Abstract
The molecular mechanisms that govern cell movement are the subject of intense study, as they impact biologically and medically important processes such as leukocyte chemotaxis and angiogenesis, among others. We demonstrate that leukocyte chemotaxis is prevented by the macrolide immunosupressant rapamycin, a specific inhibitor of the mammalian target of rapamycin (mTOR)/ribosomal p70S6 kinase (S6K) pathway. Both neutrophil chemotaxis and chemokinesis elicited by granulocyte-macrophage colony-stimulating factor (GM-CSF) were strongly inhibited by the immunosuppressant drug rapamycin with an IC50 of 0.3 nM. Inhibition, although at a higher dose, was also observed when the chemoattractant was IL-8. As for the mechanism, rapamycin targeted the increase of phosphorylation of p70S6K due to GM-CSF treatment, as demonstrated with specific anti-p70S6K immunoprecipitation and subsequent immunoblotting with anti-T421/S424 antibodies. Rapamycin also inhibited GM-CSF-induced actin polymerization, a hallmark of leukocyte migration. The specificity of the effect of rapamycin, was confirmed by the use of the structural analog FK506, which did not have a significant effect on chemotaxis but it effectively rescued rapamycin-induced p70S6K inhibition. This was expected from a competitive effect of both molecules on FK506-binding proteins (FKBP). Additionally, GM-CSF-induced chemotaxis was completely (>90%) blocked by a combination of rapamycin and the MAPK kinase (MEK) inhibitor PD-98059. In summary, the results presented here indicate for the first time that rapamycin, at sub-nanomolar concentrations, inhibits GM-CSF-induced chemotaxis and chemokinesis. This serves to underscore the relevance of the mTOR/S6K pathway in neutrophil migration.
INTRODUCTION
During inflammation and infection, neutrophil phagocytosis and killing of invading pathogens begins by adhesion of these cells to the capillary endothelium followed by diapedesis and the ultimate arrival into the inflamed/infected tissues. Chemotaxis and phagocytosis are enhanced by the presence of hematopoietic cytokines and chemokines. GM-CSF and IL-8 are particularly effective in enhancing neutrophil functionality. As for the molecular signaling mechanism of chemotaxis, a role for PI3K and Akt/PKB in regulating neutrophil migration has been demonstrated [1–4]. Despite this knowledge of the upstream members of the PI3K pathway, the physiological role of the downstream links (namely, mTOR and p70S6K), has not been examined directly in hematopoietic cells. The immunosuppresant rapamycin has emerged as a very useful agent to ascertain whether the mTOR-S6K pathway is involved in a particular signaling initiated by a stimulus of interest. For instance, thrombin-induced formation of stress fibers in 3T3 fibroblasts is associated with the presence of p70S6K and is inhibited by rapamycin [5].
Rapamycin binds in vitro to and inhibits the kinases TOR1/2 (in yeast) and mTOR (in mammalian cells) [6]. It also partially inhibits in situ insulin/mitogen-stimulated phosphorylation of eIF-4E and the ribosomal p70S6K [7]. mTOR phosphorylates p70S6K, which in turn phosphorylates the S6 protein in the 40S small ribosomal subunit. This is needed for protein translation and for new ribosome formation, hence the originally-described inhibitory effect of rapamycin in cell growth. To exert its action on mTOR, rapamycin binds to the immunophilin FK binding protein-12 (FKBP12), which is the soluble receptor for another immunosuppressant drug, FK506. FK506, an FKBP12 binding compound, related to rapamycin, does not inhibit mTOR [8,9]. Rapamycin prevents neutrophil Ca2+-associated activation [5] and infiltration that is associated with accelerated rejection in cardiac tissue transplants [10].
Rapamycin is currently being tested for its potential therapeutic properties [11–14] as an immunosuppressant for organ transplants, in cancer (currently in Phase II Clinical trials) as having anti-growth effects, directly on solid tumors and as having anti-angiogenic activity (affecting endothelial cells), in rheumatoid arthritis (as it affects synovial fibroblasts) and in heart disease, in ischemic hypertrophy and in restenosis to prevent re-occlusion of lesions by vascular smooth muscle cells after balloon angioplasty (currently in Phase III Clinical Trials). The therapeutic and the physiological mechanisms of inhibition in vivo are under current study. To this respect, Böhler et al. [15] have demonstrated that intake of a 2-hydroxy-ethyl derivative of rapamycin (SDZ RAD) by renal allograft recipients with stable graft function, results in a significant, albeit transient, reduction in T-cell proliferation. Co-stimulation of peripheral blood mononuclear cells from healthy volunteers with the rapamycin derivative and another immunosuppressant, cyclosporine A, revealed an additive effect of both agents on anti-CD3-driven T-cell proliferation [15].
With regards to cell migration and, specifically, to neutrophil chemotaxis, it should be noted that a chemotactic response to GM-CSF alone or in combination with chemoattractants from the host (like IL-8) or from the invading micro-organisms (like FMLP) would be beneficial to the phagocytic cell. Inasmuch as cell migration is crucial to the host (and sometimes can make the difference between life and death), there are well described instances in which side effects of inflammation are highly undesirable. Examples include: cellulitis, arthritis, gout, asthma, ischemia-reperfusion injury and the formation of the atherosclerotic plaque. Thus, it is understandable the interest in drugs that could be used to reduce inflammation injury in these conditions by limiting leukocyte migration. Here, we provide evidence for the first time that neutrophil migration is strongly inhibited by rapamycin and present data that help us understand the underlying cell signaling mechanism for this important leukocyte functionality.
MATERIALS AND METHODS
Materials and antibodies
GM-CSF and IL-8 were from R&D Systems (Minneapolis, MN); 24-well transwell membrane tissue plates (with 6.5 mm diameter, 5-μm pore inserts) were from Corning Costar (Cam bridge, MA); FMLP, PKA inhibitor, calphostin, anti-rabbit IgG (agarose beads), trypan blue, phalloidin-FITC conjugate from Amanita phalloides were from Sigma (St. Louis, MO); “FACS FLOW” buffer was from Fisher (Hanover Park, IL); rapamycin and FK506 were from Calbiochem (San Diego, CA); PD-98059 was from BioMol (Plymouth Meeting, PA); anti-p70S6K and anti-phospho(T421/S424)-p70S6K antibodies (polyclonal) used for immunoprecipitation and immunoblotting were from Cell Signaling (Beverly, MA).
Cells
Peripheral blood neutrophils were isolated based on a protocol described by English and Andersen [16]. Between 50–55 ml of blood were collected from the antecubital vein of healthy individuals (who signed an Institutional Review Board-approved consent form) using sodium citrate as anticoagulant. Blood was mixed with 15 ml of 6% dextran, allowed to settle, and the plasma and buffy coat were removed and spun down at 800×g for 5 min. The pellet was resuspended in 35 ml of saline and centrifuged again for 15 minutes at 10 °C in a Ficoll-Histopaque discontinuous gradient. Neutrophils were recovered and contaminating erythrocytes were lysed by hypotonic shock. Cells were washed and the pellet was resuspended at the concentration of 5 × 106 cells/ml in fresh RPMI at 2 × 106 cells/ml at the time of the experiment, and used within 5 hours after isolation.
Chemotaxis functional assay in Transwell plates
Neutrophils (3×106) resuspended in chemotaxis buffer (RPMI + 0.5% BSA) in Eppendorf tubes were incubated with inhibitors (when required) at 37 °C on a rocker platform for 30 min. After this, cells were placed in the upper chambers or “inserts” of Transwell plates that are separated from the lower wells by a 6.5-mm diameter, 5-μm pore, Nucleopore polycarbonate membrane. For the study of chemotaxis, GM-CSF or IL-8 was added in 0.5 ml chemotaxis buffer to the lower wells of Transwell plates [17]. For the study of chemokinesis, GM-CSF or IL-8 was added in 0.5 ml chemotaxis buffer to the lower chambers and in 200 μl chemotaxis buffer to the upper chambers. In either case, the Transwell plates were incubated for 45 min at 37 °C under a 5% CO2 atmosphere. The number of cells that migrated to the lower wells was calculated by counting 4 fields of duplicate 20 μl-aliquots on a microscope using a hemocytometer. Viability (by trypan blue exclusion) at the end of the assay in both chambers remained >95 ± 2%, even in the presence of the inhibitor rapamycin, ruling out a toxic effect.
F-actin measurement by flow cytometry
Neutrophils were stained with phalloidin-FITC as described [18] with some modifications. Briefly, F-actin polymerization was initiated in vivo by the addition of GM-CSF to a neutrophil cell suspension (5×106 cells/ml) for 5 min at 37 °C. After this, 0.2-ml aliquots were taken and mixed with 1 ml of pre-chilled fixing solution (two parts of double-concentrated phosphate buffer, pH 7.4, one part of 20% formaldehyde and one part of 75% glycerol in water). Samples were stored at −80 °C until ready for flow cytometry. At that time, samples were thawed and spun down for 5 min at 600×g in a refrigerated Eppendorf microfuge. Pellets were resuspended in freshly-prepared F-actin staining solution (35 μl of a 3.3-μg/ml methanol FITC-phalloidin stock plus 315 μl H20, and stained in the dark for 30 min at room temperature. Samples were centrifuged as above and pellets were resuspended in 1 ml of “FACS FLOW”. They were then analyzed by flow cytometry on a FACSCAN Becton & Dickinson flow cytometer at 488 nm excitation wavelength. Data was analyzed using Cell Quest software. The relative F-actin content was expressed in comparison with resting cells that received no treatment. Results of fluorescence intensity are shown in a logarithmic scale as in [18,19].
p70S6K analyses: [a] Phosphorylation
Stimulation of neutrophils (3×106 cells/ml in RPMI) with appropriate agents was terminated by centrifugation (14,000×g, 15 s) and resuspension of pellets in boiling SDS solution (1% SDS in 10 mM Tris, pH 7.4). Samples were boiled in a heat block for 10 min with frequent vortexing to achieve complete dissolution, taken to an ice bucket and mixed with 0.3 ml cold ddH2O and 0.4 ml cold, Triton X-100-based, lysis buffer (12 mM Tris-HCl, pH 7.2, 0.75 mM NaCl, 100 μM sodium orthovanadate, 10 mM phenylmethylsulfonyl fluoride, 0.2 mM β-glycerophosphate, 5 μg/ml each of aprotinin, pepstatin A and leupeptin, and 0.12% Triton X-100). The resulting cell lysates were used for immunoprecipitation and immunoblotting, as in [20] with anti-phospho-T421/S424-p70S6K antibodies. [b] Enzyme activity. Ribosomal p70S6K enzymatic activity was quantified by using an immunocomplex kinase assay as reported previously [17] tailored for p70S6K. Cell lysates were obtained in a Triton-X100-based lysis buffer and immunoprecipitated with anti-p70S6K antibodies. Immune complex beads were mixed with 75 μM of the phosphoacceptor peptide substrate KKRNRTLTK in freshly-prepared kinase buffer (13.4 mM HEPES, pH 7.3, 25 mM MgCl2, 30 mM Na2VO3, 5 mM p-nitrophenyl phosphate, 2 mM EGTA, 2 mM cAMP-dependent kinase inhibitor TTYADFIASGRTGRRNAIHD, 0.420μCi [γ-32P]ATP (7 nM), and 68 μM unlabeled ATP). The reaction was carried out at 37 °C for 20 min in a rotator and terminated by blotting 40 μl of the reaction mixture onto P81 ion exchange chromatography cellulose phosphate papers.
Statistical Analysis
Data are presented as the mean ± SEM. The difference between means was assessed by the Single Factor Analysis of Variance (ANOVA) test. Probabilities of less than 0.01 (p<0.01) or less than 0.05 (p<0.05) was considered to indicate significant differences.
RESULTS
Leukocyte migration is inhibited by rapamycin
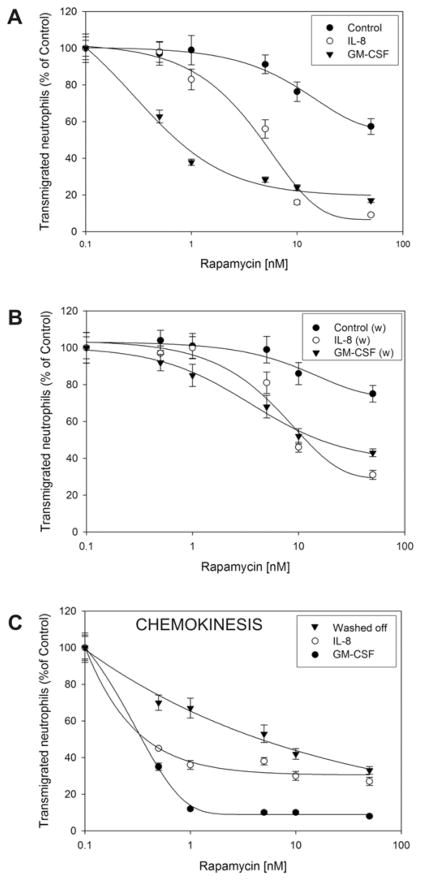
We have studied neutrophil migration in Transwell plates and have observed that preincubation of neutrophils with rapamycin at a sub-nanomolar concentration strongly inhibits chemotaxis of these cells towards either GM-CSF or IL-8. The dose-response experiment in Fig. 1A demonstrates that rapamycin inhibited GM-CSF-induced chemotaxis with an IC50 of 0.3 nM and IL-8-induced chemotaxis with an IC50 of 2 nM. Interestingly, rapamycin also inhibited control migration, particularly at 10 nM and above, with viability of cells remaining high (>90%, as per trypan blue exclusion). We next designed an experiment to ascertain whether or not the inhibition by rapamycin was reversible (Fig. 1B). After incubation with rapamycin, cells were washed and then stimulated with GM-CSF as before. In this case, the inhibitory effect of rapamycin is attenuated but never completely disappears, indicating that once it enters the cell, some of the effect is irreversible, particularly at concentrations ≥ 5 nM. Chemokinesis is also very sensitive to rapamycin, which inhibited GM-CSF-induced chemokinesis with an IC50 of 0.2 nM, and it also inhibited IL-8-induced chemokinesis with an IC50 of 0.5 nM (Fig. 1C). The inhibitory effect remained even after washing off rapamycin and allowing the cells to migrate in Transwell plates for up to 1 hour.
Figure 1. Effect of rapamycin on cell migration.
(A) Freshly isolated human neutrophil suspensions were resuspended in RPMI-based chemotaxis buffer at the density of 3×106 cells/ml and incubated with the indicated concentrations of rapamycin for 30 minutes at 37 oC. Next, aliquots containing 6×105 cells were placed in the upper “insert” wells of Transwell plates and exposed to either 7 nM GM-CSF or 10 nM IL-8 for 45 minutes at 37 °C under a 5% CO2 atmosphere. (B) Effect of washing rapamycin off (indicated in the figure by “w”). Cells were incubated with the indicated concentrations of rapamycin for 30 minutes and then spun down at 600×g for five minutes. Supernatants were decanted and pellets were resuspended in fresh chemotaxis buffer before moving them to the Transwell plates. (C) Dose-response of rapamycin on IL-8- and GM-CSF-induced chemokinesis and effect of washing off the drug. Chemokinesis was accomplished by placing the cytokine (either 7 nM GM-CSF or 10 nM IL-8) in both the lower wells and the upper inserts wells of Transwell plates. 100% represents 21±1×104 cells/ml for Control, 70±6×104 cells/ml for GM-CSF and 132±8×104 cells/ml for IL-8.
Effects of rapamycin on S6K phosphorylation, kinase activity and actin polymerization
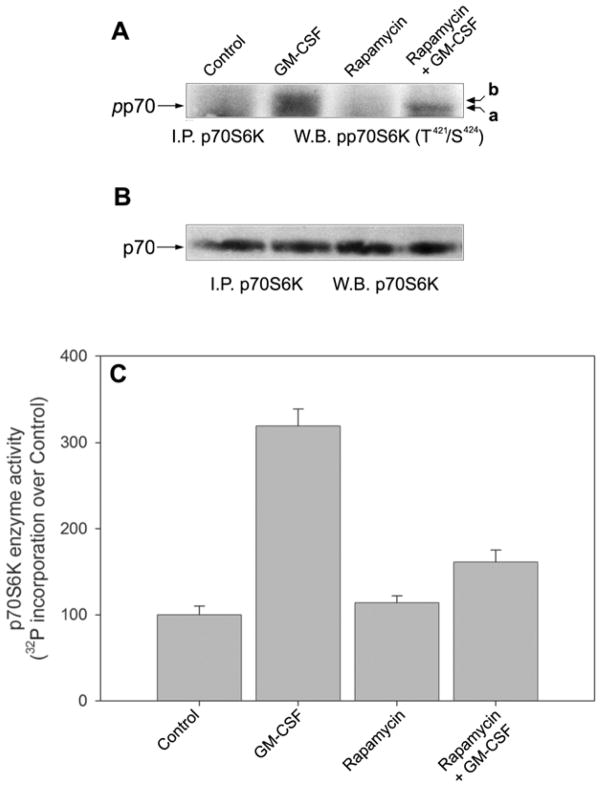
Since it is known that rapamycin inhibits the phosphorylation of mTOR as well as that of p70S6K (one of its substrates), we next run controls to ascertain if the concentration of rapamycin shown to inhibit chemotaxis was able to affect its molecular target(s). For this, we have concentrated on GM-CSF, since data in Fig. 1 indicate that chemotaxis and chemokinesis in more sensitive to rapamycin when the stimulant is GM-CSF vs. IL-8, and since chemoattractants other than GM-CSF produce a measurable, but low, effect on p70S6K phosphorylation1. Fig. 2A shows that GM-CSF induced an increase in T421/S424 phosphorylation of p70S6K and that rapamycin was able to negate that effect. This was seen in the Western blots as a change in electrophoretic mobility (indicative of a loss of phosphate, as demonstrated in [21]) and as a diminished signal to the antibody in the rapamycin + GM-CSF lane. Fig. 2B indicates that all the changes alluded to above are observed in the presence of equal levels of protein loading with an anti-p70S6K antibody that detects the unphosphorylated form.
Figure 2. Effect of rapamycin on GM-CSF-stimulated p70S6K phosphorylation and enzymatic activity.
Neutrophils were incubated with 10 nM rapamycin for 30 minutes, followed by a short (5 min.) incubation with 20 nM GM-CSF and cell lysates in boiling SDS were generated and immunoprecipitation with anti-p70S6K followed. Resulting immunocomplexes were used for immunoblotting with anti-phospho-T421/S424-p70S6K antibodies (A) or with anti-p70S6K antibodies to show equal protein loading (B). (C) Detection of p70S6K enzymatic activity and its modulation by GM-CSF and rapamycin. Cells were incubated ± rapamycin for 30 min and them subjected to GM-CSF incubation. Cells lysates were derived and immunoprecipitated. Immunocomplex beads were assayed for p70S6K activity against the S6 kinase substrate peptide KKRNRTLTK. Results are the mean ± SE of three independent experiments performed in duplicate. 100% represents 1052 ± 77 dpm.
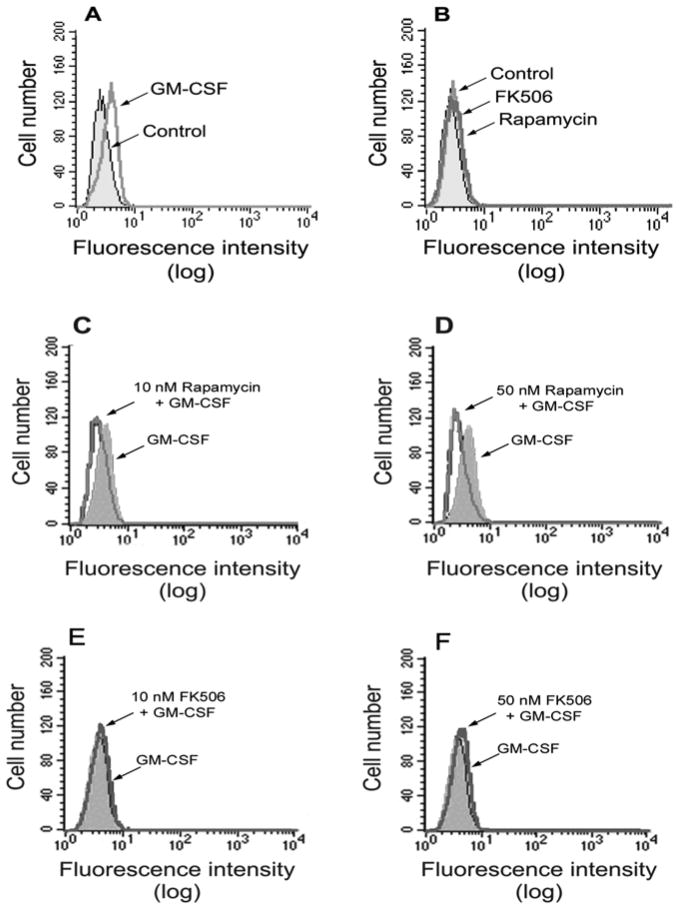
We next measured the activity of this enzyme in vitro. Incubation of neutrophils with GM-CSF resulted in an increase of p70S6K activity and pre-treatement with rapamycin caused inhibition of the enzymatic activity of p70S6K (Fig. 2C) that paralleled the results of phosphorylation indicated in Fig. 2A. All this serves to demonstrate that, in neutrophils, rapamycin targets p70S6K in conditions in which cell migration is inhibited. We next investigated if rapamycin had an inhibitory effect on actin polymerization, a hallmark of cell migration. Fig. 3A shows that GM-CSF caused an increase in F-actin polymerization as seen by a displacement to the right of the log fluorescence values in the x-axes. While rapamycin by itself had no effect on F-actin polymerization (Fig. 3B), it inhibited the GM-CSF-induced changes, as evidenced by a displacement to the left of the log fluorescence values (Fig. 3C,D). However, for the rapamycin effect to be observed, concentrations of the drug larger than those used to inhibit cell migration were needed (10 nM and above vs. 1 nM). To confirm the specificity of this effect, we repeated the experiment by preincubation of neutrophils with FK506, a structural analog of rapamycin, an FKBP12 binding compound, related to rapamycin, that lacks the ability to inhibit mTOR. In the case of FK506, no inhibition was observed at the doses tested (Fig. 3E,F).
Figure 3. Effect of rapamycin on GM-CSF-stimulated F-actin polymerization.
Several sets of neutrophil suspensions were incubated at 37 °C in the presence or the absence of 7 nM GM-CSF for 5 minutes (A); or with either 50 nM rapamycin or 50 nM FK506 (B) for 30 minutes; or with the indicated concentrations of rapamycin (C,D) or FK506 (E,F) for 30 minutes prior to the addition of GM-CSF and further incubated for 5 minutes. All stimulations were stopped at the end of the indicated lengths of time by fixing cells in formaldehyde. 200 μl-samples of 1×104 cells were incubated with phalloidin-FITC and analyzed by flow cytometry. X-axes: Log fluorescence; y-axes: Relative cell number.
FK506, but not MEKi, rescues the inhibition caused by rapamycin in chemotaxis
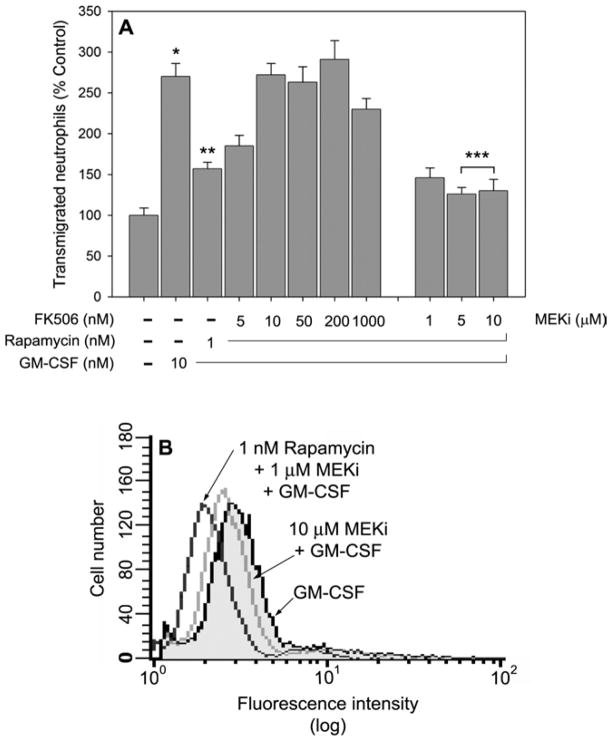
One of the hallmarks of the study of specificity of rapamycin is that its effect is prevented (or once it is produced, it can be “rescued”) by treatment of cells with the structural analog FK506 [22–25]. In our hands, such was the case: the inhibitory effect of rapamycin on chemotaxis was reversed by preincubating cells with FK506 prior to rapamycin (Fig. 4A, group of bars to the left), indicating that FK506 prevents rapamycin inhibition at a 1:10 (and greater) rapamycin:FK506 molar ratio. This action was unique to FK506, since a rescue was not observed with MEKi (Fig. 4A, group of bars to the right). In fact, the incubation with MEKi drove the existing rapamycin inhibition to pre-stimulation levels, since the difference between 5 or 10 μM MEKi and control (the two bars at the furthermost right and the bar at the furthermost left) was not significant. However, the differences between rapamycin alone and rapamcycin + 5 or 10 μM MEKi were significant (*** in Fig. 4A, with p<0.05). This might suggest that cell migration requires also a positive input from the Ras/MAPK pathway; (actin polymerization was also inhibited by MEKi and the effect was greater by a combination of MEKi and rapamycin [not shown]). We know that another element could be upregulating p70S6K activity: a positive feedback from the MAPK pathway, since we have recently demonstrated a crosstalk between those two major pathways [26]. Note, however, that rapamycin is a much stronger inhibitor of leukocyte migration, since it produced its effect in the sub-nanomolar range, while MEKi inhibited at the sub-micromolar range. A confirmation of the cooperative nature of MEKi and rapamycin is given in Fig. 4B, where actin polymerization is further inhibited when both inhibitors are together (even at low concentrations), as given by the larger difference in fluorescence intensity as compared to controls.
Figure 4. FK506 and MEKi.
(A) Effect on neutrophil chemotaxis. Neutrophils were incubated at 37 °C with the indicated concentrations of FK506 (group of bars to the left) or MEKi (group of bars to the right) for 30 minutes followed by an additional 30 minute-incubation with rapamycin. Cells were then placed in the upper wells of Transwell plates and allowed to migrate against GM-CSF (where indicated) for 45 minutes. The differences between control and GM-CSF-induced chemotaxis (*), as well as the inhibition caused by rapamycin over GM-CSF (**) were significant (p<0.01). The further inhibition by MEKi of the rapamycin inhibition (***) was also significant (p<0.05). Treatment with MEKi in the absence of GM-CSF exhibited curves indistinguishable from controls (as in Fig. 3B). (B) Effect on actin polymerization. Cells were treated with either MEKi alone or with a combination of MEKi and Rapamycin, stimulated with GM-CSF for 5 min, fixed, incubated with phalloidin-FITC and analyzed for flow cytometry.
DISCUSSION
We have shown in this study for the first time in cells of the hematopoietic system that both chemotaxis and chemokinesis were inhibited by the antibiotic macrolide and immunosuppressant rapamycin. This inhibition is very potent, particularly chemokinesis, that had an IC50 of ~300 pM and it was complete at 3–10 nM. We should highlight that the evidence for this inhibition takes place in a whole-cell setting, demonstrating an effect on a fundamental physiological function of the leukocyte. These potencies are in contrast to MEKi, the other agent that we have described here as being inhibitory of cell migration, but the concentrations of MEKi needed to hamper cell migration were in the sub-μM range [26].
A previous report has indicated a possible link to p70S6K in lipopolysaccharide-induced nitric oxide production in macrophages [27]. The authors have observed that rapamycin inhibits p70S6K (both basal and LPS-activated components) and it also inhibits mobility shift (Fig 3B of their study). Although the effects of rapamycin on p70S6K and cytoskeleton are known [27–29] this study provides the first evidence for the inhibition of cell migration by rapamycin. We have indicated here the inhibition of cell migration in conditions in which both phosphorylation and enzymatic activity are inhibited. Being rapamycin an inhibitor of the mTOR-S6K pathway, it is therefore reasonable to conclude that that pathway should have a key role in neutrophil chemotaxis and chemokinesis.
Although rapamycin is usually described as a specific inhibitor of mTOR, this kinase is a direct stimulant of p70S6K, that serves as its physiological substrate [30,31]. The fact that rapamycin binds to the immunophilin FKBP12, raises the possibility that any inhibitory effect on chemotaxis observed is through disruption of an important FKPB12 complex, independent of effects on mTOR/S6K. To address the specificity of rapamycin we treated neutrophils with FK506, an FKBP12 binding compound, related to rapamycin, that lacks the ability to inhibit mTOR [23–26]. Since FK506 did not inhibit actin polymerization, it suggested that the effect of rapamycin was directly through the mTOR pathway. Of note are also the results indicating that FK506 have a preventive effect on the inhibitory action of rapamycin (Fig. 4A) in whole cells.
We have demonstrated that rapamycin completely inhibits chemotaxis at ~1 nM (Fig. 1) but a minimum of 10 nM is required to inhibit actin polymerization (Fig. 3). The reason for this could be that there are either several pools of p70S6K inside the cell, each performing different functions and each with potentially different rapamycin sensitivities, or that the concentration of intracellular FKBP12 is much higher than that of mTOR [P. Dennis, personal communication]. In the latter scenario, rapamycin at low concentrations (<1 nM) could already saturate mTOR-dependent actin polymerization, while only minimally affecting processes dependent on FKBP12. High concentrations (>10 nM) of rapamycin can block FKBP12 and presumably, F-actin polymerization that might be dependent on both FKBP12 and mTOR. At any event, actin polymerization remains a candidate for the effect of rapamycin on neutrophils. A precedent for a connection between rapamycin and the cytoskeleton has been given in [28] who have demonstrated that rapamycin at 20 nM inhibits myogenesis and concomitant muscle cell differentiation, and by [29] who demonstrated that TOR2 in yeast is important for polarized distribution of the actin cytoskeleton, in addition to its primary function as an effector of G1 cell cycle progression.
The understanding of the blockage of neutrophil migration by inhibitors that target the mTOR/S6K pathway as demonstrated in this study, will not be confined to the physiology of leukocytes but, rather, will have a much broader effect involving the central paradigm in cell biology of cell migration. Migration of endothelial cells is key in the process of angiogenesis and tumor growth; migration of vascular smooth cells re-occludes arteries after angioplasty of atherosclerotic coronary arteries; and migration of synovial fibroblasts is a hallmark of rheumatoid arthritis.
Acknowledgments
We are grateful to Dr. Patrick Dennis for stimulating discussions on this work and to Dr. Michael Baumann for help with the flow cytometry assays. This work has been supported by grants from the National Institutes of Health (HL056653) and the American Heart Association 0250417N to J.G.-C.
Abbreviations
- IL-8
interleukin-8
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- p70S6K
ribosomal p70-S6 kinase
- mTOR/FRAP
mammalian target of rapamycin
- FKBP12
FK506-binding protein
- PI3K
phosphatidylinositol 3-kinase
- MEKi
mitogen-activated protein kinase kinase inhibitor (PD-98059)
- FITC
fluorescein isothiocyanate
Footnotes
Gomez-Cambronero, J., Horn, J., Paul, C. and Baumann, M., unpublished observations
References
- 1.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 4.Nijhuis E, Lammers JW, Koenderman L, Coffer PJ. J Leukocyte Biol. 2002;71:115–124. [PubMed] [Google Scholar]
- 5.Crouch MF. Biochem Biophys Res Commun. 1997;233:193–199. doi: 10.1006/bbrc.1997.6419. [DOI] [PubMed] [Google Scholar]
- 6.Dennis PB, Thomas G. Curr Biol. 2002;12:269. doi: 10.1016/s0960-9822(02)00796-0. [DOI] [PubMed] [Google Scholar]
- 7.Hara K, Yonezawa K, Kozlowski MT, Sugimoto T, Andrabi K, Weng QP, Kasuga M, Nishimoto I, Avruch J. J Biol Chem. 1997;272:26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Donahoe PK, Zervos AS. Science. 1994;265:674–676. doi: 10.1126/science.7518616. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Schreiber SL, Clardy J. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 10.Wieder KJ, Hancock WW, Schmidbauer G, Corpier CL, Wieder I, Kobzik L, Strom TB, Kupiec-Weglinski JW. J Immunol. 1993;51:1158–1166. [PubMed] [Google Scholar]
- 11.Kahan BD. Expert Opin Pharmacother. 2001;2:1903–1917. doi: 10.1517/14656566.2.11.1903. [DOI] [PubMed] [Google Scholar]
- 12.Dumont FJ. Curr Opin Investig Drugs. 2001;2:1220–1234. [PubMed] [Google Scholar]
- 13.Garber K. J Natl Cancer Inst. 2001;93:1517–1519. doi: 10.1093/jnci/93.20.1517. [DOI] [PubMed] [Google Scholar]
- 14.Kahan BD. Transplant Proc. 1994;26:3203–3204. [PubMed] [Google Scholar]
- 15.Bohler T, Waiser J, Budde K, Lichter S, Jauho A, Fritsche L, Korn A, Neumayer HH. Transplant Proc. 1998;30:2195–197. doi: 10.1016/s0041-1345(98)00588-0. [DOI] [PubMed] [Google Scholar]
- 16.English D, Andersen BR. J Immunol Methods. 1974;5:249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- 17.Lehman JA, Paul CC, Baumann MA, Gomez-Cambronero J. Am J Physiol Cell Physiol. 2001;280:183–191. doi: 10.1152/ajpcell.2001.280.1.C183. [DOI] [PubMed] [Google Scholar]
- 18.Egger G, Burda A, Glasner A. Virchows Arch. 2001;438:394–397. doi: 10.1007/s004280000321. [DOI] [PubMed] [Google Scholar]
- 19.Hua J, Sakamoto K, Nagaoka I. J Leukocyte Biol. 2002;71:632–640. [PubMed] [Google Scholar]
- 20.Joseph DE, Paul CC, Baumann MA, Gomez-Cambronero J. J Biol Chem. 1996;22:13088–13093. doi: 10.1074/jbc.271.22.13088. [DOI] [PubMed] [Google Scholar]
- 21.Lehman J, Calvo V, Gomez-Cambronero J. J Biol Chem. 2003 doi: 10.1074/jbc.M300376200. (Papers in Press, M300376200) [DOI] [PubMed] [Google Scholar]
- 22.Chung J, Kuo CJ, Crabtree GR, Blennis J. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 23.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumont FJ, Melino MR, Staruch MJ, Koprak SL, Fischer PA, Sigal NH. J Immunol. 1990;144:1418–1424. [PubMed] [Google Scholar]
- 25.Mukhopadhyay NK, Price DJ, Kyriakis JM, Pelech S, Sanghera J, Avruch J. J Biol Chem. 1992;267:3325–3335. [PubMed] [Google Scholar]
- 26.Lehman JA, Gomez-Cambronero J. Biochem Biophys Res Commun. 2002;293:463–469. doi: 10.1016/S0006-291X(02)00238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein SL, Finn AJ, Dave SH, Meng F, Lowell CA, Sanghera JS, DeFranco AL. J Leukocyte Biol. 2000;67:405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- 28.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 29.Gingras AC, Raught B, Sonenberg N. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 30.Erbay E, Chen J. J Biol Chem. 2001;276:36079–36082. doi: 10.1074/jbc.C100406200. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A, Kunz J, Hall MN. Proc Natl Acad Sci USA. 1996;93:13780–13785. doi: 10.1073/pnas.93.24.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]