Summary
Signal transduction following binding of lipopolysaccharide (LPS) to Toll-like receptor 4 (TLR4) is an essential aspect of host innate immune responses to infection by Gram-negative pathogens. Here, we describe a novel molecular mechanism used by a prevalent human bacterial pathogen to evade and subvert the human innate immune system. We show that the oral pathogen, P. gingivalis, uses endogenous lipid A 1- and 4'-phosphatase activities to modify its LPS, creating immunologically silent, non-phosphorylated lipid A. This unique lipid A provides a highly effective mechanism employed by this bacterium to evade TLR4 sensing and to resist killing by cationic anti-microbial peptides. In addition, lipid A 1- phosphatase activity is suppressed by hemin, an important nutrient in the oral cavity. Specifically, P. gingivalis grown in the presence of high hemin produces lipid A that acts as a potent TLR4 antagonist. These results suggest that hemin-dependent regulation of lipid A 1-dephosphorylation can shift P. gingivalis lipid A activity from TLR4 evasive to TLR4 suppressive, potentially altering critical interactions between this bacterium, the local microbial community, and the host innate immune system.
Introduction
Interactions between commensal bacteria and corresponding host tissues are considered essential for the normal development and health of the host organism (Rakoff-Nahoum et al., 2004, Tlaskalova-Hogenova et al., 2004). However, certain chronic inflammatory diseases are the result of a change in the commensal community's composition and its relationship with the host. The commensal-host relationship involves environmentally-induced alterations in the microbial community's composition and the presentation of associated virulence factors. Imbalances in this relationship can alter the specific host innate immune response to bacteria, promoting the progression of disease (Liljemark et al., 1996, Pumbwe et al., 2007, Ramsey et al., 2009).
The oral microbial community found on the tooth and tooth root surface, commonly referred to as dental plaque, is the most highly characterized microbial consortium associated with the human host. Numerous studies have characterized the bacterial species found in dental plaque and found a significant difference in both the numbers and types of bacteria associated with either clinically healthy or diseased sites (Socransky et al., 1994). This has led to a hypothesis that attributes the characteristic tissue and bone destruction found in periodontitis to the type of microbial community residing in the gingival pocket that is in closest proximity to periodontal tissue (Socransky et al., 1994). The causative basis for periodontitis likely relates to an excessive host innate immune response to a unique bacterial consortium and the virulence factors produced by certain pathogenic members of this community (Page et al., 1997). More recently, it has been recognized that the innate host response to commensal bacteria found within dental plaque proximal to clinically healthy sites contributes to oral health (Roberts et al., 2002). Consistent with this idea, clinically healthy sites display a highly orchestrated expression of select innate host defense mediators (Kornman et al., 1997). Furthermore, an extensive body of evidence has shown that the innate host defense status of periodontal tissue is highly dependent upon the composition of the oral microbial consortium (Socransky et al., 1998).
P. gingivalis, a Gram-negative, anaerobic bacterium that is greatly enriched in diseased periodontal sites, expresses multiple virulence factors, and is the most widely known periopathogen (Yilmaz, 2008). Lipopolysaccharide (LPS), specifically, the lipid A portion, is a key Gram-negative bacterial component recognized by TLR4 of the host innate immune system (Beutler, 2000), and its recognition by TLR4 facilitates a robust pro-inflammatory response that promotes the removal of bacteria from protected host tissues (Munford et al., 2006). It is believed that P. gingivalis LPS, which displays weak agonism and strong antagonism at TLR4, is a critical virulence factor involved in altering responses of the host innate defense system, presumably due to both dephosphorylation and deacylation of its lipid A (Dixon et al., 2005). We previously suggested that hemin, a clinically relevant environmental nutrient, is involved in the transition of healthy sites to diseased sites, due to increased vascular ulceration and the release of blood into gingival tissues, and that its increased presence can alter the ability of P. gingivalis LPS to activate TLR4 (Al-Qutub et al., 2006, Reife et al., 2006). Bacteria grown in low hemin conditions produce a weak LPS agonist consisting of a mono-phosphorylated, penta-acylated lipid A (Chen et al., 2007, Reife et al., 2006), whereas in high hemin conditions, they produce a LPS antagonist bearing a mono-phosphorylated tetra-acylated lipid A structure (Al-Qutub et al., 2006, Chen et al., 2007, Coats et al., 2005, Darveau et al., 1995, Reife et al., 2006). Independent studies using synthetic penta-acylated (Sawada et al., 2007) and tetra-acylated (Zhang et al., 2008) P. gingivalis lipid A structures have confirmed our observations of weak TLR4 agonism and potent antagonism, respectively. P. gingivalis, similar to other Gram-negative bacteria, synthesizes lipid A that initially contains phosphate moieties at both the 1- and -4' position of the di-glucosamine backbone (Kumada et al., 1995, Raetz et al., 2007). The recent report by Rangarajan et. al. that P. gingivalis contains lipid A structures lacking phosphates (Rangarajan et al., 2008), and the observation by Wang et. al. that lipid A phosphate removal can facilitate lipid A deacylation (Wang et al., 2007) suggests that hemin-dependent lipid A phosphatases regulate the generation of non-phosphorylated, deacylated lipid A in P. gingivalis. We hypothesized that 1- and 4'-lipid A phosphatases determine the ability of P. gingivalis LPS to function as a weak agonist or a strong antagonist at TLR4 depending upon hemin conditions.
Results
P. gingivalis lipid A structure is modified by novel lipid A phosphatases
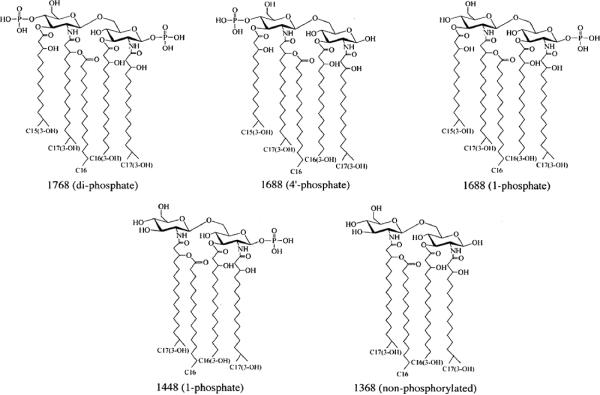
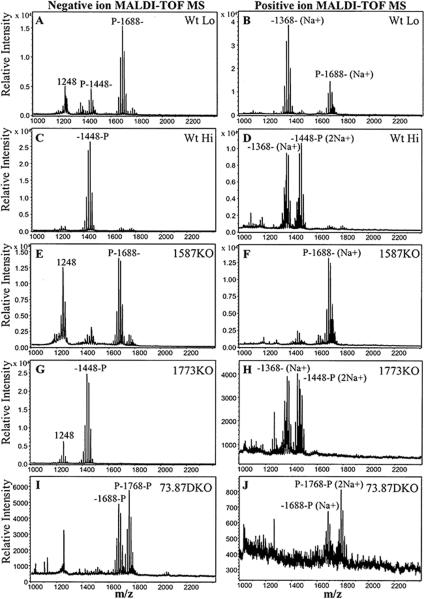
Initially, major lipid A structures that have been previously elucidated in P. gingivalis (Kumada et al., 1995)(Fig. 1) were examined in samples derived from wild-type bacteria (strain ATCC 33277) grown in low hemin conditions (1 μg ml−1) and high hemin conditions (10 μg ml−1) (denoted Wt Lo and Wt Hi, respectively). These lipid A isolates were analyzed by matrix-assisted laser desorption time-of-flight (MALDI-TOF) mass spectrometry (MS) in negative and positive ion modes in order to detect both the phosphorylated and non-phosphorylated lipid A structures (Fig. 2A–D). In addition, MALDI-TOF/TOF tandem MS analyses were performed to assign the position of phosphate groups in the observed major peaks of the resulting mass spectra (Figs. S1–S5). Negative ion MALDI-TOF MS analysis of lipid A derived from Wt Lo (Fig. 2A) confirmed our previous observations that Wt Lo P. gingivalis produce a major lipid A cluster centered around m/z 1688, bearing a 4'-phosphate (Fig. S1) (Al-Qutub et al., 2006). This lipid A species represents a significant portion of the total lipid A as judged by thin layer chromatography (TLC) analysis (Fig. S6). In addition, a lipid A cluster centered around m/z 1448 bearing a 4'-phosphate was observed in this preparation (Fig. S2). However, this lipid A is a minor species that is undetectable by TLC (Fig. S6). In contrast, Wt Hi bacteria (Fig. 2C) display a major cluster centered at m/z 1448, bearing a 1-phosphate (Fig. S4)(Al-Qutub et al., 2006) that represents a major lipid A species as judged by TLC analysis (Fig. S6). Previous analyses by others indicated that these lipid A structures represent mono-phosphorylated, penta-acylated lipid A (m/z 1688) and mono-phosphorylated, tetra-acylated lipid A (m/z 1448) structures, respectively, although both fatty acid and phosphate heterogeneity was found (Kumada et al., 1995). We have shown that the fatty acid heterogeneity manifests as lipid A structural “clusters” due to the relaxed acyl chain specificity found in P. gingivalis LpxA and LpxD lipid A acyltransferases (Bainbridge et al., 2008). When lipid A preparations from either Wt Lo or Wt Hi bacteria were examined in the positive ion mode, a major lipid A structure at m/z 1391, corresponding to the sodium adduct (a difference of 23 m/z units) of a non-phosphorylated, tetra-acylated lipid A species (m/z 1368) was observed (Fig. 2B and Fig. S3; Fig. 2D and Fig. S5). However, we were unable to determine the relative ratio of non-phosphorylated lipid A to phosphorylated lipid A in Wt P. gingivalis by TLC since the non-phosphorylated lipid A did not resolve into a discrete spot, and it appeared to migrate with the solvent used to develop the phosphorylated lipid A species (Fig. S6). These data support the conclusion that both Wt Lo and Wt Hi P. gingivalis produce non-phosphorylated, tetra-acylated lipid A (m/z 1368). Therefore, both lipid A 1- and 4'- phosphatases are active in P. gingivalis at both low and high hemin conditions. Furthermore, positive ion MALDI-TOF MS analyses suggest that the major tetra-acylated lipid A structure observed in Wt Lo bacteria is non-phosphorylated (Fig. 2B) while the preparations obtained from Wt Hi bacteria are enriched for tetra-acylated lipid A bearing a 1-phosphate group (Fig. 2D). These combined data suggest that lipid A 1-phosphatase activity is suppressed in Wt Hi P. gingivalis, resulting in the accumulation of both di-phosphorylated, penta-acylated lipid A and mono-phosphorylated lipid A structures (Fig. S6).
Fig. 1.
The Major lipid A structures examined by MALDI-TOF MS and MALDI-TOF tandem MS in this study have been identified in P. gingivalis as previously described (Kumada et al., 1995).
Fig. 2.
P. gingivalis expresses both lipid A 4'- and 1-phosphatase activities. Lipid A isolated from wild-type P. gingivalis 33277 or mutant bacteria bearing deletions in PG1587 and PG1773 gene loci were examined by MALDI-TOF MS and MALDI-TOF/TOF tandem MS (Figs. S1–S9) to elucidate the position of the lipid A phosphates. Phosphate positions are designated as `P' at the left (4'-position) or the right (1-position) of the representative lipid A mass ion for each cluster in the MALDI-TOF mass spectra. Lipid A samples were examined in the negative ion mode (A, C, E, G, and I) or the positive ion mode (B, D, F, H, and J). (A and B) Wild-type P. gingivalis grown in (1 μg ml−1) hemin (Wt Lo). (C and D) Wild-type P. gingivalis grown in 10 μg ml−1 hemin (Wt Hi). (E and F) PG1587-deficient (1587KO) bacteria grown in (1 μg ml−1) hemin (G and H) PG1773-deficient (1773KO) bacteria grown in (1 μg ml−1) hemin. (I and J) PG1587/PG1773-deficient (73.87DKO) bacteria grown in (1 μg ml−1) hemin.
To assess the contribution of lipid A phosphatases to hemin-induced remodeling of P. gingivalis lipid A structures, we engineered mutant strains with deletions of putative lipid A 1- and 4'-phosphatase coding sequences and examined the structural characteristics of their lipid A. The candidate lipid A phosphatases were identified by performing a BLAST search that compared the amino acid sequence of F. novicida LpxE, a lipid A 1-phosphatase (Wang et al., 2004), against the P. gingivalis (strain W83) proteome (J. Craig Venter Institute). This search identified PG1587 and PG1773 as the highest scoring subjects, yielding E values of 0.00059 and 0.013 respectively, and indicating that these hypothetical proteins belong to the PAP2 phosphatase superfamily which includes known lipid A phosphatases (Carman et al., 2006). Subsequently, amino acid sequence alignments of PG1773 and PG1587 against the amino acid sequences of F. novicida LpxE (lipid A 1-phosphatase) (Wang et al., 2004) and LpxF (lipid A 4'-phosphatase) (Wang et al., 2006)were performed (Fig. S7). Based upon these alignments, PG1587 displayed more identity to LpxF than to LpxE (13.5% vs. 10% respectively). Conversely, the amino-terminal half of PG1773 exhibited a higher degree of identity to LpxE than to LpxF (16.5% vs. 13.1% respectively). The carboxy-terminal half of PG1773 did not align with either of these lipid A phosphatases and it appears to be a novel protein domain that is evolutionarily restricted to the genus, Porphyromonas, as determined by BLAST searches (data not shown).
Consistent with lipid A 1-phosphatase activity being suppressed in high hemin, the lipid A structural profile of P. gingivalis deficient in the putative lipid A 1-phosphatase, PG1773 (1773KO), resembled the profile obtained from Wt Hi bacteria in MALDI-TOF MS analyses (Compare Fig. 2G and H to C and D) independent of the hemin concentration in the growth media (Fig. S8 E–H). The 1773KO strain exhibited growth characteristics characteristic of Wt bacteria grown on TYHK-agar plates or in liquid broth media. Notably, the lipid A profile of 1773KO was identical to Wt Hi lipid A as determined by TLC (Fig. S6), displaying increased accumulation of both di-phosphorylated, penta-acylated lipid A, and mono-phosphorylated, tetra-acylated lipid A as compared to lipid A expressed in Wt Lo bacteria. In addition, both the tetra-acylated, non-phosphorylated (m/z 1368) (Fig. 2H), and the tetra-acylated, 1-phosphate lipid A structures (m/z 1448) (Fig. 2G and Fig. S9) predominated in this mutant strain as determined by MALDI-TOF MS. These data indicate that the function of PG1773 is essential for the efficient removal of the 1-phosphate from lipid A from tetra-acylated lipid A, as is observed for Wt Lo P. gingivalis. However, the persistence of tetra-acylated lipid A structures lacking both the 1- and 4'-phosphates in the 1773KO strain and Wt Hi bacteria indicates that P. gingivalis harbors an additional, unidentified phosphatase(s) capable of removing the 1-phosphate of lipid A. Further examination of the BLAST search performed using the F. novicida LpxE amino acid sequence against the P. gingivalis proteome revealed that the locus, PG1738, encodes a third potential PAP2 domain-containing phosphatase (Expect = 0.020). However, a strain bearing a deletion of this genetic locus was still able to modulate its lipid A structure in response to hemin, similar to Wt P. gingivalis (data not shown). In addition, a strain bearing deletions for both the PG1773 and PG1738 loci exhibited a lipid A profile that was identical to the 1773KO strain (i. e. they still display prominent tetra-acylated lipid A clusters that are de-phosphorylated at both the 1- and 4'-positions). We conclude that PG1738 does not encode the additional lipid A 1-phosphatase activity. Instead, it is possible that P. gingivalis contains a novel lipid A 1-phosphatase that was non-detectable by our BLAST search strategy. Alternatively, the putative lipid A phosphatase encoded by PG1587, which is still present in the PG1738-PG1773 double knockout, is capable of removing both the 1- and 4'-phosphates of lipid A.
Bacteria deficient for the putative lipid A 4'-phosphatase, PG1587(1587KO), were unable to alter their lipid A structure (Fig. 2E and F) in response to hemin (Fig. S8 A–D) as revealed by negative and positive ion mode MALDI-TOF MS. Importantly, the tetra-acylated lipid A 1-phosphate structure (m/z 1448) was essentially eliminated in this strain, even when it was grown in high hemin (Fig. S8 C and D). This strain also exhibited viability that was similar to Wt bacteria when grown on TYHK-agar plates. However, it achieved a higher optical density (measured at 600 nm) more rapidly than other strains when cultured in liquid TYHK media (data not shown). Lipid A isolated from the 1587KO bacteria demonstrated a major peak cluster corresponding to penta-acylated 4'-phosphate lipid A (m/z 1688) (Fig. S10), the characteristic structure observed in the negative ion mode for Wt Lo P. gingivalis. TLC anlayses of total lipid A confirmed that the mono-phosphorylated, penta-acylated lipid A is a major component of the 1587KO strain (Fig. S6). However, in contrast to Wt Lo bacteria, the tetra-acylated, non-phosphorylated lipid A (m/z 1368) was entirely absent from the 1587KO strain as determined by positive ion MALDI-TOF MS (Fig. 2F). These data indicate that the removal of the 4'-phosphate from penta-acylated lipid A precursors by the putative phosphatase, PG1587, is required for efficient production of both the non-phosphorylated lipid A (m/z 1368), and the tetra-acylated lipid A 1-phosphate species (m/z 1448) that is observed in either low or high hemin conditions respectively. Presumably, lipid A 4'-phosphate removal from the penta-acylated, bis-phosphate lipid A generates an optimal substrate for lipid A 3'-O- deacylation (Wang et al., 2007). In consideration of this putative requirement, detection of the minor mono-phosphorylated, tetra-acylated lipid A 4'-phosphate (Fig. 2A and Fig. S2) suggests that at least two lipid A deacylase activities having distinct substrate specificities exist in P. gingivalis.
A P. gingivalis double mutant, lacking both PG1587 and PG1773 (73.87DKO) gene loci exhibited a unique lipid A structural profile consistent with the combined inactivation of endogenous lipid A 4'- and 1- phosphatase activities (Fig. 2I and J). The mass spectra displayed di-phosphorylated, penta-acylated lipid A ions (m/z 1768) including both bis-phosphate and 1-pyrophosphate linkages (Jones et al., 2008) (Fig. S11), as well as mono-phosphorylated, penta-acylated lipid A bearing a 1-phosphate (m/z 1688) (Fig. S12). The di-phosporylated, penta-acylated lipid A (m/z 1768) represents a major component of total lipid A in this strain as determined by TLC, whereas the mono-phosphorylated, tetra-acylated lipid A is undetectable (Fig. S6). Similar to the 1587KO strain, neither the non-phosphorylated, nor the mono-phosphorylated, tetra-acylated lipid A was observed in 73.87DKO LPS isolates as determined by MALDI-TOF MS (Fig. 2I and J). The absence of penta-acylated lipid A lacking the 1-phosphate in the 73.87DKO strain is intriguing since the accumulation of tetra-acylated lipid A lacking the 1-phosphate in 1773KO indicates that P. gingivalis harbors additional unidentified lipid A 1-phosphatase activity (see above). At least two explanations can reconcile this apparent discrepancy in the lipid A profile that we observed for the 73.87 DKO mutant. Firstly, the postulated, additional lipid A 1-phosphatase activity might be specific for a tetra-acylated lipid A substrate as opposed to a penta-acylated substrate. The tetra-acylated substrate fails to accumulate in 73.87DKO due to the loss of the PG1587 4'-phosphatase activity, since as noted earlier, this activity is necessary for efficient 3'-O-deacylation of the penta-acylated lipid A precursor. Secondly (but not mutually exclusive to the first hypothesis), as suggested above, the PG1587 protein might be capable of de-phosphorylating both the 1- and 4'-positions of lipid A in P. gingivalis. In this scenario, one would predict that in the absence of both the PG1773 and PG1587 gene products, lipid A 1-phosphatase activity is completely eliminated, as is observed for the mutant strain,73.87DKO by MALDI-TOF MS.
The detection of penta-acylated lipid A lacking the 4'-phosphate (m/z 1688) in73.87DKO indicates that P. gingivalis encodes an additional lipid phosphatase(s) capable of removing the 4'-phosphate group from the di-phosphorylated, penta-acylated lipid A precursor (m/z 1768), in the absence of both PG1587 and PG1773 activities. We observed that a strain deficient for both the PG1587 and PG1738 loci accumulated mono-phosphorylated penta-acylated lipid A (m/z 1688) bearing a 4'-phosphate rather than a 1-phosphate (data not shown). These data provide strong evidence that the putative lipid phosphatase, PG1738, accounts for the additional 4'-phosphate removal from the penta-acylated lipid A precursor (m/z 1768) that is observed in the 73.87DKO strain. However, the failure of the penta-acylated 1-phosphate lipid A to undergo 3'-O-deacylation following 4'-dephosphorylation by PG1738 in 73.87DKO (Fig. 2I and J) is paradoxical given the apparent necessity for 4'-dephosphorylation by PG1587 preceding 3'-O-deacylation (Fig. 2E and F). This discrepancy can be explained if the removal of the lipid A 4'-phosphate by PG1738 occurs in a sub-cellular compartment of the bacterium that is inaccessible to the lipid A 3'-O-deacylase activity.
Non-Phosphorylated Lipid A Determines the Reduced Ability of P. gingivalis and Its LPS to Activate the Host Innate Immune System via TLR4
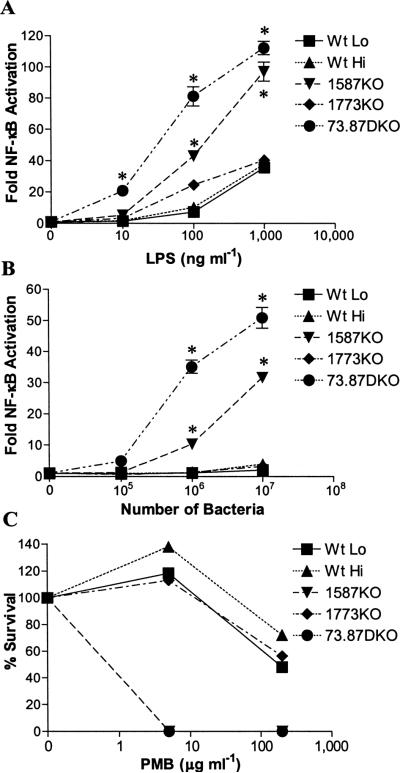
We determined the contribution of different lipid A structures upon TLR4 activation by examining the abilities of LPS preparations or intact P. gingivalis to stimulate NF-κB activation in HEK 293 cells expressing recombinant human TLR4 and MD-2 (Fig. 3A and B). Specifically, we were interested in determining if the non-phosphorylated lipid A (m/z 1368), found in wild-type P. gingivalis LPS preparations, contributed to poor TLR4 activation. We noted that the 1587KO mutant contains a lipid A agonist (m/z 1688) similar to Wt Lo bacteria with the important exception that it lacks non-phosphorylated lipid A (m/z 1368) (compare Fig. 2A and B to E and F). Examination of TLR4 activation in HEK 293 cells treated with isolated LPS (Fig. 3A) or intact bacteria (Fig. 3B) revealed that 1587KO bacteria and LPS were significantly more potent TLR4 agonists as compared to Wt Lo bacteria and LPS. Moreover, P. gingivalis 73.87DKO bacteria and LPS exhibited the most potent ability to activate TLR4 (Fig. 3A and B). This mutant strain lacks non-phosphorylated lipid A and is enriched in the di-phosphorylated, penta-acylated lipid A (m/z 1768), (See Fig. 2I and J). These results agree with the potent TLR4 activity of synthetic lipid A (m/z 1768) (Kumada et al., 2008), and reveal that the ability of intact P. gingivalis to activate TLR4 in HEK 293 cells correlates well with the observed potency of its isolated LPS. Similar results were obtained from experiments conducted using primary human endothelial cells which respond to bacterial LPS by activation of TLR4-dependent E-selectin expression (Fig. S13). Taken together, these data establish that the non-phosphorylated, deacylated lipid A produced by this bacterium dramatically reduces the potency of P. gingivalis and its LPS at human TLR4, promoting a robust evasion of the host innate immune system. These results also demonstrate that the lipid A phosphatase, PG1587, is required to generate non-phosphorylated lipid A, allowing P. gingivalis to resemble Gram-positive oral commensal bacteria that fail to alert the innate host defense system through TLR4 (Dixon, 2005).
Fig. 3.
Lipid A phosphatases control the ability of P. gingivalis to evade host innate immune defenses. P. gingivalis evades TLR4 sensing through its LPS. HEK293 cells expressing human TLR4 and MD-2 were exposed to the indicated doses of LPS (A), or whole bacteria (B), for 4 hours. The fold activation of NF-κB over the media control was determined by measuring inducible Fire-fly luciferase activity. The results shown are means ±SD of triplicate samples from one of three independent experiments. Asterisks indicate statistically significant differences (P < 0.001; two-tailed unpaired t tests) in the potency of 1587KO LPS and 73.87DKO LPS relative to Wt LPS or the potency of 1587KO bacteria and 73.87DKO bacteria relative to Wt bacteria. (C) Lipid A phosphatases confer P. gingivalis resistance to killing by antimicrobial cationic peptides. The indicated strains of P. gingivalis were plated on TYHK-agar plates containing polymyxin B (PMB) (0, 5, and 200 μg ml−1) and viable colonies were counted after 8–10 days of growth in an anaerobic chamber. The percent survival for each strain was based upon the ratio of the number of counted colonies from the experimental plates containing PMB to the number of counted colonies from the control plate that did not contain PMB. The results shown are representative of two independent determinations. Similar results were obtained using multiple determinations of a liquid culture PMB sensitivity assay (data not shown).
Phosphatase-dependent production of non-phosphorylated lipid A provides a potent resistance mechanism to cationic anti-microbial peptides
Modification of lipid A phosphate groups can alter susceptibility of pathogenic Gram-negative bacteria such as Helicobacter pylori, Francisella novicida, and Salmonella enterica to the killing activity of cationic antimicrobial peptides (Kawasaki et al., 2007, Tran et al., 2006, Wang et al., 2007). Therefore, we examined the contribution of non-phosphorylated lipid A in P. gingivalis to the cationic anti-microbial peptide, polymyxin B (PMB), susceptibility (Fig. 3C). We observed that 1587KO and 73.87DKO mutant strains of P. gingivalis, lacking both non-phosphorylated or tetra-acylated lipid A structures, were highly susceptible to PMB killing, and were completely eliminated at low concentrations (5 μg ml−1) of the drug. In contrast, the Wt P. gingivalis and the 1773KO mutant strains were strikingly resistant to high doses of PMB (200 μg ml−1). These data demonstrate that the expression of PG1587, in addition to facilitating evasion of the host TLR4 response by P. gingivalis, performs a key role in determining the resistance of this organism to host cationic antimicrobial peptides via the regulation of lipid A dephosphorylation and deacylation.
Increased hemin induces the production of a TLR4 antagonist
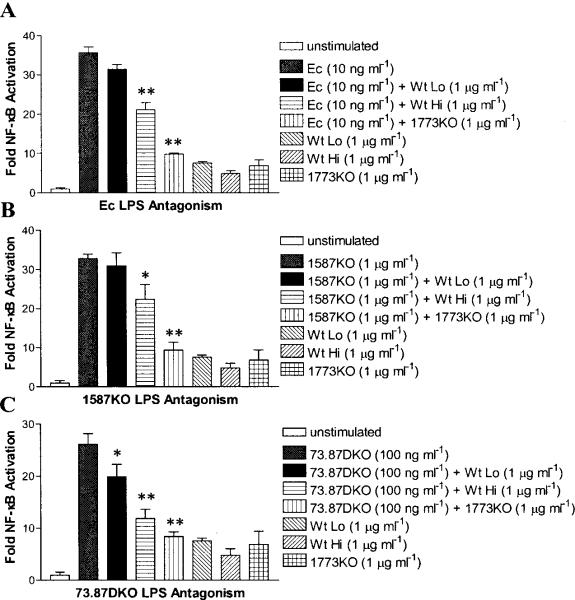
Hemin-dependent suppression of lipid A 1- dephosphorylation is required to generate the TLR4 lipid A antagonist (m/z 1448) (Fig. 2C and D) (Fig. S6). In support of this concept, when the 1-phosphate moiety of tetra-acylated 1ipid A is retained, as in 1773KO bacteria, the lipid A antagonist (m/z 1448) accumulates due to the loss of lipid A 1-phosphatase expression (Fig. 2G and H) (Fig. S6). The effect of hemin-dependent lipid A 1-phosphatase regulation can be seen in Fig. 4, where LPS obtained from P. gingivalis grown in high hemin significantly increases functional TLR4 antagonism, as compared with LPS isolated from bacteria grown in low hemin. Specifically, Wt Hi LPS, which is enriched for the lipid A antagonist (m/z 1448) relative to Wt Lo LPS (Fig. S6), was significantly more potent than Wt Lo LPS in antagonizing Escherichia coli LPS (Fig. 4A), 1587KO LPS (Fig. 4B), and 73.87DKO LPS (Fig. 4C). In agreement with these data, LPS isolated from the 1773KO mutant, which is also enriched for the lipid A antagonist (Fig. S6), was the most potent inhibitor of TLR4 activation in response to the different TLR4 agonists (Fig. 4A–C).
Fig. 4.
Hemin-dependent control of lipid A phosphatase modulates the ability of P. gingivalis LPS to suppress host TLR4 activation. HEK293 cells expressing human TLR4 and MD-2 and harboring a NF-κB-luciferase reporter were exposed to the indicated doses of LPS for 4 hours, and TLR4-dependent NF-κB activation was determined by Fire-fly luciferase assay. The relative abilities of Wt Lo LPS, Wt Hi LPS, and 1773KO LPS to antagonize (A) Ec LPS-dependent, (B) 1587KO LPS-dependent, or (C) 73.87DKO LPS-dependent activation of TLR4. The results shown are means ±SD of triplicate samples from one of three independent experiments. Asterisks indicate significant differences (* P < 0.05; ** P < 0.01; two-tailed unpaired t tests) for the level of TLR4 activation achieved for LPS agonist alone vs. the level achieved for LPS agonist combined with either Wt Hi LPS or 1773KO LPS.
Discussion
In this study, we performed a combined application of both negative and positive ion mode MALDI-TOF MS to analyze various forms of lipid A isolated from the Gram-negative pathogen, P. gingivalis. The inclusion of positive ion mode MALDI-TOF MS in this report represents a novel strategy that permitted the efficient detection of non-phosphorylated lipid A that is not readily detectable in samples examined in the standard negative ion mode MALDI-TOF MS. To the best of our knowledge, this approach has not been extensively utilized to examine lipid A structures present in important human pathogenic bacterial species, and it suggests that important details concerning the lipid A characteristics of many Gram-negative pathogens needs to be re-examined. The results presented in this study also demonstrate that P. gingivalis uses novel lipid A 1- and 4'- phosphatases to generate a unique non-phosphorylated lipid A. This lipid A is strikingly inert and provides a succinct mechanism to evade TLR4 activation and to resist killing by cationic anti-microbial peptides. Although direct quantitative evidence regarding the relative amounts of non-phosphorylated lipid A and phosphorylated lipid A structures was not obtained in this study, cummulative evidence in the form of structural and functional data presented here strongly suggest that the silent, non-phosphorylated lipid A (m/z 1368) contributes significantly to the characteristic low potency of the lipid A derived from P. gingivalis grown in low hemin conditions. This conclusion is based upon the failure of both Wt Lo LPS and bacteria (which contain both the penta-acylated lipid A (m/z 1688) and non-phosphorylated lipid A (m/z 1368) to activate TLR4, as well as the pronounced abiltity of these bacteria to resist PMB killing. In contrast, the 1587KO mutant, which displays the m/z 1688 ion but does not display detectable m/z 1368 ion, is a relatively potent activator of TLR4 and is extremely sensitive to PMB killing. We are currently developing procedures aimed at determining the relative quantities of non-phosphorylated and phosphorylated lipid A structures in P. gingivalis to resolve this important issue.
In addition to P. gingivalis employing non-phosphorylated lipid A to evade host TLR4 activation, we observed that hemin-dependent reduction of the lipid A 1-phosphatase activity, PG1773, leads to the production of a mono-phosphorylated lipid A antagonist capable of suppressing TLR4 responses. It is possible that high hemin suppresses PG1773 activity via a post-transcriptional mechanism since increases in hemin lead to increased rather than decreased PG1773 mRNA expression (data not shown). Since P. gingivalis has multiple mechanisms for hemin binding and uptake into the periplasmic and cytoplasmic compartments (Olczak et al., 2005), hemin could transduce conformational changes in PG1773 via a hemin receptor (eg. HmuR). Alternatively, post-translational modification of PG1773 (eg. phosphorylation) by a hemin-regulated kinase might regulate its function. In either case, the novel carboxy-terminal domain of PG1773 provides a potential regulatory target for mediating the hemin-dependent functions of this phosphatase.
With regard to the biological significance of hemin-dependent lipid A modification in P. gingivalis, locally available hemin, in the form of hemoglobin, is known to significantly increase in periodontal tissue during infection and correlates with the progression of disease and inflammation (Hanioka et al., 1990, Hanioka et al., 1991). A number of potential virulence factors including LPS, gingipains (Takii et al., 2005)and fimbriae (Hajishengallis et al., 2008) have been implicated in the mechanism used by P. gingivalis to evade the host innate immune system mechanism. We suggest that increased environmental hemin, contributes to shifting P. gingivalis interaction from an evasive, commensal state towards a pathologic, pro-inflammatory state with the host. We hypothesize that this is in part achieved by altering the relative amounts of LPS bearing silent, non-phosphorylated lipid A (m/z 1368) to LPS bearing antagonistic, mono-phosphorylated lipid A (m/z 1448), via hemin-dependent suppression of the lipid A 1-phosphatase activity, PG1773 (Fig. 5). LPS enriched in antagonistic lipid A content can suppress TLR4 activation in response to LPS derived from multiple bacteria (Darveau et al., 1995) through competitive binding to MD-2 (Coats et al., 2007, Coats et al., 2005). Also, the lipid A antagonist exhibited the most potent activity against the `oral bacterium-type' LPS consisting of di-phosphorylated lipid A bearing long chain fatty acids. In contrast, it was less potent in its ability to inhibit a pro-inflammatory `enteric bacterium-type' LPS bearing short chain fatty acids. Since P. gingivalis releases LPS that can penetrate gingival tissue (Schwartz et al., 1972), the lipid A antagonist can potentially dampen TLR4 responses for the entire oral microbial community. This concept is especially relevant to the concept that TLR4 sensing prevents invasion into sub-mucosal tissue by mucosal Gram-negative bacteria (Munford et al., 2006). Therefore, P. gingivalis may represent a member of periopathogenic dental plaque that can inhibit TLR4 responses to other microbial members of the local community.
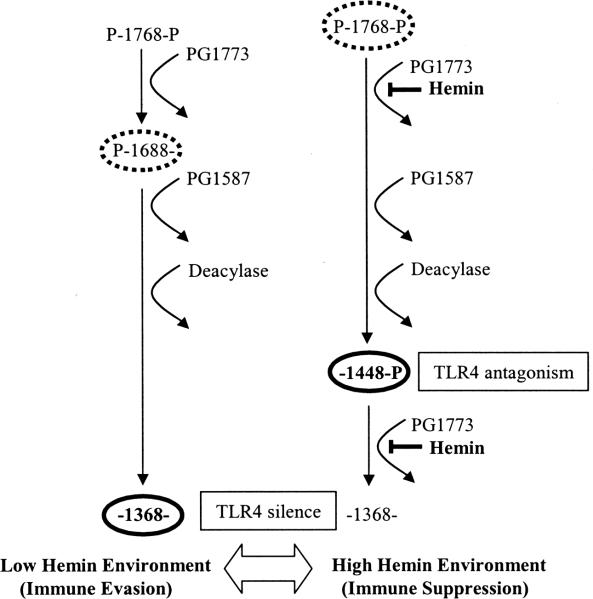
Fig. 5.
Model for modulation of TLR4 responses via the regulation of lipid A phosphatase activity in P. gingivalis. In a low hemin environment (left), penta-acylated, di-phosphorylated lipid A is sequentially processed by lipid A 1-phosphatase, lipid A 4'-phosphatase, and deacylase activities producing LPS bearing a non-phosphorylated lipid A (bold-type oval) that is inactive at TLR4. We propose that in a high hemin environment (right), lipid A 1-phosphatase activity is suppressed leading to the accumulation of a TLR4 antagonist (bold-type oval). Although penta-acylated, mono- and di-phosphorylated lipid A structures (TLR4 agonists) are detectable by MALDI-TOF MS and TLC (stippled-type ovals, left and right), LPS isolates from wild-type bacteria grown in either high or low hemin fail to strongly activate TLR4. The relative amounts of non-phosphorylated and phosphorylated lipid A structures in P. gingivalis are presently unknown. However, the empirical data presented in this report strongly suggest that silent, non-phosphorylated lipid A (low hemin) and mono-phosphorylated tetra-acylated lipid A (high hemin) exert functional dominance over the lipid A agonists present in either the Wt Lo or Wt Hi bacteria respectively.
In a broader context of host-pathogen interactions, lipid A 1- and 4'-phosphatase-like activities are found in a variety of Gram-negative bacteria (Karbarz et al., 2003, Price et al., 1995, Tran et al., 2004, Wang et al., 2006). In addition, pathogens such as Bordetella parapertussis, F. novicida, and H. pylori are known to produce immunologically silent or antagonistic LPS (Hajjar et al., 2006, Mann et al., 2005, Pece et al., 1997). Similarly to P. gingivalis, it is possible that lipid A phosphatases in these bacteria are regulated by their respective local microenvironmental conditions. Regulation of phosphatases by microenvironmental factors, such as hemin, or other local nutrients might control the balance between lipid A structures that fail to activate TLR4 or function as agonists or antagonists at TLR4. It will be necessary to re-examine the lipid A from known pathogens to determine if this paradigm is applicable to these bacteria. If this model represents a widely distrubuted biological strategy, then it should be useful to design anti-microbial drugs with increased hydrophobic features to overcome the pronounced non-polar characteristics of the cell walls from Gram-negative pathogens that employ this type of evasion strategy.
Experimental procedures
Bacterial growth conditions
P. gingivalis strain ATCC 33277 was obtained from our stock collection. Bacteria were grown in TYHK medium consisting of trypticase soy broth (30 g l−1) (Becton Dickinson, Sparks, MD), yeast extract 5 g l−1 (Becton Dickinson, Sparks, MD), and vitamin K3 (menadione) (Sigma-Aldrich, St. Louis, MO). The basal TYHK medium was sterilized by autoclaving, followed by the addition of filter-sterilized hemin (Sigma-Aldrich, St. Louis, MO) to the desired final concentration of either (1 μg ml−1) or (10 μg ml−1) as indicated in the text and figure legends. Cultures were grown in an anaerobic growth chamber (5% H2, 5% CO2, 90% N2) and maintained at 37°C on TYHK-agar plates.
Gene Deletions in P. gingivalis 33277
The genomic nucleotide sequences encoding the putative lipid A 1-phosphatase, PG1773, and the putative lipid A 4'-phosphatase, PG1587, were obtained from searches of the annotated P. gingivalis W83 genome at The Comprehensive Microbial Resource (http://cmr.jcvi.org/tigr-scripts/CMR/CmrHomePage.cgi). Gene deletions were created by introducing either a tetracycline resistance cassette (tetQ) in place of the coding region for PG1773 or an erythromycin resistance cassette (ermF/AM) in place of the coding region for PG1587. Polymerase chain reaction (PCR) amplification of genomic DNA from P. gingivalis 33277 was performed using primer sets designed against the W83 sequence to amplify 1000 base-pairs upstream and 1000 base-pairs downstream from the regions adjacent to the PG1773 and PG1587 coding regions respectively. The amplified 5' and 3' flanking regions for PG1773 and PG1587 respectively, were co-ligated with the tetQ and ermF/AM cassettes respectively into pcDNA3.1(−) to generate the gene disruption plasmids, p1773 5'flank:tetQ:3'flank and p1587 5'flank:erm:3'flank. P. gingivalis 33277 deficient in either PG1587 (1587KO) or PG1773 (1773KO) was generated by introducing either p1587 5'flank:erm:3'flank or p1773 5'flank:tetQ:3'flank into P. gingivalis 33277 by electroporation in a GenePulser Xcell (BioRad, Hercules, CA). Bacteria were plated on TYHK/agar plates containing the appropriate selective medium which included either erythromycin (5 μg ml−1) or tetracycline (1 μg ml−1) and incubated anaerobically. One week later, colonies were selected for characterization. Loss of the PG1587 and PG1773 coding sequences were confirmed in all clones by PCR analyses using primers designed to detect the coding sequences. The doubly deficient strain, 73.87DKO, was generated by introducing p1587 5'flank:erm:3'flank into 1773KO by electroporation, and processing as described above.
Isolation of LPS and Lipid A
Bacteria were cultured for 48 hours in TYHK medium containing hemin at a concentration of either (1 μg ml−1) or (10 μg ml−1). LPS was isolated using a modified version of the Tri-reagent protocol for LPS isolation as previously described (Al-Qutub et al., 2006). To generate lipid A, dried LPS samples were resuspended in 10 mM sodium acetate [pH 4.5] containing 1% sodium dodecyl sulfate (w/v). The solution was heated 100°C for 1 hour followed by lyophilization overnight. The resulting lipid A pellets washed once in ice-cold 95% ethanol containing 0.02 N HCl, three times in 95% ethanol, followed by a final extraction with 1160 μl of chloroform-methanol-water (1:1:0.9, v:v:v) to remove residual carbohydrate contaminants. The chloroform layer containing the lipid A was dried and used for MALDI-TOF MS or MALDI-TOF/TOF tandem MS analyses.
MALDI-TOF MS Analyses
For MALDI-TOF MS analyses, lipid A samples were dissolved in 10 μl of a mixture of 5-chloro-2-mercaptobenzothiazole (20 mg ml−1) in chloroform/methanol 1:1 (v/v) and 0.5 μl of each sample was analyzed in both positive and negative ion modes on an AutoFlex Analyzer (Bruker Daltonics). Data were acquired with a 50 Hz repletion rate and up to 3000 shots were accumulated for each spectrum. Instrument calibration and all other tuning parameters were optimized using HP Calmix (Sigma-Aldrich, St. Louis, MO). Data was acquired and processed using flexAnalysis software (Bruker Daltonics).
MALDI-TOF/TOF Tandem MS Analyses
For MALDI-TOF/TOF tandem MS analyses, lipid A was analyzed by a MALDI-TOF mass spectrometer in the positive and negative ion modes on a 4700 Proteomics Analyzer (Applied Biosystems, Framingham, MA). Samples were dissolved in 10 μl of a mixture of 5-chloro-2-mercaptobenzothiazole (20 mg ml−1) in chloroform/methanol/water 4:4:1 (v/v/v), and 0.5 μl of sample was analyzed by MALDI-TOF MS and MALDI-TOF/TOF tandem MS. Both MALDI-TOF MS and MALDI-TOF/TOF tandem MS data were acquired in reflectron mode with a Nd:YAG laser with 200 Hz repetition rate and up to 3750 shots were accumulated for each spectrum. The precursor isolation window was set to +/− 5 Da. MALDI-TOF/TOF tandem mass spectra were acquired with collision energies of 1 keV and air was used as the collision gas. Instrument calibration and all other tuning parameters were optimized using HP Calmix (Sigma-Aldrich, St. Louis, MO). Data was acquired and processed using Data Explorer (Applied Biosystems, Framingham, MA).
Thin layer chromotography (TLC) analyses
Lipid A samples derived from 200 ml cultures of P. gingivalis strains (Wt Lo, Wt Hi, 1587KO Lo, 1773KO Lo, and 73.87DKO Lo) were suspended in chloroform and spotted on a TLC plate (Silica Gel 60, EMD Biosciences). The samples were developed using a solvent system consisting of chloroform, pyridine, 88% formic acid, and H2O (50:50:16:5, v/v). After air drying, the plate was sprayed with H2O to visualize lipid spots. The major spots (indicated in Fig. S6) were scraped and eluted from the silica using a mixture of chlorform and methanol (50:50, v/v). The recovered lipid A species were subsequently identified by MALDI-TOF MS.
HEK293 TLR4 Activation Assays
HEK293 cells were plated in 96-well plates at a density of 4 × 104 cells per well and transfected the following day with plasmids bearing firefly luciferase, Renilla luciferase, human TLR4 and MD-2 by a standard calcium phosphate precipitation method as described previously (Coats et al., 2005). The test wells were stimulated in triplicate for 4 hours at 37°C with the indicated doses of LPS or intact bacteria that had been suspended by vortexing in DMEM containing 10% human serum. Following stimulation, the HEK293 cells were rinsed with phosphate-buffered saline and lysed with 50 μl of passive lysis buffer (Promega, Madison, WI). Luciferase activity was measured using the Dual Luciferase Assay Reporter System (Promega, Madison, WI). Data are expressed as fold increase of NF-κB-activity which represents the ratio of NF-κB-dependent fire-fly luciferase activity to β-Actin promoter-dependent Renilla luciferase activity.
HUVEC E-selectin assay
Primary human umbilical endothelial cells (passage 3–5) (Clonetics, San Diego, CA) were plated in a 96-well plate format. Endothelial cells were stimulated (in triplicate) for 4 hours at 37°C with either isolated LPS or intact cells (as indicated in the figure legend), that had been mixed by vortexing in cell stimulation medium containing 5% human serum. Endothelial cell E-selectin expression was detected by a previously described ELISA protocol (Reife et al., 2006) and data were plotted with GraphPad Prism. Data sets were expressed as relative E-selectin expression units for comparison.
Polymyxin B Sensitivity Assays
Overnight cultures of Wt P. gingivalis 33277 and its isogenic putative phosphatase mutants were grown in THYK media containing hemin (1 μg ml−1) or (10 μg ml−1) as indicated in the text. Liquid cultures that had been grown for 24 hours in an anaerobic growth chamber were diluted to a starting optical density (OD600) of 1.0, which represents approximately 1 × 109 cfu/ml for all of the strains examined (data not shown). Subsequently, 10−3 to 10−8 dilutions of each strain were plated on TYHK-agar plates containing PMB (0, 5, and 200 μg ml−1). After 8–10 days of incubation in an anaerobic chamber, the resulting colonies were counted to determine the viability at the different PMB treatments. The results for each strain were plotted as percent survival which was derived from the ratio of the number of colonies detected on the experimental plates containing polymyxin B (5 or 200 μg ml−1) to the number of colonies detected on the control plates that did not contain polymyxin B.
Statistical Analyses
Data were analyzed by two-tailed unpaired t tests (GraphPad Prism) where indicated. P < 0.05 was considered indicative of statistical significance.
Supplementary Material
Acknowledgements
This work was supported by NIH grant DE12768 (R.P.D).
References
- Al-Qutub MN, Braham PH, Karimi-Naser LM, Liu X, Genco CA, Darveau RP. Hemin-Dependent Modulation of the Lipid A Structure of Porphyromonas gingivalis Lipopolysaccharide. Infect Immun. 2006;74:4474–4485. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge BW, Karimi-Naser L, Reife R, Blethen F, Ernst RK, Darveau RP. Acyl chain specificity of the acyltransferases LpxA and LpxD and substrate availability contribute to lipid A fatty acid heterogeneity in Porphyromonas gingivalis. J Bacteriol. 2008;190:4549–4558. doi: 10.1128/JB.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci. 2006;31:694–699. doi: 10.1016/j.tibs.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Coats SR, Bumgarner RE, Darveau RP. Hierarchical gene expression profiles of HUVEC stimulated by different lipid A structures obtained from Porphyromonas gingivalis and Escherichia coli. Cell Microbiol. 2007;9:1028–1038. doi: 10.1111/j.1462-5822.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- Coats SR, Do CT, Karimi-Naser LM, Braham PH, Darveau RP. Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cell Microbiol. 2007;9:1191–1202. doi: 10.1111/j.1462-5822.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- Coats SR, Pham TT, Bainbridge BW, Reife RA, Darveau RP. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol. 2005;175:4490–4498. doi: 10.4049/jimmunol.175.7.4490. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Cunningham MD, Bailey T, Seachord C, Ratcliffe K, Bainbridge B, et al. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311–1317. doi: 10.1128/iai.63.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DR. Thesis. 2005. Lipid A Heterogeneity within Porphyromonas gingivalis and Other Oral Bacteria: Effect of Lipid A Content on hTLR4 Utilization and E-selectin Expression. [Google Scholar]
- Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res. 2005;84:584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, et al. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun. 2006;74:6730–6738. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanioka T, Shizukuishi S, Tsunemitsu A. Hemoglobin concentration and oxygen saturation of clinically healthy and inflamed gingiva in human subjects. J Periodontal Res. 1990;25:93–98. doi: 10.1111/j.1600-0765.1990.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Hanioka T, Shizukuishi S, Tsunemitsu A. Changes in hemoglobin concentration and oxygen saturation in human gingiva with decreasing inflammation. J Periodontol. 1991;62:366–369. doi: 10.1902/jop.1991.62.6.366. [DOI] [PubMed] [Google Scholar]
- Jones JW, Shaffer SA, Ernst RK, Goodlett DR, Turecek F. Determination of pyrophosphorylated forms of lipid A in Gram-negative bacteria using a multivaried mass spectrometric approach. Proc Natl Acad Sci U S A. 2008;105:12742–12747. doi: 10.1073/pnas.0800445105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbarz MJ, Kalb SR, Cotter RJ, Raetz CR. Expression cloning and biochemical characterization of a Rhizobium leguminosarum lipid A 1-phosphatase. J Biol Chem. 2003;278:39269–39279. doi: 10.1074/jbc.M305830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, China K, Nishijima M. Release of the lipopolysaccharide deacylase PagL from latency compensates for a lack of lipopolysaccharide aminoarabinose modification-dependent resistance to the antimicrobial peptide polymyxin B in Salmonella enterica. J Bacteriol. 2007;189:4911–4919. doi: 10.1128/JB.00451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Kumada H, Haishima Y, Umemoto T, Tanamoto K. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J Bacteriol. 1995;177:2098–2106. doi: 10.1128/jb.177.8.2098-2106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada H, Haishima Y, Watanabe K, Hasegawa C, Tsuchiya T, Tanamoto K, Umemoto T. Biological properties of the native and synthetic lipid A of Porphyromonas gingivalis lipopolysaccharide. Oral Microbiol Immunol. 2008;23:60–69. doi: 10.1111/j.1399-302X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- Liljemark WF, Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7:180–198. doi: 10.1177/10454411960070020601. [DOI] [PubMed] [Google Scholar]
- Mann PB, Wolfe D, Latz E, Golenbock D, Preston A, Harvill ET. Comparative toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect Immun. 2005;73:8144–8152. doi: 10.1128/IAI.73.12.8144-8152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford RS, Varley AW. Sield as signal: lipopolysaccharides and the evolution of immunity to Gram-negative bacteria. PLoS Pathog. 2006;2:e67. doi: 10.1371/journal.ppat.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olczak T, Simpson W, Liu X, Genco CA. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev. 2005;29:119–144. doi: 10.1016/j.femsre.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Pece S, Giuliani G, Di Leo A, Fumarola D, Antonaci S, Jirillo E. Role of lipopolysaccharide and related cytokines in Helicobacter pylori infection. Recenti Prog Med. 1997;88:237–241. [PubMed] [Google Scholar]
- Price NP, Jeyaretnam B, Carlson RW, Kadrmas JL, Raetz CR, Brozek KA. Lipid A biosynthesis in Rhizobium leguminosarum: role of a 2-keto-3-deoxyoctulosonate-activated 4' phosphatase. Proc Natl Acad Sci U S A. 1995;92:7352–7356. doi: 10.1073/pnas.92.16.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumbwe L, Skilbeck CA, Nakano V, Avila-Campos MJ, Piazza RM, Wexler HM. Bile salts enhance bacterial co-aggregation, bacterial-intestinal epithelial cell adhesion, biofilm formation and antimicrobial resistance of Bacteroides fragilis. Microb Pathog. 2007;43:78–87. doi: 10.1016/j.micpath.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Whiteley M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci U S A. 2009;106:1578–1583. doi: 10.1073/pnas.0809533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan M, Aduse J, Paramonov N, Hashim A, Bostanci N, Fraser OP, et al. Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50. J Bacteriol. 2008;190:2920–2932. doi: 10.1128/JB.01868-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reife RA, Coats SR, Al-Qutub M, Dixon DM, Braham PA, Billharz RJ, et al. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition. Cell Microbiol. 2006;8:857–868. doi: 10.1111/j.1462-5822.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- Roberts FA, Darveau RP. Beneficial bacteria of the periodontium. Periodontol 2000. 2002;30:40–50. doi: 10.1034/j.1600-0757.2002.03004.x. [DOI] [PubMed] [Google Scholar]
- Sawada N, Ogawa T, Asai Y, Makimura Y, Sugiyama A. Toll-like receptor 4-dependent recognition of structurally different forms of chemically synthesized lipid As of Porphyromonas gingivalis. Clin Exp Immunol. 2007;148:529–536. doi: 10.1111/j.1365-2249.2007.03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Stinson FL, Parker RB. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972;43:270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994;5:7–25. doi: 10.1111/j.1600-0757.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Takii R, Kadowaki T, Baba A, Tsukuba T, Yamamoto K. A functional virulence complex composed of gingipains, adhesins, and lipopolysaccharide shows high affinity to host cells and matrix proteins and escapes recognition by host immune systems. Infect Immun. 2005;73:883–893. doi: 10.1128/IAI.73.2.883-893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Tran AX, Karbarz MJ, Wang X, Raetz CR, McGrath SC, Cotter RJ, Trent MS. Periplasmic cleavage and modification of the 1-phosphate group of Helicobacter pylori lipid A. J Biol Chem. 2004;279:55780–55791. doi: 10.1074/jbc.M406480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AX, Whittimore JD, Wyrick PB, McGrath SC, Cotter RJ, Trent MS. The lipid A 1-phosphatase of Helicobacter pylori is required for resistance to the antimicrobial peptide polymyxin. J Bacteriol. 2006;188:4531–4541. doi: 10.1128/JB.00146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Karbarz MJ, McGrath SC, Cotter RJ, Raetz CR. MsbA transporter-dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane: topography of francisella novicida LpxE expressed in Escherichia coli. J Biol Chem. 2004;279:49470–49478. doi: 10.1074/jbc.M409078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McGrath SC, Cotter RJ, Raetz CR. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4'-phosphatase LpxF. J Biol Chem. 2006;281:9321–9330. doi: 10.1074/jbc.M600435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ribeiro AA, Guan Z, Abraham SN, Raetz CR. Attenuated virulence of a Francisella mutant lacking the lipid A 4'-phosphatase. Proc Natl Acad Sci U S A. 2007;104:4136–4141. doi: 10.1073/pnas.0611606104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gaekwad J, Wolfert MA, Boons GJ. Synthetic tetra-acylated derivatives of lipid A from Porphyromonas gingivalis are antagonists of human TLR4. Org Biomol Chem. 2008;6:3371–3381. doi: 10.1039/b809090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.