Abstract
Objectives
To define pathogen tests and device specifications needed for emerging point-of-care (POC) technologies used in disasters.
Design
Surveys included multiple-choice and ranking questions. Multiple-choice questions were analyzed with the χ2 test for goodness-of-fit and the binomial distribution test. Rankings were scored and compared using analysis of variance and Tukey's multiple comparison test.
Participants
Disaster care experts on the editorial boards of the American Journal of Disaster Medicine and the Disaster Medicine and Public Health Preparedness, and the readers of the POC Journal.
Results
Vibrio cholera and Staphylococcus aureus were top-ranked pathogens for testing in disaster settings. Respondents felt that disaster response teams should be equipped with pandemic infectious disease tests for novel 2009 H1N1 and avian H5N1 influenza (disaster care, p < 0.05; POC, p < 0.01). In disaster settings, respondents preferred self-contained test cassettes (disaster care, p < 0.05; POC, p < 0.001) for direct blood sampling (POC, p < 0.01) and disposal of biological waste (disaster care, p < 0.05; POC, p < 0.001). Multiplex testing performed at the POC was preferred in urgent care and emergency room settings.
Conclusions
Evidence-based needs assessment identifies pathogen detection priorities in disaster care scenarios, in which Vibrio cholera, methicillin-sensitive and methicillin-resistant Staphylococcus aureus, and Escherichia coli ranked the highest. POC testing should incorporate setting-specific design criteria such as safe disposable cassettes and direct blood sampling at the site of care.
Keywords: cassette, direct sampling, Haiti, Katrina, pathogen detection, tsunami, Vacutainer
Introduction
The Southeast Asia Tsunami of 2004 and the Hurricane Katrina in 2006 illustrated the need for and the feasibility of point-of-care testing (POCT) in emergency and disaster care.1 However, disaster response teams in both settings were ill-equipped to meet the diagnostic demands. Time-critical tests for bloodstream pathogen detection were lacking. Emerging POC device designs should be based on stakeholder needs to ensure maximum clinical impact and minimum time to implementation.2-4 The objective of this article is to define pathogen priorities and design specifications for emerging POC technologies to bridge gaps for emergency and disaster testing at the point of service.
Methods
Respondents
Forty-six disaster care professionals were randomly selected from the editorial boards of the American Journal of Disaster Medicine and the Disaster Medicine and Public Health Preparedness by assigning random numbers (Minitab, State College, PA) to public published lists. Two hundred Point of Care Journal subscribers were invited to participate. Contact information for the POC Journal was provided by the publisher, Lippincott Williams and Wilkins (Philadelphia, PA). The UC Davis Institutional Review Board approved this research.
Survey development
Surveys were developed through literature review and multidisciplinary consultations that included experienced professionals in bioengineering, emergency medicine, infectious diseases, and critical care medicine. Survey questions used visual logistics, that is, graphics and pictures, to present questions and concepts without the need for lengthy text descriptions. The surveys had three sections: demographics (profession, work setting, and geographic location), pathogen detection (bacterial, viral, and fungal), and device design (preanalytical processing, sample type, disposal, and user interface). To encourage participation, simplify distribution, and facilitate return, we developed a web-based platform (SurveyMonkey, Portland, OR). The participants were sent personalized invitations through e-mail and the US Postal Service. To achieve geographic representation, we made follow-up phone calls and sent paper-based surveys through the Federal Express with a self-addressed return envelope. To view survey questions, readers can type http://www.surveymonkey.com/s/AJDM into any web browser.
Statistical analysis
We conducted statistical analysis for identical multiple-choice and ranking questions to compare the responses. We used SPSS 15 (SPSS, Chicago, IL) statistical software to analyze multiple-choice questions with the nonparametric χ2 test for goodness-of-fìt (when more than two choices) and the binomial distribution test (when two choices). Ranked responses were scored and analyzed using analysis of variance and Tukey's multiple comparison tests. Nonparametric data were analyzed with the Mann-Whitney U-test. Statistical significance was defined as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
Ranks were evaluated by assigning each factor (eg, Vibrio cholera) an overall score. Survey respondents assigned ranks, Rj, with j = [1, nr], where nr is the number of possible ranks for each factor. Ranks were inverted so that the choice most preferred had the highest numerical value: . The score, Si, was determined by summing the product of each inverted rank and its corresponding frequency: . The frequency, Fij, is the number of times survey respondents assigned an individual factor with a specific rank, with i = [1, nf], where nf is the number of factors given for selection. When a respondent designated the same rank for two or more factors, the average was assigned.
Definitions
We used the following terms to describe device design: (a) test cassette collection, biological sample collection using a self-contained cartridge that directly interfaces with an analyzer; (b) Vacutainer collection, biological sample collection using a Vacutainer; (c) direct sampling, sample collection that requires no manipulation or transfer to another container for analysis; and (d) coupled direct sampling, direct sampling using a needle and hub coupled to a test cassette. For clarity, each design was presented visually.
Results
Demographics
Of 46 disaster care editorial board members, 31 responded (response rate 67 percent), including medical doctors (14/31, 45 percent), disaster responders (9/31, 29 percent), research scientists (5/31, 16.1 percent), laboratorians (2/31, 6.5 percent), and industry (1/31, 3.2 percent). Of 200 POC Journal readers surveyed, 100 responded (response rate 50 percent), including laboratorians (55/100, 55 percent), medical doctors (13/100,13 percent), POC coordinators (12/100, 12 percent), industries (10/100,10 percent), research scientists (7/100,7 percent), and nurses (3/100,3 percent).
Pathogen detection
When queried with “yes” or “no” choices for avian H5N1 influenza, novel 2009 H1N1 (Swine flu) influenza, and severe acute respiratory disease, disaster care (n = 11) and POC (n = 67) respondents felt it necessary that response teams should be equipped with novel 2009 H1N1 influenza and avian H5N1 influenza testing at the POC (disaster care, p < 0.05; POC, p < 0.01). The disaster care experts and POC respondents preferred the emergency room, disaster setting, outpatient clinics, and urgent care clinics as the top four settings for these pandemic infectious disease tests, with sputum, blood, and swabs as preferred sample types (Table 1).
Table 1.
Pandemic pathogen testing preferences
| Respondents (n) |
Testing location | Percentage of respondents who preferred location |
|---|---|---|
| Disaster care (26) | ||
| Emergency room | 73.1 | |
| Disaster | 65.4 | |
| Outpatient clinic | 57.7 | |
| Urgent care clinic | 57.7 | |
| Rural area | 42.3 | |
| Intensive care unit | 7.7 | |
| Operating room | 0 | |
| Point-of-care (72) | ||
| Emergency room | 59.7 | |
| Urgent care clinic | 50 | |
| Disaster | 47.2 | |
| Outpatient clinic | 44.4 | |
| Rural area | 26.4 | |
| Intensive care unit | 4.2 | |
| Operating room | 1.4 | |
| Sample type* | Percentage of respondents who preferred sample type |
|
| Disaster care (22) | ||
| Sputum | 40.9 | |
| Blood | 31.8 | |
| Swab | 18.2 | |
| Point-of-care (63) | ||
| Blood | 42.9 | |
| Sputum | 36.5 | |
| Swab | 22.2 | |
Note: Respondents rarely selected nasal wash/secretion, nasopharyngeal aspirate, saliva, urine, or expired air for preferred sample types.
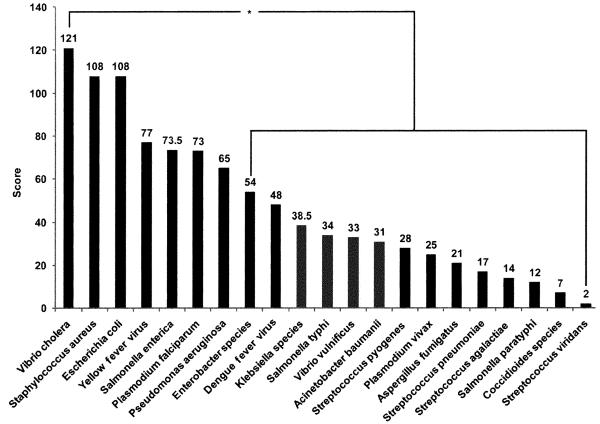
Figure 1 shows that disaster care respondents (24) ranked Vibrio cholera, Staphylococcus aureus, and Escherichia coli as the three most important pathogens to test at the POC in a general disaster setting, with Vibrio cholera ranking higher in pairwise comparison when compared with other pathogens except Staphylococcus aureus, Escherichia coli, yellow fever virus, Plasmodium falciparum, Salmonella enterica, and Pseudomonas aeruginosa (p < 0.05). POC survey respondents (46) ranked Staphylococcus aureus higher in pairwise comparison when compared with other pathogens, except Vibrio cholera and Salmonella typhi, for general disaster settings (p < 0.01).
Figure 1.
Priorities for pathogen testing in disaster settings. Disaster care respondents (n = 24) ranked pathogens in the order displayed in this Pareto plot for disaster settings, and they chose Vibrio cholera higher in statistical pairwise comparisons when compared with other pathogens, demarcated on the right: *p < 0.05.
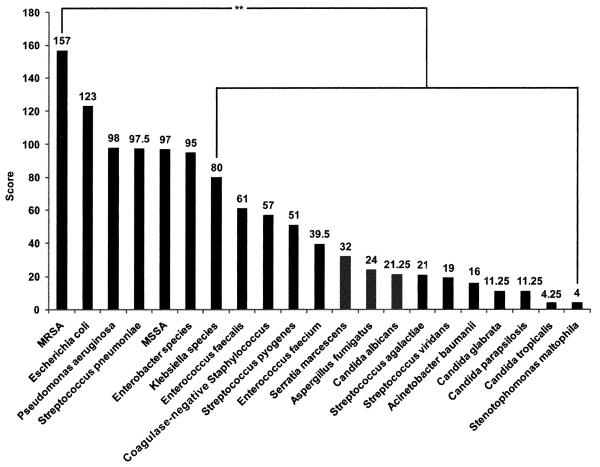
Disaster care respondents ranked methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa, and Streptococcus pneumonia as the top four bloodstream pathogens for detection at the POC (Figure 2). POC respondents (47) ranked MRSA, Streptococcus pneumonia, Staphylococcus aureus, and Escherichia coli as the top four bloodstream pathogens for POCT. MRSA was ranked higher in pairwise comparison when compared with the other pathogens for bloodstream infection (p < 0.01).
Figure 2.
Bloodstream pathogen testing priorities. Disaster care respondents (n = 21) ranked methicillin-resist-ant Staphylococcus aureus number one and also ranked this organism higher in pairwise comparison when compared with other pathogens, demarcated on the right: **p < 0.01.
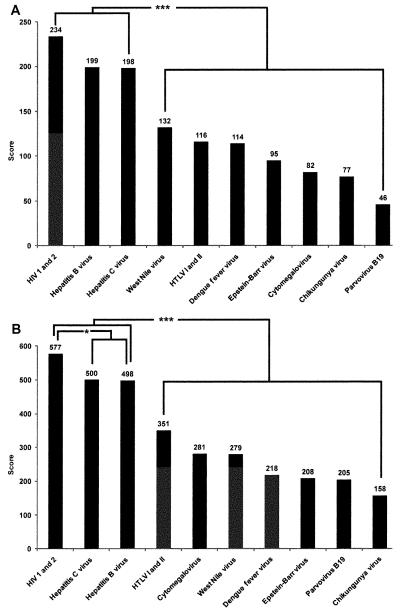
For emergency blood donor screening, disaster care (24) and POC (61) respondents ranked HIV 1 and 2 and Hepatitis B and C higher in pairwise comparison when compared with other pathogens (disaster care, p < 0.01; POC, p < 0.01). Figure 3 shows that POC respondents ranked HIV 1 and 2 higher in pairwise comparison when compared with both Hepatitis B and C (p < 0.05).
Figure 3.
Emergency blood donor screening. Panel A illustrates that disaster care respondents (n = 24) ranked HIV 1 and 2, Hepatitis B virus, and Hepatitis C virus higher in pairwise comparison when compared with other pathogens, demarcated on the right. Panel B shows that point-of-care respondents (n = 61) also ranked HIV 1 and 2, Hepatitis B virus, and Hepatitis C virus higher in pairwise comparison when compared with other pathogens. Additionally, point-of-care respondents ranked HIV 1 and 2 higher in pairwise comparison when compared with Hepatitis B virus and Hepatitis C virus, as shown on the left: ***p < 0.001 and *p < 0.05.
Device design
Disaster care and POC respondents selected handheld devices more frequently than portable or transportable for disaster settings (disaster care: 27/28, 96 percent; POC: 90/94, 96 percent). However, disaster care respondents selected handheld devices less frequently in urgent care (8/28, 29 percent) and emergency room settings (7/28, 25 percent).
Both groups of respondents preferred test cassette over Vacutainer sample collection in disaster settings (26 disaster care respondents, p < 0.05; 88 POC respondents, p < 0.001). Disaster care respondents showed no preference for test cassette versus Vacutainer collection for urgent care and emergency room settings. For urgent care settings, POC respondents preferred Vacutainer collection (87 POC respondents, p < 0.05), but showed no preference in emergency room settings.
Disaster care survey respondents showed no preference for direct sampling or coupled direct sampling in disaster, urgent care, and emergency room settings. POC respondents (84) preferred direct sample collection to coupled direct sample collection in disaster settings (p < 0.01), but they (83) preferred coupled direct sample collection to direct sample collection in urgent care and emergency room settings (p < 0.05).
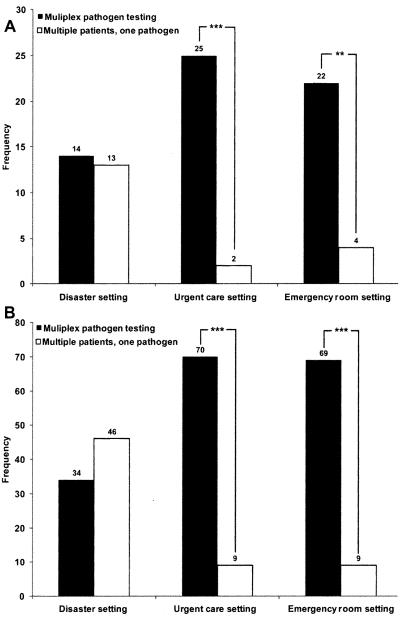
In both urgent care and emergency room settings (Figure 4), both surveys showed a preference for a device that processes a single patient sample for multiple pathogens over a device that processes multiple patient samples for a single pathogen (26 disaster care respondents, p ≤ 0.001; 78 POC respondents, p < 0.001). Respondents showed no preference in disaster settings.
Figure 4.
Sample processing. Respondents selected two testing options: tests for multiple pathogens in a single patient sample versus multiple patient samples tested for a single pathogen. Panel A illustrates that disaster care respondents preferred multiplex pathogen testing in urgent care and emergency room settings (n = 26). Panel B shows that point-of-care respondents also preferred multiplex pathogen testing in these same settings (n = 78). ***p < 0.001, **p < 0.01.
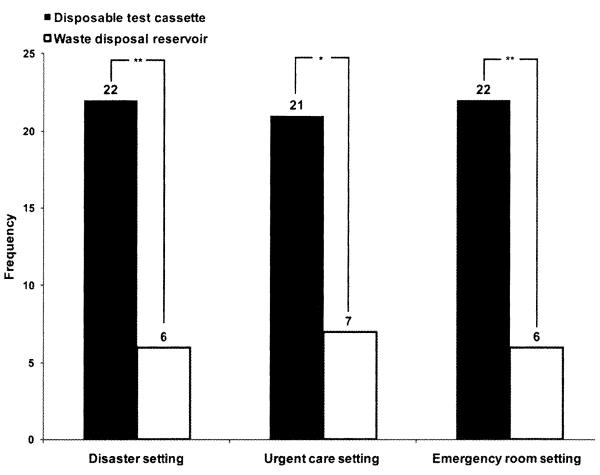
Figure 5 shows that in disaster, emergency room, and urgent care settings, respondents from both surveys preferred a device that stores biohazard waste within a test cassette versus a device that stores waste in a reservoir that is emptied periodically (28 disaster care respondents, p < 0.05; 83 POC respondents, p < 0.001).
Figure 5.
Biohazard waste disposal. Disaster care respondents (n = 28) chose one of the two biohazard waste disposal methods: a device that stores biohazard waste in a reservoir versus a device that stores biohazard waste in a disposable test cassette, and preferred the latter in all three settings, **p < 0.01, *p < 0.05.
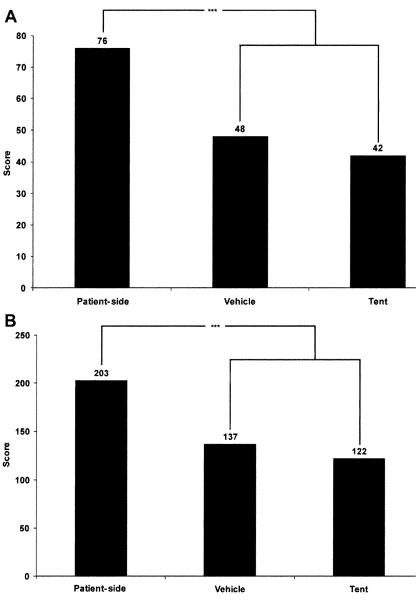
For POCT disaster scenarios, disaster care and POC respondents ranked testing at the patient-side higher in pairwise comparison when compared with vehicle or tent locations (28 disaster care respondents, p < 0.001; 80 POC respondents, p < 0.001; Figure 6).
Figure 6.
Field testing location. Panel A shows that disaster care respondents (n = 28) ranked patient-side testing higher in pairwise comparison when compared with other testing locations. Panel B shows comparable results for point-of-care respondents (n = 80). ***p < 0.001.
Discussion
Documentation of pathogens isolated following floods, hurricanes, tsunamis, and earthquakes revealed substantial agreement with survey results. Specifically, Vibrio cholera, methicillin-sensitive Staphylococcus aureus (MSSA), MRSA, Escherichia coli, and Streptococcus pneumonia, all ranked highly by survey respondents, have been observed in several different disaster settings (Table 2).5-18 POC technologies for the rapid diagnosis of Vibrio cholera are available, although none appear to be approved by the Food and Drug Administration.19-24 However, rapid POC detection of the others are not yet available.
Table 2.
Top-ranked pathogens found in major disasters
| Pathogen | Disaster | Location (year) | Isolation site/path of infection |
|---|---|---|---|
| Vibrio cholerae | |||
| Flooding | Bangladesh (2004)5 | Blood/water, food borne | |
| Hurricane Katrina | Louisiana (2005)6,7 | Blood/water borne | |
| MSSA and MRSA | |||
| Earthquake | Turkey (1999)8,9 | Wound/wound | |
| India and Pakistan (2005)10 | Wound, pus/wound | ||
| China (2008)11 | Wound, pus/wound | ||
| Haiti (2010)12 | Wound/wound | ||
| Hurricane Katrina | Louisiana (2005)13 | Wound/wound | |
| Tsunamis | Thailand (2004)14 | Wound/wound | |
| Escherichia coli | |||
| Earthquake | Indonesia (2005)15 | Blood/water borne | |
| India and Pakistan (2005)10 | Wound, pus/wound | ||
| China (2008)11 | Wound, pus/wound | ||
| Bangladesh (2004)5 | Stool/water, food borne | ||
| Hurricane Katrina | Louisiana (2005)13 | Canal water/water borne | |
| Tsunami | Thailand (2004)16 | Stool/water, food borne | |
| Streptococcus | |||
| Earthquake | India and Pakistan (2005)10 | Wound, pus/wound | |
| China (2008)11 | Wound, pus/wound | ||
| Haiti (2010)12 | Wound/wound | ||
| Flooding | Nonspecific17,18 | Blood/inhalation | |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Pandemic infectious diseases represent both public health and global challenges.25 The World Health Organization recommends the use of POC testing for influenza.26 Respondents chose novel 2009 H1N1 influenza (“swine flu”) and avian H5N1 influenza testing as necessary resources for disaster response teams. Blood and sputum were selected as sample types for pandemic infectious disease testing. For disaster settings, respondents who chose multiple patient samples processing for a single pathogen elaborated with comments about the usefulness in biothreat or pandemic scenarios.
Both disaster care experts and POC respondents cite the need for handheld devices to facilitate patientside testing. They preferred self-contained test cassettes for direct blood sampling, storage, and disposal of biohazard samples, and they identified the need for rapid, simple, and traceable sample collection methods that can be easily adapted to disaster-focused tests.
Guidelines for performance and environmental robustness should be developed to increase user's confidence and to benefit victims and patients by improving the environmental stress tolerances27 of POC reagents in field settings. Emerging technologies can be properly integrated at the local level through small-world networks to ensure appropriate regional coordination and competent use from frequent practice.28,29
Conclusions
The high priorities for disaster POC testing are Vibrio cholera, MSSA, MRSA, and Escherichia coli. Pandemic infectious disease tests, specifically novel 2009 H1N1 and H5N1 influenza, are needed in both emergency rooms and disaster settings. Respondents preferred self-contained test cassettes for direct blood sampling, storage, and disposal of biohazard materials to minimize contamination. Ultimately, the usefulness of future POC testing for disaster care will depend on how well these needs of current and future stakeholders are addressed.
Policy recommendations
This research supports the following policy recommendations:
■ POC pathogen testing for use in disaster care should be guided by objective needs assessment that delineates device design specifications and pathogen targets.
■ Setting- and scenario-specific pathogen detection at the point of service will enable timely community surveillance, outbreak trending, and crisis management for targeted therapy.
■ Funding should be made available for the rapid development of handheld and highly portable devices that facilitate emergency and disaster preparedness.
■ Imbedding emerging POC devices at a local level will encourage high user competency, matured practical designs, and robust reagent supplies.
■ Industry must respond by helping to address national preparedness with novel POC devices that will endure harsh conditions.
Acknowledgments
The authors thank the survey responders, and Lippincott Williams and Wilkins (LWW, Philadelphia, PA) and Laurel Beckett, PhD, Biostatistician, UC Davis, for their valuable contributions. This study was supported by the Point-of-Care Testing Center for Teaching and Research (POCT-CTR) and by the National Institute for Biomedical Imaging and Bioengineering (NIBIB) Point-of-Care Technologies Center grant (Dr. Kost, PI, NIH U54 EB007959). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIBIB or the National Institutes of Health. The figures and tables were provided with permission and courtesy of Knowledge Optimization®, Davis, CA.
References
- 1.Kost GJ, Tran NK, Tuntideelert M, et al. Katrina, the tsunami and point-of-care testing: Optimizing rapid response in diagnosis in disasters. Am J Clin Pathol. 2006;126:513–520. doi: 10.1309/NWU5E6T0L4PFCBD9. [DOI] [PubMed] [Google Scholar]
- 2.Kost GJ, Hale KN, Brock TK, et al. Point-of-care testing for disasters: Needs assessment, strategic planning, and future design. Clin Lab Med. 2009;29(3):583–605. doi: 10.1016/j.cll.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mecozzi DM, Brock TK, Tran NK, et al. Evidence-based point-of-care device design for emergency and disaster care. Point Care. 2010;9:65–69. doi: 10.1097/POC.0b013e3181d9d47a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hale KN, Brock TK, Mecozzi D, et al. Point-of-care for disaster and pandemic preparedness. In: Price CP St., John A, Kricka LJ, editors. Point-of-Care Testing. 3rd ed. AACC Press; Washington, DC: 2010. pp. 355–371. [Google Scholar]
- 5.Qadri F, Khan A, Faruque A, et al. Enterotoxigenic Escherichia coli and Vibrio cholerae diarrhea, Bangladesh, 2004. Emerg Infect Dis. 2005;11:1104–1107. doi: 10.3201/eid1107.041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention [Accessed June 28, 2010];Update on CDC's response to Hurricane Katrina. 2005 September 19; Available at http:/www.cdc.gov/od/Katrina/09-19-05.htm.
- 7.Sinigalliano C, Gidley M, Shibata T, et al. Impacts of Hurricanes Katrina and Rita on the microbial landscape of the New Orleans area. Proc Natl Acad Sci USA. 2007;104:9029–9034. doi: 10.1073/pnas.0610552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulut M, Fedakar R, Akkose S, et al. Medical experience of university hospital in Turkey after the 1999 Marmara earthquake. Emerg Med J. 2005;22:494–498. doi: 10.1136/emj.2004.016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazancioglu R, Cagatay A, Colangu S, et al. The characteristics of infections in crush syndrome. Clin Microbiol Infect. 2002;8:202–206. doi: 10.1046/j.1469-0691.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- 10.Kiani QH, Amir M, Ghazanfar MA, et al. Microbiology of wound infections among hospitalised patients following the 2005 Pakistan earthquake. J Hosp Infect. 2009;73:71–78. doi: 10.1016/j.jhin.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Tao C, Kang M, Chen Z, et al. Microbiologic study of the pathogens isolated from wound culture among Wenchuan earthquake survivors. Diagn Microbiol Infect Dis. 2009;63:268–270. doi: 10.1016/j.diagmicrobio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Pape JW, Rouzier V, Ford H, et al. The GHESKIO field hospital and clinics after the earthquake in Haiti—Dispatch 3 from Port-au-Prince. NEngl J Med. 2010;362:e34. doi: 10.1056/NEJMpv1001787. [DOI] [PubMed] [Google Scholar]
- 13.MMWR Norovirus out-break among evacuees from Hurricane Katrina—Houston, TX, September 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1016–1018. [PubMed] [Google Scholar]
- 14.Uckay I, Sax H, Harbarth S, et al. Multi-resistant infections in repatriated patients after natural disasters: Lessons learned from the 2004 tsunami for hospital infection control. J Hosp Infect. 2008;68:1–8. doi: 10.1016/j.jhin.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Gupta SK, Suantio A, Gray A, et al. Factors associated with E. coli contamination of household drinking water among tsunami and earthquake survivors, Indonesia. Am J Trop Med Hyg. 2007;76:1158–1162. [PubMed] [Google Scholar]
- 16.Hiransuthikul N, Tantisiriwat W, Lertutsahakul K, et al. Skin and soft tissue infections among tsunami survivors in Southern Thailand. Clin Infect Dis. 2005;41:e93–e96. doi: 10.1086/497372. [DOI] [PubMed] [Google Scholar]
- 17.Vernon D, Banner W, Cantwell P, et al. Streptococcus pneumoniae bacteremia associated with near-drowning. Crit Care Med. 1990;18:1175–1176. doi: 10.1097/00003246-199010000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Ender P, Dolan M. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;25:896–907. doi: 10.1086/515532. [DOI] [PubMed] [Google Scholar]
- 19.Cholera DFA, Bengal DFA. New Horizons Diagnostic Inc.; [Accessed June 28, 2010]. Web site. Available at http://www.nhdiag.com/ [Google Scholar]
- 20.New Horizons Diagnostic Inc. Cholera 0139 SMART™ II [package insert] New Horizons Diagnostic Inc.; Maryland, USA: 2008. [Google Scholar]
- 21.New Horizons Diagnostic Inc. Cholera SMART™ II [package insert] New Horizons Diagnostic Inc.; Maryland, USA: 2004. [Google Scholar]
- 22.New Horizons Diagnostic Inc. SMART™ II Choler 01 Water Test [package insert] New Horizons Diagnostic Inc.; Maryland, USA: 2006. [Google Scholar]
- 23.SmarTest Diagnostics . Smart Q™ [package insert] SmarTest Diagnostics; Yavne, Israel: 2006. [Google Scholar]
- 24.Span Diagnostics Limited . Span Diagnostics Limited; Crystal VC. Gujarat, India: [Accessed June 28, 2010]. Available at http://www.span.co.in. [Google Scholar]
- 25.Louie RF, Kitano T, Brock TK, et al. Point-of-care testing for pandemic influenza and biothreats. Disaster Med Public Health Prep. 2009;3(Suppl 2):S193–S202. doi: 10.1097/DMP.0b013e3181be6dc4. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization [Accessed June 28, 2010];WHO recommendations on the use of rapid testing for influenza diagnosis. 2005 July; Available at http://www.who.int/csr/disease/avian_influenzalguidelines/rapid_testinglenlindex.html.
- 27.Louie RF, Sumner SL, Belcher S, et al. Thermal stress and point-of-care testing performance: Suitability of glucose test strips and blood gas cartridges for disaster response. Disaster Med Public Health Prep. 2009;3:13–17. doi: 10.1097/DMP.0b013e3181979a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kost GJ. Newdemics, public health, small-world networks, and point-of-care testing. Point Care. 2006;5:138–144. [Google Scholar]
- 29.Kost GJ, Kost L, Suwanyangyuen A, et al. Emergency cardiac biomarkers and point-of-care testing: Optimizing acute coronary syndrome care using small-world networks in rural settings. Point Care. 2010;9:53–64. doi: 10.1097/POC.0b013e3181d9d45c. [DOI] [PMC free article] [PubMed] [Google Scholar]