modification and were employed in studies of the inhibition of the binding of cholera toxin B pentamer (CT B5) to glycoplipid-modified surfaces. The inhibition exhibited by these multivalent glycopolypeptides showed 20–400-fold enhancements over that of monovalent galactose, likely due to in part to the statistical effects of displaying multiple ligands but also likely due to efficient multivalent binding that was possible via the control of ligand placement and number on these scaffolds (Figure 5B,C). Glycopolypeptides with saccharide ligands placed nominally 35 Å apart, a distance which matches that between two adjacent binding sites on CT B5, showed significantly enhanced inhibition over that of glycopolypeptides with shorter interligand distances, suggesting the role of multivalent binding mechanisms and underscoring the importance of the control of spacing on binding. In addition, polypeptides with 35 Å spacing but lower hydrodynamic volume showed similar or improved inhibition relative to glycopolymers of greater hydrodynamic volume, contrary to most previously reported trends. These effects further support optimization of ligand efficacy through appropriate display on a well-defined scaffold and suggest the value of employing these methods in other studies of multivalent binding.83,101,102
Evaluation of Multivalent Interactions
Characterization of the target behaviors of polymeric systems and evaluation of their inflammatory and immunological properties must be balanced during their development, in order to optimize what can be learned about polymer design principles for a given clinical application. The evaluation of binding by polymeric ligands can be achieved via multiple methods, such as affinity chromatography, capillary electrophoresis (CE), surface plasma resonance (SPR), two-dimensional NMR spectroscopy, electron energy mapping, X-ray diffraction, isothermal titration calorimetry (ITC), and differential scanning calorimetry (DSC).103–105 The association and dissociation constants of the binding event can be obtained from SPR or NMR experiments, while thermodynamic parameters can be obtained from ITC and DSC experiments.106 Advanced imaging techniques and other characterization methods, such as SAXS and SANS, may improve molecular-level characterization of polymeric therapeutics and, in concert with assays designed to test functional activity in a desired model, will provide useful information to guide the design of macromolecular therapeutics. Various in vitro bioassays have therefore also been developed for evaluating multivalent interactions, such as enzyme-linked immunosorbent assays (ELISA), immunological precipitation, hemagglutination assay, and fluorescence titration.107
To evaluate the impact of polymeric therapeutics on cell responses, a large variety of cell-based assays have been developed. For example, glycoconjugates have been synthesized for the study of monocyte and macrophage activation, and monocyte- and macrophage-based cell assays have been conducted. Upon activation, glycoconjugates can be uptaken by monocyte and macrophages; therefore, these glycoconjugates, either radio-labeled or fluorescently labeled, can be tracked to study the internalization process.108 L-Selectin shedding and regulation effects of a series of glycopolymers were observed in leukocyte rolling inhibition assay and L-selectin shedding assay followed by ELISA and Western blot.86 Ultimately, the utility of these macromolecules in therapeutic applications must be evaluated in vivo, and accordingly, animal studies will be required to evaluate functions such as inhibition or adjuvant effects.109
Many challenges remain in the development of active macromolecular structures via either chemical or biological methods. Controlled trafficking of the macromolecular drugs and their interaction with biological targets will require continued optimization. Methods to characterize and/or predict the interactions of the macromolecular drugs with their targets on the molecular level will also need to be developed; advanced imaging techniques and single molecule characterization methods certainly offer promising approaches in addressing these challenges. Systems biology approaches to the prediction of complicated cellular outcomes as a result of manipulation of specific signaling pathways will also facilitate advances in this area. With the continued convergence in these areas, the prospects are excellent for the design and synthesis of polymers of specified structure, responsiveness, and biological activity for the treatment of human disease.
Biographies
 Shuang Liu is currently a Ph.D. candidate in Materials Science and Engineering at the University of Delaware. She earned her B.E. degree in Polymer Science and Engineering and an M.S. degree in Polymer Chemistry and Physics from the Zhejiang University, China, where she was awarded the Zhejiang University Excellent Student Award each year of her bachelor’s studies. She began a doctoral program in Materials Science and Engineering at the University of Delaware in 2004 under the instruction of Prof. Kristi L. Kiick. Her Ph.D. program is about the study of multivalent interactions between glycopolypeptide-based multivalent ligands and protein targets or cell surface receptors, targeted for elucidation of structure–function relationships and for future therapeutic applications. In addition to presentation of her research results in multiple publications and at national meetings, her research and teaching efforts have been recognized by the awarding of a University of Delaware Graduate Fellowship, the UD MSEG Chairperson’s Award, and a UD MSEG Graduate Student Teaching Award. She also has served as vice president of the UD student chapter of the Materials Research Society.
Shuang Liu is currently a Ph.D. candidate in Materials Science and Engineering at the University of Delaware. She earned her B.E. degree in Polymer Science and Engineering and an M.S. degree in Polymer Chemistry and Physics from the Zhejiang University, China, where she was awarded the Zhejiang University Excellent Student Award each year of her bachelor’s studies. She began a doctoral program in Materials Science and Engineering at the University of Delaware in 2004 under the instruction of Prof. Kristi L. Kiick. Her Ph.D. program is about the study of multivalent interactions between glycopolypeptide-based multivalent ligands and protein targets or cell surface receptors, targeted for elucidation of structure–function relationships and for future therapeutic applications. In addition to presentation of her research results in multiple publications and at national meetings, her research and teaching efforts have been recognized by the awarding of a University of Delaware Graduate Fellowship, the UD MSEG Chairperson’s Award, and a UD MSEG Graduate Student Teaching Award. She also has served as vice president of the UD student chapter of the Materials Research Society.
 Ronak Maheshwari is currently a final year Ph.D student in the laboratories of Dr. Kristi L. Kiick in the Department of Materials Science and Engineering at the University of Delaware. He earned his dual B.S and M.S. degrees in Materials Science and Metallurgical Engineering from the Indian Institute of Technology–Bombay. His Masters research was conducted under the guidance of Dr. T. R. Rama Mohan and Dr. B. T. Rao on the synthesis of Ti/AW bio-glass composites for biomedical applications, chosen for presentation at the 2003 International Conference on Biomedical Materials held in Cardiff, U.K. Ronak began his doctoral program in Materials Science and Engineering at University of Delaware in 2003. His doctoral research under the guidance of Dr. Kiick focuses on controlling multiple aspects of multivalent interactions in the inhibition of toxins via the use of well-defined glycopolypeptides synthesized to contain both natural and non-natural amino acids. This approach permits control of ligand presentation as well as additional secondary interactions with multivalent targets and will be useful for developing new multivalent platforms in biology.
Ronak Maheshwari is currently a final year Ph.D student in the laboratories of Dr. Kristi L. Kiick in the Department of Materials Science and Engineering at the University of Delaware. He earned his dual B.S and M.S. degrees in Materials Science and Metallurgical Engineering from the Indian Institute of Technology–Bombay. His Masters research was conducted under the guidance of Dr. T. R. Rama Mohan and Dr. B. T. Rao on the synthesis of Ti/AW bio-glass composites for biomedical applications, chosen for presentation at the 2003 International Conference on Biomedical Materials held in Cardiff, U.K. Ronak began his doctoral program in Materials Science and Engineering at University of Delaware in 2003. His doctoral research under the guidance of Dr. Kiick focuses on controlling multiple aspects of multivalent interactions in the inhibition of toxins via the use of well-defined glycopolypeptides synthesized to contain both natural and non-natural amino acids. This approach permits control of ligand presentation as well as additional secondary interactions with multivalent targets and will be useful for developing new multivalent platforms in biology.
 Kristi L. Kiick is an Associate Professor of Materials Science and Engineering at the University of Delaware. She earned her B.S. degree in Chemistry from the University of Delaware and an M.S. degree in Bioinorganic Chemistry from the University of Georgia as a National Science Foundation Graduate Fellow. Kiick began a doctoral program in Polymer Science and Engineering at the University of Massachusetts Amherst in 1996, funded by a National Defense Science and Engineering Graduate Fellowship. Her doctoral research, conducted at the California Institute of Technology under the direction of David A. Tirrell, pioneered new strategies for incorporating non-natural amino acids into protein-based materials. Dr. Kiick joined the UD faculty in the Department of Materials Science and Engineering as an Assistant Professor in August 2001 and was promoted to the rank of Associate Professor in 2007. Her research programs have been funded in part by a Camille and Henry Dreyfus Foundation New Faculty Award, a Beckman Young Investigator Award, an NSF CAREER Award, and a DuPont Young Professor Award, and she has served as a project leader on an NIH Center of Biomedical Research Excellence awarded to the University of Delaware in 2002 and renewed in 2008. Her current research programs combine biosynthetic techniques, chemical methods, and bioinspired assembly strategies for producing novel materials with long-term applications in medicine and/or device technologies.
Kristi L. Kiick is an Associate Professor of Materials Science and Engineering at the University of Delaware. She earned her B.S. degree in Chemistry from the University of Delaware and an M.S. degree in Bioinorganic Chemistry from the University of Georgia as a National Science Foundation Graduate Fellow. Kiick began a doctoral program in Polymer Science and Engineering at the University of Massachusetts Amherst in 1996, funded by a National Defense Science and Engineering Graduate Fellowship. Her doctoral research, conducted at the California Institute of Technology under the direction of David A. Tirrell, pioneered new strategies for incorporating non-natural amino acids into protein-based materials. Dr. Kiick joined the UD faculty in the Department of Materials Science and Engineering as an Assistant Professor in August 2001 and was promoted to the rank of Associate Professor in 2007. Her research programs have been funded in part by a Camille and Henry Dreyfus Foundation New Faculty Award, a Beckman Young Investigator Award, an NSF CAREER Award, and a DuPont Young Professor Award, and she has served as a project leader on an NIH Center of Biomedical Research Excellence awarded to the University of Delaware in 2002 and renewed in 2008. Her current research programs combine biosynthetic techniques, chemical methods, and bioinspired assembly strategies for producing novel materials with long-term applications in medicine and/or device technologies.
References and Notes
- 1.Fischbach C, Mooney DJ. Biomaterials. 2007;28(12):2069. doi: 10.1016/j.biomaterials.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Tirrell DA. Nature (London) 2004;428(6982):487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Lutolf MP, Hubbell JA. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 4.Kiick KL. Science. 2007;317(5842):1182–1183. doi: 10.1126/science.1145951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan R. Nat Rev Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 6.Watson KJ, Anderson DR, Nguyen ST. Macromolecules. 2001;34(11):3507–3509. [Google Scholar]
- 7.Gillies ER, Goodwin AP, Frechet JMJ. Bioconjugate Chem. 2004;15(6):1254–1263. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]
- 8.Kim YS, Gil ES, Lowe TL. Macromolecules. 2006;39(23):7805–7811. [Google Scholar]
- 9.York AW, Kirkland SE, McCormick CL. Adv Drug Delivery Rev. 2008;60(9):1018–1036. doi: 10.1016/j.addr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Dhal PK, Holmes-Farley SR, Huval CC, Jozefiak TH. Adv Polym Sci. 2006;192:9. [Google Scholar]
- 11.Kiessling LL, Gestwicki JE, Strong LE. Angew Chem, Int Ed. 2006;45(15):2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichterle O, Lim D. Nature (London) 1960;185(4706):117. [Google Scholar]
- 13.Lim F, Sun AM. Science. 1980;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 14.Byrne ME, Park K, Peppas NA. Adv Drug Delivery Rev. 2002;54(1):149. doi: 10.1016/s0169-409x(01)00246-0. [DOI] [PubMed] [Google Scholar]
- 15.Keys KB, Andreopoulos FM, Peppas NA. Macromolecules. 1998;31(23):8149–8156. [Google Scholar]
- 16.Torchilin VP. Adv Drug Delivery Rev. 2006;58(14):1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Park H, Park K. Proc Int Symp Control Rel Bioact Mater. 1998;25:60–61. [Google Scholar]
- 18.Oh JK, Siegwart DJ, Lee H-i, Sherwood G, Peteanu L, Hollinger JO, Kataoka K, Matyjaszewski K. J Am Chem Soc. 2007;129(18):5939–5945. doi: 10.1021/ja069150l. [DOI] [PubMed] [Google Scholar]
- 19.Gindy ME, Panagiotopoulos AZ, Prud’homme RK. Langmuir. 2008;24(1):83–90. doi: 10.1021/la702902b. [DOI] [PubMed] [Google Scholar]
- 20.Hubbell JA. Science. 2003;300(5619):595–596. doi: 10.1126/science.1083625. [DOI] [PubMed] [Google Scholar]
- 21.Torchilin VP. Nat Rev Drug Discovery. 2005;4(2):145. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 22.Torchilin VP. J Controlled Release. 2001;73(2–3):137. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 23.Accardo A, Tesauro D, Aloj L, Tarallo L, Arra C, Mangiapia G, Vaccaro M, Pedone C, Paduano L, Morelli G. ChemMedChem. 2008;3(4):594–602. doi: 10.1002/cmdc.200700269. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Proc Natl Acad Sci USA. 2008;105(18):6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo D, Saltzman WM. Nat Biotechnol. 2000;18(1):33. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 26.Ahn CH, Chae SY, Bae YH, Kim SW. J Controlled Release. 2004;97(3):567. doi: 10.1016/j.jconrel.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Wood KC, Azarin SM, Arap W, Pasqualini R, Langer R, Hammond PT. Bioconjugate Chem. 2008;19(2):403–405. doi: 10.1021/bc700408r. [DOI] [PubMed] [Google Scholar]
- 28.Wood KC, Steven RL, Langer R, Hammond PT. Angew Chem, Int Ed. 2005;44(41):6704–6708. doi: 10.1002/anie.200502152. [DOI] [PubMed] [Google Scholar]
- 29.Pack DW, Hoffman AS, Pun S, Stayton PS. Nat Rev Drug Discovery. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 30.Varde NK, Pack DW. J Controlled Release. 2007;124(3):172–180. doi: 10.1016/j.jconrel.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasachari S, Liu Y, Prevette LE, Reineke TM. Biomaterials. 2007;28(18):2885. doi: 10.1016/j.biomaterials.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasachari S, Liu Y, Zhang G, Prevette L, Reineke TM. J Am Chem Soc. 2006;128(25):8176–8184. doi: 10.1021/ja0585580. [DOI] [PubMed] [Google Scholar]
- 33.Verbaan FJ, Oussoren C, van Dam IM, Takakura Y, Hashida M, Crommelin DJ, Hennink WE, Storm G. Int J Pharm. 2001;214(1–2):99–101. doi: 10.1016/s0378-5173(00)00642-6. [DOI] [PubMed] [Google Scholar]
- 34.Fichter KM, Zhang L, Kiick KL, Reineke TM. Bioconjugate Chem. 2008;19(1):76–88. doi: 10.1021/bc0701141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan R. Nat Rev Drug Discovery. 2003;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 36.Rosenbaum D, Holmes-Farley S, Mandeville W, Pitruzzello M, Goldberg D. Nephrol Dial Transplant. 1997;12(5):961–964. doi: 10.1093/ndt/12.5.961. [DOI] [PubMed] [Google Scholar]
- 37.Ringsdorf H. J Polym Sci Polym Symp. 1975;51:135–153. [Google Scholar]
- 38.Duncan R, Kopecek J. Soluble Synthetic Polymers As Potential Drug Carriers. 1984;57:51–101. [Google Scholar]
- 39.Duncan R. Anti-Cancer Drugs. 1992;3:175–210. doi: 10.1097/00001813-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Greenwald RB. J Controlled Release. 2001;74(1–3):159–171. doi: 10.1016/s0168-3659(01)00331-5. [DOI] [PubMed] [Google Scholar]
- 41.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. J Biol Chem. 1977;252(11):3582–3586. [PubMed] [Google Scholar]
- 42.Hoffman AS. J Controlled Release. 1987;6(1):297. [Google Scholar]
- 43.Monji N, Cole CA, Hoffman AS. J Biomater Sci, Polym Ed. 1994;5:407. doi: 10.1163/156856294x00112. [DOI] [PubMed] [Google Scholar]
- 44.Duncan R. Nat Rev Drug Discovery. 2003;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 45.Tugulu S, Silacci P, Stergiopulos N, Klok HA. Biomaterials. 2007;28(16):2536–2546. doi: 10.1016/j.biomaterials.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Bull SR, Guler MO, Bras RE, Venkatasubramanian PN, Stupp SI, Meade TJ. Bioconjugate Chem. 2005;16(6):1343–1348. doi: 10.1021/bc050153h. [DOI] [PubMed] [Google Scholar]
- 47.Löwik DWPM, Ayres L, Smeenk JM, Van Hest JCM. Synthesis of Bio-Inspired Hybrid Polymers Using Peptide Synthesis and Protein Engineering. Vol. 202 Springer; Berlin: 2006. [Google Scholar]
- 48.Kovacs EW, Hooker JM, Romanini DW, Holder PG, Berry KE, Francis MB. Bioconjugate Chem. 2007;18(4):1140–1147. doi: 10.1021/bc070006e. [DOI] [PubMed] [Google Scholar]
- 49.Chilkoti A, Christensen T, MacKay JA. Curr Opin Chem Biol. 2006;10(6):652–657. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright ER, Conticello VP. Adv Drug Delivery Rev. 2002;54(8):1057–1073. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 51.Herrero-Vanrell R, Rincon AC, Alonso M, Reboto V, Molina-Martinez IT, Rodriguez-Cabello JC. J Controlled Release. 2005;102(1):113–122. doi: 10.1016/j.jconrel.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Shamji MF, Chen J, Friedman AH, Richardson WJ, Chilkoti A, Setton LA. J Controlled Release. 2008;129(3):179–186. doi: 10.1016/j.jconrel.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. J Controlled Release. 2006;115(2):175. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 54.Shen WC, Ryser HJP. Biochem Biophys Res Commun. 1981;102(3):1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- 55.Ulbrich K, Subr V. Adv Drug Delivery Rev. 2004;56(7):1023–1050. doi: 10.1016/j.addr.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 56.Etrych T, Chytil P, Jelínková M, Ríhová B, Ulbrich K. Macromol Biosci. 2002;2(1):43–52. [Google Scholar]
- 57.Kratz F, Beyer U, Thomas SM. Crit Rev Ther Drug Carrier Syst. 1999;16(3):245–288. doi: 10.1615/critrevtherdrugcarriersyst.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- 58.Duncan R. Biochem Soc Trans. 2007;035(1):56–60. doi: 10.1042/BST0350056. [DOI] [PubMed] [Google Scholar]
- 59.Vicent MJ, Duncan R. Trends Biotechnol. 2006;24(1):39–47. doi: 10.1016/j.tibtech.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Vicent MJ, Greco F, Nicholson RI, Paul A, Griffiths PC, Duncan R. Angew Chem, Int Ed. 2005;44(26):4061–4066. doi: 10.1002/anie.200462960. [DOI] [PubMed] [Google Scholar]
- 61.Kopecek J, Rejmanova P, Strohalm J, Ulbrich K, Rihova B, Chytry V, Lloyd JB, Duncan R. Synth Polym Drugs. 1991:000. [Google Scholar]
- 62.Ulbrich K, Etrych T, Chytil P, Jelínková M, Ríhová B. J Controlled Release. 2003;87(1–3):33–47. doi: 10.1016/s0168-3659(02)00348-6. [DOI] [PubMed] [Google Scholar]
- 63.Duncan R. Nat Rev Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 64.Duncan R, Cable HC, Lloyd JB, Rejmanová P, Kopecek J. Makromol Chem. 1983;184(10):1997–2008. [Google Scholar]
- 65.Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. Oxford University Press; New York: 2000. [Google Scholar]
- 66.Chau Y, Tan FE, Langer R. Bioconjugate Chem. 2004;15(4):931–941. doi: 10.1021/bc0499174. [DOI] [PubMed] [Google Scholar]
- 67.Gopferich A, Tessmar J. Adv Drug Delivery Rev. 2002;54(7):911–931. doi: 10.1016/s0169-409x(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 68.Carbone AL, Song M, Uhrich KE. Biomacromolecules. 2008;9(6):1604–1612. doi: 10.1021/bm8000759. [DOI] [PubMed] [Google Scholar]
- 69.Prudencio A, Schmeltzer RC, Uhrich KE. Macromolecules. 2005;38(16):6895–6901. doi: 10.1021/ma048051u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmeltzer RC, Schmalenberg KE, Uhrich KE. Biomacromolecules. 2005;6(1):359–367. doi: 10.1021/bm049544+. [DOI] [PubMed] [Google Scholar]
- 71.Whitaker-Brothers K, Uhrich K. J Biomed Mater Res, Part A. 2006;76A(3):470–479. doi: 10.1002/jbm.a.30356. [DOI] [PubMed] [Google Scholar]
- 72.Whitaker-Brothers K, Uhrich K. J Biomed Mater Res, Part A. 2004;70A(2):309–318. doi: 10.1002/jbm.a.30083. [DOI] [PubMed] [Google Scholar]
- 73.Schmeltzer RC, Uhrich KE. Polym Bull. 2006;57(3):281–291. doi: 10.1007/s00289-006-0561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee Y, Sampson NS. Curr Opin Struct Biol. 2006;16(4):544–550. doi: 10.1016/j.sbi.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 75.Farokhzad OC, Langer R. Adv Drug Delivery Rev. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Wolfenden ML, Cloninger MJ. Bioconjugate Chem. 2006;17(4):958–966. doi: 10.1021/bc060107x. [DOI] [PubMed] [Google Scholar]
- 77.Mammen M, Choi SK, Whitesides GM. Angew Chem, Int Ed. 1998;37(20):2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 78.Ambrosi M, Cameron NR, Davis BG. Org Biomol Chem. 2005;3(9):1593–1608. doi: 10.1039/b414350g. [DOI] [PubMed] [Google Scholar]
- 79.Fan E, Zhang Z, Minke WE, Hou Z, Verlinde CLMJ, Hol WGJ. J Am Chem Soc. 2000;122(11):2663–2664. [Google Scholar]
- 80.Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, Read RJ, Bundle DR. Nature (London) 2000;403(6770):669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 81.McGavin RS, Gagne RA, Chervenak MC, Bundle DR. Org Biomol Chem. 2005;3(15):2723–2732. doi: 10.1039/b416105j. [DOI] [PubMed] [Google Scholar]
- 82.Rai PR, Saraph A, Ashton R, Poon V, Mogridge J, Kane RS. Angew Chem, Int Ed. 2007;46(13):2207–2209. doi: 10.1002/anie.200604317. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Kiick KL. J Am Chem Soc. 2005;127(47):16392–16393. doi: 10.1021/ja055102+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baessler KA, Lee Y, Roberts KS, Facompre N, Sampson NS. Chem Biol. 2006;13(3):251–259. doi: 10.1016/j.chembiol.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. ACS Chem Biol. 2007;2(4):252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 86.Mowery P, Yang ZQ, Gordon EJ, Dwir O, Spencer AG, Alon R, Kiessling LL. Chem Biol. 2004;11(5):725–732. doi: 10.1016/j.chembiol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 87.Ragupathi G, Koide F, Sathyan N, Kagan E, Spassova M, Bornmann W, Gregor P, Reis CA, Clausen H, Danishefsky SJ, Livingston PO. Cancer Immunol Immunother. 2003;52(10):608–616. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sen Gupta S, Raja KS, Kaltgrad E, Strable E, Finn MG. Chem Commun. 2005;(34):4315–4317. doi: 10.1039/b502444g. [DOI] [PubMed] [Google Scholar]
- 89.Kaltgrad E, Sen Gupta S, Punna S, Huang C-Y, Chang A, Wong C-H, Finn MG, Blixt O. ChemBioChem. 2007;8(12):1455–62. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 90.Illes Z, Stern JNH, Reddy J, Waldner H, Mycko MP, Brosnan CF, Ellmerich S, Altmann DM, Santambrogio L, Strominger JL, Kuchroo VK. Proc Natl Acad Sci USA. 2004;101(32):11749–11754. doi: 10.1073/pnas.0403833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gujraty KV, Joshi A, Saraph A, Poon V, Mogridge J, Kane RS. Biomacromolecules. 2006;7(7):2082–2085. doi: 10.1021/bm060210p. [DOI] [PubMed] [Google Scholar]
- 92.Ladmiral V, Mantovani G, Clarkson GJ, Cauet S, Irwin JL, Haddleton DM. J Am Chem Soc. 2006;128(14):4823–4830. doi: 10.1021/ja058364k. [DOI] [PubMed] [Google Scholar]
- 93.Wolfenden ML, Cloninger MJ. J Am Chem Soc. 2005;127(35):12168–12169. doi: 10.1021/ja053008n. [DOI] [PubMed] [Google Scholar]
- 94.Pochechueva TV, Galanina OE, Bird MI, Nifantiev NE, Bovin NV. Chem Biol. 2002;9(6):757–762. doi: 10.1016/s1074-5521(02)00157-6. [DOI] [PubMed] [Google Scholar]
- 95.Carrico ZM, Romanini DW, Mehlc RA, Francis MB. Chem Commun. 2008:1205–1207. doi: 10.1039/b717826c. [DOI] [PubMed] [Google Scholar]
- 96.Romanini DW, Francis MB. Bioconjugate Chem. 2008;19(1):153–157. doi: 10.1021/bc700231v. [DOI] [PubMed] [Google Scholar]
- 97.Sharma N, Furter R, Kast P, Tirrell DA. FEBS Lett. 2000;467(1):37–40. doi: 10.1016/s0014-5793(00)01120-0. [DOI] [PubMed] [Google Scholar]
- 98.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Proc Natl Acad Sci USA. 2002;99(1):19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kwon I, Kirshenbaum K, Tirrell DA. J Am Chem Soc. 2003;125(25):7512–7513. doi: 10.1021/ja0350076. [DOI] [PubMed] [Google Scholar]
- 100.Hendrickson TL, de Crecy-Lagard V, Schimmel P. Annu Rev Biochem. 2004;73:147–176. doi: 10.1146/annurev.biochem.73.012803.092429. [DOI] [PubMed] [Google Scholar]
- 101.Liu S, Kiick KL. Macromolecules. 2008;41(3):764–772. doi: 10.1021/ma702128a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polizzotti BD, Maheshwari R, Vinkenborg J, Kiick KL. Macromolecules. 2007;40(20):7103–7110. doi: 10.1021/ma070725o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eva Åström SO. J Mol Recognit. 2006;19(4):282–286. doi: 10.1002/jmr.786. [DOI] [PubMed] [Google Scholar]
- 104.Reine Johansson LCGMOSO. J Mol Recognit. 2006;19(4):275–281. doi: 10.1002/jmr.794. [DOI] [PubMed] [Google Scholar]
- 105.Johansson R, Gunnarsson LC, Ohlin M, Ohlson S. J Mol Recognit. 2006;19(4):275–281. doi: 10.1002/jmr.794. [DOI] [PubMed] [Google Scholar]
- 106.Dam TK, Gerken TA, Cavada BS, Nascimento KS, Moura TR, Brewer CF. J Biol Chem. 2007;282(38):28256–28263. doi: 10.1074/jbc.M704677200. [DOI] [PubMed] [Google Scholar]
- 107.Pieters RJ. Med Res Rev. 2007;27(6):796–816. doi: 10.1002/med.20089. [DOI] [PubMed] [Google Scholar]
- 108.Ortega-Munoz M, Morales-Sanfrutos J, Perez-Balderas F, Hernandez-Mateo F, Giron-Gonzalez MD, Sevillano-Tripero N, Salto-Gonzalez R, Santoyo-Gonzalez F. Org Biomol Chem. 2007;5(14):2291–2301. doi: 10.1039/b706331h. [DOI] [PubMed] [Google Scholar]
- 109.Rai P, Padala C, Poon V, Saraph A, Basha S, Kate S, Tao K, Mogridge J, Kane RS. Nat Biotechnol. 2006;24(5):582–586. doi: 10.1038/nbt1204. [DOI] [PubMed] [Google Scholar]