Abstract
The first 2–4 days after an Anopheles gambiae female mosquito emerges are critical to her survival and reproductive success. Yet, the order of behavioural events (mating, sugar feeding, blood feeding) during this time has received little attention. We discovered that among female cohorts sampled from emergence, sugar feeding had a higher probability than blood feeding of occurring first, and mating rarely occurred before a meal was taken. The night after emergence, 48% of females fed on sugar in mesocosms, and 25% fed on human blood; in the absence of sugar, 49% of females fed on human blood. After 5 days, 39% of the sugar-supplied females had blood fed and mated, and were fructose negative, whereas only 8% of the sugar-denied females had both blood fed and mated by this time. The model that best explained the transitions suggests that females made use of two distinct behavioural pathways, the most common one being to sugar-feed, then mate, and then seek blood. Other females sought blood first, then mated, and forwent a sugar meal. Lipid levels were higher in females with access to sugar than in females without access to sugar, particularly for those in later gonotrophic stages, while glycogen levels in the sugar-supplied group were higher throughout. In single-night experiments with females having had access to sucrose since emergence, those given a blood meal 1 day before spending a night with males had higher insemination rates than those not receiving the blood meal. These results indicate that the trade-off between survival and immediate reproduction is resolved by young adult females in accordance with availability of resources and gonotrophic state.
Keywords: Anopheles gambiae s.s., behavioural sequence, energetic reserve, mating, sugar feeding
The acquisition and maintenance of energetic reserves by animals serve as insurance against starvation. Reserve size is expected to vary with the costs and benefits of its maintenance (Witter & Cuthill 1993). For example, juvenile salmon, Salmo salar Linnaeus, are thought to regulate energy intake according to a low target level of lipid reserves at the end of the winter according to a trade-off between starvation and predation risk (Bull et al. 1996; Finstad et al. 2010). Predictability of the food supply also exerts an influence on the optimal reserve level, allowing, for instance, dominant great tits, Parus major L., to maintain lower lipid reserves, and be less exposed to predation, than subordinates (Gentle & Gosler 2001).
For females of most anautogenous mosquitoes (i.e. requiring a blood meal to develop their first batch of eggs) the most efficient way to create and maintain reserves is by feeding on plant sugar (Van Handel 1965). The main cost of recent sugar feeding is a reduction in subsequent blood meal size, and therefore egg batch size, as both compete for space in the mosquito abdomen (Mostowy & Foster 2004). Thus, females are faced with a choice between sugar feeding to rapidly increase their energetic reserves, or blood feeding, which contributes to fecundity through the development of eggs and to a smaller extent also supplements energetic reserves (Nayar & Sauerman 1975). Optimal diet choice of mosquitoes will thus have to balance somatic and reproductive demands (Roitberg & Friend 1992). Females of certain species may fare just as well, or better, by excluding sugar from their diets. Aedes aegypti L., the yellow fever mosquito, appears to fit this last description. Life table experiments show that age-specific survivorship (Scott et al. 1997), reproductive potential and the basic reproduction rate (Scott et al. 1997; Harrington 2001) are all higher in females offered only blood and water than in females offered both blood and continuous access to a 10% sugar solution. This phenomenon was also shown by Braks et al. (2006) for A. albopictus Skuse.
Similarly, females of the highly anthropophilic species Anopheles gambiae Giles s.s. have a lower survival rate in the absence of sugar, but an increased blood-feeding rate (Straif & Beier 1996; Gary & Foster 2001). As a result, lifetime fecundity is roughly equivalent with and without sugar, but females lacking sugar achieve this fecundity in a shorter time frame. For growing populations (Houston & McNamara 1999), sugar feeding therefore appears to have a fitness cost, raising the question: why partake in it?
Several studies indicate that some A. gambiae females do indeed use sugar. Beier (1996) found that 10–17% of female A. gambiae s.l. collected during indoor biting catches in western Kenya tested positive for fructose, indicating recent plant-sugar ingestion. Foster & Takken (2004) found in olfactometer experiments that 1-day-old females strongly preferred honey-related volatiles over human-related volatiles, while the reverse was true for 5-day-old sugar-fed females. Several cage studies showed an increased survival of this species when offered sugar solution (Gary & Foster 2001) or access to nectar-bearing plants (Gary & Foster 2004; Impoinvil et al. 2004; Manda et al. 2007). And females with access to putative host plants had higher fecundity after one blood meal (compared to blood-only access), yet not after three blood meals (Manda et al. 2007). Thus, feeding on sugar before the first blood meal may be advantageous for females of this species. In laboratory cages, females fed on sugar between gonotrophic cycles and showed higher feeding frequencies when access to oviposition sites or blood meals was delayed (Gary & Foster 2006). A recent theoretical study (Ma & Roitberg 2008) supported the expectation that sugar feeding in this species is most prevalent after emergence and after oviposition, but field-based confirmation of this pattern of behaviour is lacking.
The following hypotheses, summarized in Table 1, may explain female A. gambiae’s use of plant sugar early in life.
Table 1.
Hypotheses for initial meal choice of female A. gambiae and predicted outcomes of the behavioural-sequences experiments that would lend support to them.
| Hypothesis for initial meal choice | Prediction |
|---|---|
| 1: females favour sugar | Behavioural sequences in mesocosms with sugar will all start with females taking a sugar meal. |
| 2: females favour blood | The proportion of females choosing blood as their initial meal will be the same in mesocosms with and without sugar. |
| 3: females are opportunistic | If both resources are available, sequences starting with sugar- and blood meals will both be observed; in mesocosms without sugar the proportion of females taking blood for their 1st meal will be greater than in mesocosms with sugar. |
(1) Females predominantly ingest sugar at the first available opportunity and prefer sugar over a blood meal, possibly to increase expected survival directly by supplementing meager energy reserves. The sugar priority may be related to blood-host-seeking behaviour if the expression of host seeking (Clements 1999) is enabled by sugar feeding. Alternatively, the sugar preference may stem from its facilitation not just of survival, but also of mating behaviour (reviewed by Clements 1999; Yuval 2006). It fuels the swarming flight of males (Gary et al. 2009; Stone et al. 2009b) and, possibly, the mating activity of females, such as locating swarms and selecting mates (Gibson & Russell 2006; Cator et al. 2009; Warren et al. 2009; Pennetier et al. 2010). If females favour sugar initially, then behavioural sequences should start with sugar feeding when both blood and sugar sources are present in the environment.
(2) Females should not feed on sugar unless opportunities to blood-feed are uncommon. Thus, only those that would otherwise remain unfed and starve should accept a sugar meal; the proportion of females taking a blood meal initially should be independent of the availability of sugar in the environment.
(3) Females are opportunistic feeders. Limiting the time spent searching for a sugar or blood meal is the most important factor, because both types of meals have similar fitness values early in life: they both supplement energetic reserves, allow for development of ovarian follicles to the resting stage (Fernandes & Briegel 2004) and provide fuel for flight. Thus, food preference will merely reflect abundance of host types, ease in locating them, or access to them. If females are opportunistic, behavioural sequences should start equally with either meal type when both meal types are available in the environment. Without access to sugar, the proportion of females feeding on blood should initially increase, compared to a situation where both resources are available.
The principal objective of this study was to establish the behavioural routine of young adult female A. gambiae under simulated natural conditions. This allowed us to determine whether they are obligate sugar-feeders, opportunistic sugar-feeders, or preferential blood-feeders. By also investigating two plausible reasons that females include sugar in their diet, to enhance energy reserves or to facilitate mating, we gained further insight into how this species resolves the trade-off between enhanced survival prospects and earlier reproduction.
METHODS
The mosquitoes used for these experiments came from a colony established in 2001 by staff at the International Centre of Insect Physiology and Ecology from the local population of A. gambiae s.s. in Mbita Point, Suba District, Nyanza, Kenya, and identified by PCR. This Mbita strain has been maintained in acrylic cages at 26.6±1 °C and 80±5 % RH since 2006. Water and 10% sucrose solution were available to colony adults ad libitum. Blood feeding of adults on humans, for colony maintenance and for experiments, was covered under The Ohio State University’s Biosafety protocol No. 2005R0020 and Biomedical IRB protocol No. 200440193, FWA No. 00006378. Experimental mosquitoes were reared by transferring 100 newly hatched first-instar larvae into 22.8 × 33 cm pans filled with 450 ml of aged tap water and feeding them 0.2 mg of finely ground Tetramin™ fish flakes per larva during the first 3 days of larval development, 0.4 mg on each of the next 3 days, and 0.8 mg on subsequent days until pupation.
Experimental mosquitoes inhabited two mesocosms at The Ohio State University, moist-floored 9.1 m3 greenhouse enclosures designed to simulate conditions of endemic habitats in equatorial Africa. A detailed description is given in Stone et al. (2009a), with a few adjustments described here. A container of water was placed inside a chimney-like construct that housed resting sites, to elevate humidity within this structure. Mosquitoes rested in 30 black cardboard tubes projecting into the chimney. To compare the behaviour and die-off of mosquitoes in the large mesocosm environment to that in laboratory cages, a clear acrylic cage (36 × 52 × 46 cm) was situated on three bricks in a water-filled tray on the floor of each mesocosm in which the experiment was performed simultaneously. A black cup (10 cm in diameter, 8 cm in depth) oriented sideways and glued to the wall served as a resting site.
Lights in the mesocosms came on at 0700 hours and remained on until 1800 hours, leaving enough time for the natural, diminishing light of the evening crepuscular period at this latitude to facilitate mating.
Treatments consisted of the presence or absence of sugar in the environment. In the sugar-supplied mesocosm, four potted plants with extrafloral nectaries (two Senna didymobotrya and two Ricinus communis) were placed in aluminium-wrapped trays on the floor; water in the trays served as a moat to keep ants from competing with the mosquitoes for nectar. The Ricinus plants produced a large amount of visible extrafloral nectar. To ensure that sugar availability would not be limited in the sugar treatment, in addition to the plants, wicks of sucrose and honey solution were suspended from a bank of lights in the mesocosm. Wicks protruded from six vials of 10% sucrose solution with 0.001 mg/litre verbenone added for scent (Gary & Foster 2006), and six wicks protruded from vials of 10% multifloral honey solution. In cages, one sucrose wick and one honey wick were available to mosquitoes. These solutions and wicks were replenished every 2 days. In the sugar-denied mesocosm and cage, the wicks held water, and in the mesocosm, we placed an artificial (plastic) Ficus tree to provide a plant-like structure.
Behavioural Sequences and Reserve Acquisition with Blood but with or without Sugar
At the start of each replicate experiment, 400 pupae were placed in each mesocosm, and 200 in each cage. They emerged as adults overnight. Females were allowed to blood-feed for 30 min on a human host (C.S.) each evening between 2100 and 2200 hours, starting the day after emergence. The order in which mesocosms were given access to a host was switched each evening.
Every morning, between 0700 and 0800 hours, 20 females per mesocosm, and 10 per cage, were removed from resting sites by mouth aspirator, killed and fixed at −40 °C for later dissection and chemical analysis. After 5 days of sampling, the number of females in the water-only mesocosm had become depleted (only with considerable effort could a full sample be obtained), and the experiment was stopped at this point. It is likely that some predation occurred, because a small plump spider was found in one mesocosm on one occasion, and a centipede was found outside the perimeter of another mesocosm.
In addition to the 400 pupae, on the first night, 200 5-day-old virgin males that had been kept in cages with ad libitum access to sugar (i.e. sexually mature and well fed) were released into the mesocosms. To ensure that adequate numbers of competent males would be present throughout the experimental period in both treatments (preliminary cage studies had revealed good survival of such males for only 2 days after sugar was withheld), on the third night another 150 5-day-old sugar-fed males were released into both mesocosms. The same procedures were applied to the cages, but with half the number of males.
Because females taking blood one or two nights after emergence would have been gravid before the end of the experiment, an oviposition site (a plastic tray with a layer of water in the mesocosms; a petri dish with water in the cages) was made available to avoid behavioural aberrations associated with delayed oviposition. In the mesocosms, females could also use the moats holding the plants or the moist carpet on the floor as oviposition sites.
We gathered the following information for each individual female removed from the experiment: (1) Sella’s stage of blood digestion and gonotrophic progress, based on the relative sizes of the digesting blood meal and swelling ovaries; (2) Christopher’s stage of a few primary ovarian follicles, to further assess whether and when a blood meal was taken; (3) wing length, as an indicator of body size; (4) presence or absence of tracheolar skeins on an ovary of any non-blood-fed female (Detinova method), to distinguish nulliparous from parous females (only of females collected on days 4 and 5); (5) the presence or absence of sperm in the spermatheca; and (6) energetic reserves: on days 4 and 5, we collected females, excised the ovaries of non-blood-fed and nongravid (i.e. ‘empty’) females, then stored the bodies individually in an Eppendorf tube and froze them until energetic reserves were measured, using the methods of Van Handel (1985) and Van Handel & Day (1988). We crushed females in 0.2 ml of 2% sodium sulphate solution, then added 1.5 ml of chloroform:methanol (1:2) to each tube and centrifuged the tubes for 15 min. Half of the supernatant was used for lipid analysis, half for fructose, and the precipitate for glygocen analysis. Lipids were assayed by evaporating the supernatant set aside for this, adding 0.2 ml sulfuric acid and heating it at 90 °C for 10 min. After adding 4.8 ml of vanillin-phosphoric acid reagent, absorbance was read at 525 nm on a spectrophotometer. The amount of fructose was determined by evaporating the second half of the supernatant to about 0.1 ml, adding 4.9 ml of anthrone reagent and incubating at 26 °C for 1 h, after which the absorbance was read at 625 nm. Glycogen was determined by adding 5 ml of anthrone reagent to the precipitate, heating at 90 °C for 15 min, and measuring absorbance at 625 nm. Standards were glucose, sucrose and soybean oil solutions. Amounts (μg) of carbohydrates and lipids were transformed into Joules by multiplying by 0.016 and 0.037, respectively. Fructose quantities, which indicate undigested crop sugar from recent sugar feeding, were measured for all females; lipid and glycogen amounts per female were measured for the final two replicates. The glycogen and lipid values obtained included both the maternal reserves and, in the case of blood-fed and gravid females, also those materials contained within the developing egg follicles and fully developed eggs.
Females that had taken a blood meal, regardless of the state of digestion of the meal and consequent development of vitellogenic eggs, were scored as blood-fed, as were females that appeared unfed but were parous, having already laid the eggs. Females were classified according to their internal state: eight combinations (N1–N8), consisting of their blood-feeding (gonotrophic and postgonotrophic) status, insemination status and fructose positivity. Altogether, four replicates of the experiment were performed. For analysis of behavioural sequences, population vectors for the 5 days of the experiment were based on the proportions of females in each internal state. The corresponding behavioural transitions between internal states over 5 days were estimated by utilizing Wood’s quadratic programming method (Caswell 2001). A number of different models were considered, and the best among these was selected by projecting the population using the estimated transition matrix, obtaining the residual sum of squares by comparing the real with the simulated data, and calculating the Akaike’s Information Criterion (AICc) for small samples for each model. AIC is a relative measure of the information lost when using a model, which can then be used to select among alternative models. The model or models with the lowest AIC values are those that best explain the data with a minimum of free parameters. It allows for a ranking of competing models based on fit, while accounting for the number of estimated parameters (Burnham & Anderson 2002).
Bootstrap selection frequencies were calculated to provide information on the uncertainty of the best model. Bootstrap samples were obtained by combining data from all four replicates and sampling with replacement from this data set for each day separately. A single data point consisted of the scored internal state of a female. One bootstrap sample then consisted of a matrix of proportions of eight states for each of 5 days. Subsequently, for each of 2000 bootstrap samples a transition matrix and an AICc value were calculated for each model, and the best model selected. The selection frequencies were obtained by tallying the number of times each model was selected as the best model.
Effect of Blood Meal or Sugar Feeding on Insemination
Further experiments were done to obtain independent evidence that blood-fed only, sugar-and-blood-fed, and sugar-fed only females are equally capable of mating while digesting a meal. We separated males and females 1 day after emergence and provided them with continuous access to 10% sucrose in clear acrylic cages. Of 100 sugar-fed females, 50 were allowed to take a replete blood meal on day 3. In the late afternoon of day 4, 100 males and all 100 females were released into one mesocosm with the four Ricinus and Senna plants in it. The following morning all mosquitoes were collected by aspirator and frozen. Presence or absence of blood in the midgut indicated the meal history of each female. The spermatheca was removed and inspected by compound microscope for the presence or absence of sperm. This experiment was replicated five times. In a variation of that experiment, 50 females had access only to sucrose from day 1, whereas the other 50 had no sucrose but took their blood meals on day 2. Then all 100 females were released into the mesocosm along with 100 same-age sugar-fed males on day 3 and were collected the following morning. This experiment also was replicated five times.
Statistics and Data Analysis
Differences in energy reserves and proportions of females in different behavioural states were tested for normality and analysed with t tests or Mann–Whitney U tests. An effect of fructose content on insemination status in mesocosms was analysed with binary logistic regression. We analysed differences in insemination rates between females that had and had not taken blood and in the proportions of females surviving for 5 days with Pearson’s chi-square tests. All facets of the analysis employed SPSS v.16 (2007) software. Calculations of AICc values and bootstrap selection frequencies were performed in MatLab (2009, Mathworks, Natick, MA, U.S.A.).
RESULTS
Mesocosms with Sugar
Sugar feeding in the sugar-supplied mesocosm was observed during early scotophase. It occurred mainly on the plants rather than on the sugar and honey wicks. Mosquitoes probed near the tips of Senna leaflets, as well as on the extrafloral nectaries located at the base of the petioles. The number of males and females probing on the plants increased after swarming had stopped. Approximately 1 h after sunset large numbers of mosquitoes were on the plants, primarily Senna, upon the leaflets of which tiny droplets of sweet liquid were visible.
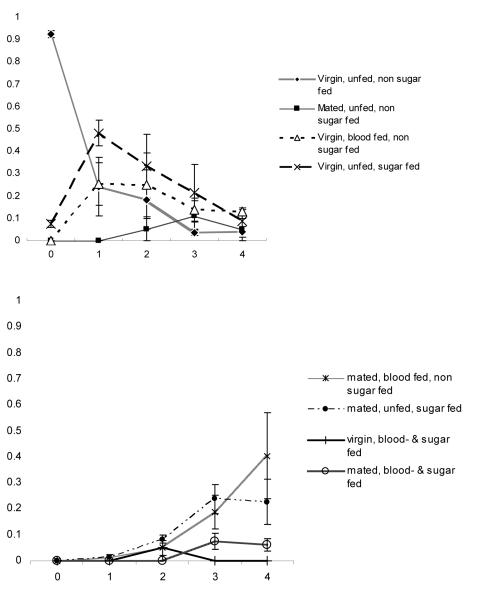
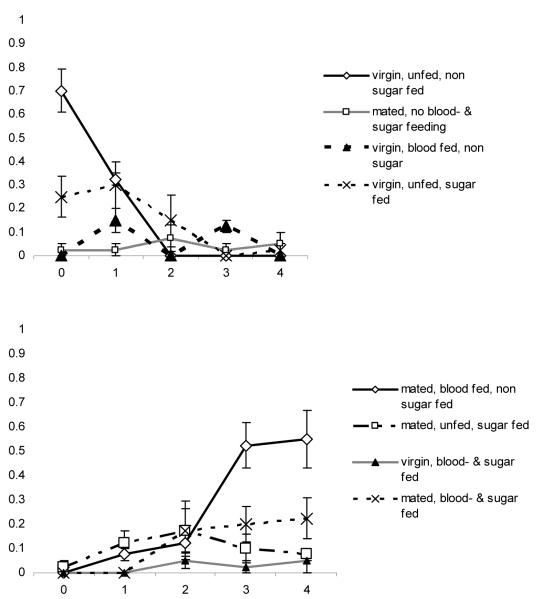
Judging from the internal states on day 1 (Fig. 1), a small proportion of females already had taken sugar during the night of emergence, but the vast majority (92.5%) had not engaged in any of the three behaviours under study. After the following night, 24% remained in the initial state, but the majority had at this point acquired a meal, either blood (25%) or sugar (48%); the differences in proportions of females feeding on either blood or sugar was not significant (t6 = −2.05, P = 0.086). On day 3 the largest proportions of females were those that were both fructose positive and had mated (24%), and those that were fructose positive but had not mated (21%). Females that had taken a blood meal and mated successfully made up 19% of the population at this time, while 14% had taken a blood meal without having mated. By day 4 the largest proportion consisted of females that had mated and blood-fed and were fructose negative (39%), which was not significantly different from the 23% of females that had mated and were fructose positive, but had not fed on blood (t6 = 0.96, P = 0.37).
Figure 1.
Mean proportions + SE (four replicates) of females in internal state categories per day after emergence in the mesocosms with sugar available: (a) ≤1 action; (b) 2–3 actions.
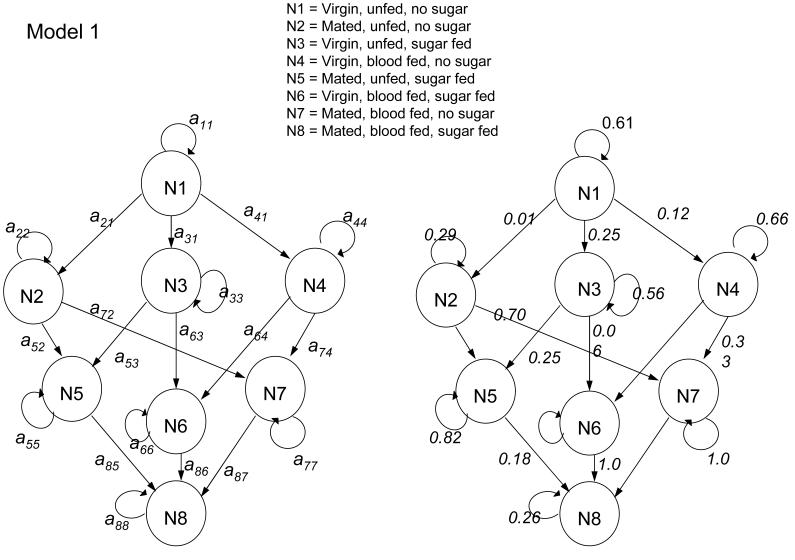
Several models were constructed to elucidate the transitions between these states. The initial model (Model 1) allowed females to perform one action (blood-feed, sugar-feed or mate) per night, or to remain within their current states. The transitions for this model are shown in Fig. 2. Notable was the low estimate for transition a21, suggesting that mating before taking a meal was rare. Low values for transitions a63 and a64 confirm that feeding on sugar was typically not followed directly by feeding on blood, and vice versa, while the value of transition a88 appears artificially low (which might be interpreted as low survival rate of state N8 = blood-fed, mated, fructose positive).
Figure 2.
Path diagrams of internal states and transition matrix values for Model 1. Arrows without a value indicate zero.
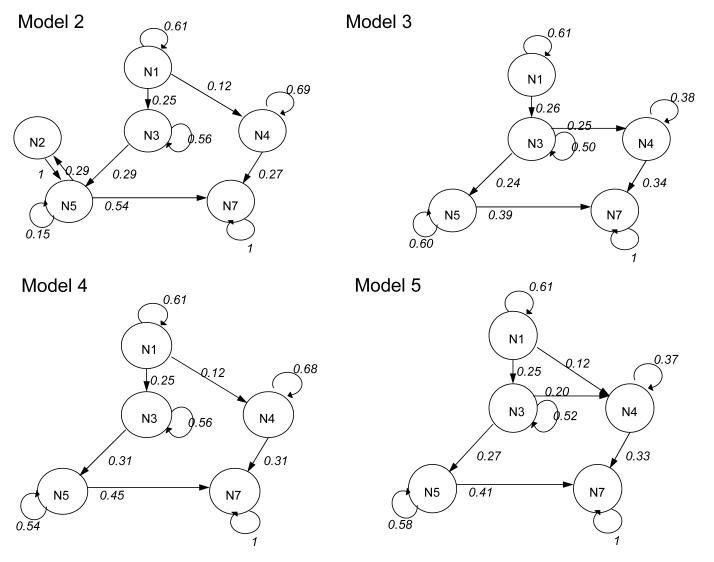
Model 2 (Fig. 3) was used to refine Model 1, by removing the pathways to states N6 (blood-fed, virgin, fructose positive) and N8 (blood-fed, mated, fructose positive) from other states, and from the unfed-virgin to unfed-mated state (a21). As the anthrone test detects only recent sugar meals, Model 2 allowed for the possibility that females could digest a sugar meal completely, and it opened the pathways from the sugar-fed-mated state to the mated state (a25), and also to the blood-fed-mated state (a75).
Figure 3.
Path diagrams of internal states and transition matrix values for Models 2, 3, 4 and 5.
The following three models differed slightly and were constructed to answer three questions: (1) whether sugar feeding necessarily precedes blood feeding, which would suggest that sugar has a role in supplying energy for host-seeking behaviour (Model 3); (2) whether sugar and blood feeding are both viable options that precede mating (Model 4), which is the same as Model 2 but lacks the pathway from the sugar-fed-mated state to the unfed-mated state; and (3) whether females are entirely opportunistic (i.e. unfed virgins seek either sugar or blood), and those that take sugar subsequently mate or take a blood meal (Model 5).
Among these five models, Model 4 had the most support, based on the AICc values (Table 2). Model 5, where sugar feeding preceded either mating or a blood meal, had considerably less support. The first three models had virtually no support. That mating occurred only following a meal suggests that the expression of mating behaviour depends on energy reserves. The strong support for Model 4 suggests that feeding on sugar specifically enables the behavioural sequence of mating followed by host seeking, rather than providing energy for opportunistically mating or finding a blood meal. It also suggests that both blood and sugar are viable options for an initial meal, although feeding on sugar first is more likely under the experimental circumstances.
Table 2.
Values for the number of model parameters (K-1), residual sum of squares (RSS), AICc, AIC differences (Δi), and bootstrap selection frequency. Models are ordered in terms of Δi for AICc.
| Model | K-1 | RSS | AICc | Δ i | Selection frequency (%) |
|---|---|---|---|---|---|
| 4 | 10 | 3435 | 209.6 | 0 | 80.5 |
| 5 | 11 | 3399 | 213.3 | 3.7 | 18.2 |
| 2 | 12 | 3576 | 219.7 | 10.1 | 0 |
| 3 | 10 | 5307 | 226.9 | 17.3 | 1.3 |
| 1 | 20 | 3608 | 273.4 | 63.8 | 0 |
The bootstrap selection frequencies of the five tested models reinforce this conclusion and provide information on the robustness of Model 4, which was selected as the best model in 80.5% of bootstrap samples, whereas Model 5, the model that allowed for more opportunistic behaviour, was selected as the best model in 18.2% of the samples. Model 3 was the best model in only 1.3% of the cases, while Models 1 and 2 were never selected.
This analysis suggests that in A. gambiae females the most common sequence of behaviours during the first 5 days of life is to rest on the night of emergence, feed on sugar on the following night, then find a mate and take a blood meal. However, a fairly large proportion appears to make use of a different pathway, by taking blood first, then mating, and forsaking sugar altogether. Among females that, during the first 3 days, had taken sugar but had not yet mated, the mean wing length (2.92 mm) did not differ significantly from that of females that fed on blood instead (2.87 mm) (ANOVA: F1, 96 = 1.46, P = 0.229). This indicates that female size was not a factor that determined initial meal choice.
Mesocosm without Sugar
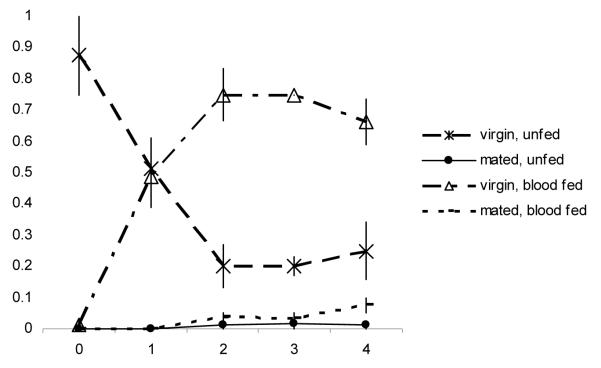
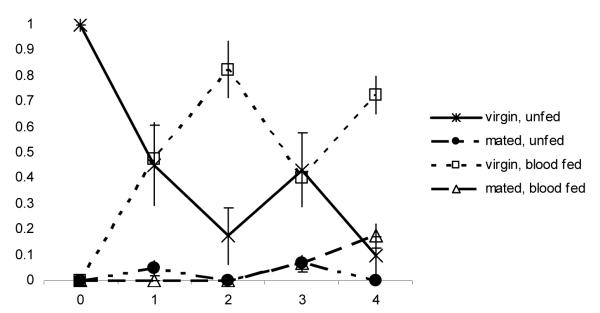
The behavioural pattern of females in mesocosms lacking sugar was more straightforward (Fig. 4). On the night of emergence, most females (99%) again engaged in neither mating nor blood feeding. On the following night, however, 49% had taken a blood meal, which was a significantly greater proportion than the percentage of females that had taken a blood meal on that night when sugar was available (Mann–Whitney U test: U = 16, N1= N2 = 4, P < 0.02). On days 3 and 4, 75% were blood-fed virgins. On day 5, 8% of females were blood-fed and had successfully mated; this value did not differ significantly from the percentage of females in that state when sugar was available (Mann–Whitney U test: U = 13.5, N1= N2 = 4, P = 0.09). Females that mated before obtaining a meal were very rare throughout, as in the sugar-supplied mesocosms.
Figure 4.
Mean proportions + SE (four replicates) of females in internal state categories per day after emergence in the mesocosms without sugar.
Cages with and without Sugar
In the cage study, behavioural transition patterns were largely the same as in mesocosms, with a few marked differences (Fig. 5). Where sugar was available, a significantly greater percentage of females had already taken a sugar meal (25%) on the night of emergence (Mann–Whitney U test: U = 16, N1= N2 = 4, P < 0.02). On the last 2 days, more than 50% of females tested negative for fructose, but they had taken a blood meal and had mated. Thus, the pattern was similar to that in the mesocosm, but the end point, at which females had mated and obtained a blood meal, was reached 1 day faster in the cages than in the larger space of the mesocosm. The main difference between females in cages and in mesocosms with access only to water and blood was that the percentage of females that had both blood-fed and mated was only marginally higher in cages (Mann–Whitney U test: U = 2, N1= N2 = 4, P = 0.074). On day 4, 18% of the females had both blood-fed and mated (cf. 8% for females in mesocosms) (Fig. 6).
Figure 5.
Mean proportions + SE (of four replicates) of females in internal state categories per day after emergence in the cages with sugar: (a) ≤1 action; (b) 2–3 actions.
Figure 6.
Mean proportions + SE (of four replicates) of females in internal state categories per day after emergence in the cages without sugar.
Energy Reserves
The mean glycogen and lipid reserves per day differed sharply between females in mesocosms with and without sugar (Table 3). Throughout the experimental period, lipid levels of females with access to sugar were higher than those without, the difference being most distinct on the last day. Glycogen levels also were substantially higher in females in the presence of sugar sources from day 1 onwards. The same trend occurred in females kept in cages. Here, however, on the last 2 days of the experiment, glycogen levels did not differ significantly between treatments.
Table 3.
Mean glycogen and lipid reserves of females, and fructose content of virgin and mated females, according to day after emergence
| Glycogen | Lipid | Fructose | ||||
|---|---|---|---|---|---|---|
| Mesocosm | ||||||
| with sugar | without sugar | with sugar | without sugar | virgin | mated* | |
| Day | ||||||
| 0 | 0.03 ± 0.00 | 0.07 ± 0.01* | 0.19 ± 0.01 | 0.08 ± 0.01* | 0.18 ± 0.08 | 0 |
| 1 | 0.13 ± 0.02 | 0.04 ± 0.01* | 0.19 ± 0.01 | 0.14 ± 0.01* | 0.43 ± 0.07 | 0.13 |
| 2 | 0.18 ± 0.03 | 0.06 ± 0.01* | 0.29 ± 0.04 | 0.20 ± 0.02* | 0.39 ± 0.08 | 0.18 ± 0.04 |
| 3 | 0.14 ± 0.01 | 0.06 ± 0.01* | 0.36 ± 0.04 | 0.29 ± 0.03 | 0.42 ± 0.09 | 0.30 ± 0.06 |
| 4 | 0.16 ± 0.02 | 0.09 ± 0.01* | 0.57 ± 0.06 | 0.29 ± 0.04* | 0.48 ± 0.15 | 0.19 ± 0.03 |
| Cage | ||||||
|---|---|---|---|---|---|---|
| with sugar | without sugar | with sugar | without sugar | virgin | mated | |
| Day | ||||||
| 0 | 0.06 ± 0.01 | 0.11 ± 0.02* | 0.18 ± 0.01 | 0.22 ± 0.02 | 0.31 ± 0.13 | 0.72 |
| 1 | 0.13 ± 0.03 | 0.05 ± 0.01* | 0.26 ± 0.04 | 0.09 ± 0.01* | 0.42 ± 0.11 | 1.00 ± 0.42 |
| 2 | 0.24 ± 0.06 | 0.13 ± 0.02* | 0.43 ± 0.05 | 0.36 ± 0.05 | 0.20 ± 0.03 | 0.30 ± 0.07 |
| 3 | 0.16 ± 0.02 | 0.12 ± 0.02 | 0.76 ± 0.05 | 0.28 ± 0.04* | 0.06 | 0.09 ± 0.03 |
| 4 | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.75 ± 0.10 | 0.27 ± 0.04* | 0.17 ± 0.08 | 0.16 ± 0.07 |
Mean glycogen and lipid reserves (cal/female) in mesocosms and cages with and without plants with sugar available, per day. Significant differences (t tests, P < 0.05) in reserve levels between treatments are indicated by a * (N = 40 / day, except without sugar on d 0, where N = 20 for mesocosms, half that in cages). Fructose content (cal/female) of fructose-positive females in mesocosms with nectariferous plants, significantly affects their probability of insemination (binary logistic regression, Wald χ2 = 6.4, P = 0.011), but not in cages (Wald χ2 = 3.6, P = 0.058).
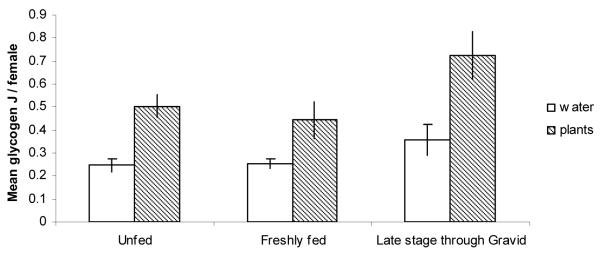
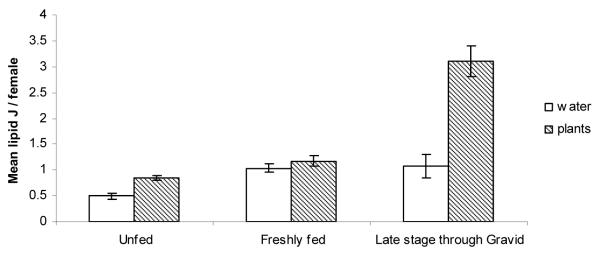
A comparison of energetic reserves of females according to gonotrophic state in sugar-supplied and sugar-denied treatments showed that glycogen and lipid levels were usually higher in females with environmental sugar (Figs 7, 8). The exception was the lipid levels of freshly blood-fed females, which were insignificantly different.
Figure 7.
Mean glycogen levels + SE of females in different stages of blood meal digestion, for mesocosms with sugar/nectar or mesocosms with only water and blood. *P < 0.05 (t tests: N = 46–166).
Figure 8.
Mean lipid content + SE of females in different stages of blood meal digestion, for mesocosms with sugar/nectar or mesocosms with only water and blood. *P < 0.05 (t tests: N = 41–160).
In mesocosms, fructose levels of fructose-positive females that had mated were lower than those of fructose-positive females that had not mated (Table 3). The most likely explanation is that mated females took their sugar meals earlier, and had used or converted more of it, which is congruent with the interpretation that females usually took a sugar meal before mating. This pattern was not evident for females in cages.
Survival
Daily survival values were derived from the average number of surviving females after 5 days, assuming a constant rate of mortality and accounting for removal through sampling. Females in mesocosms containing and lacking sugar had daily survival values of 0.94 and 0.86, respectively. The difference in the number of survivors was significant (χ21 = 95.1, P<0.05). Females in cages containing and lacking sugar had daily survival values of 0.96 and 0.94, respectively, and the number of surviving females differed significantly (χ21 = 7.67, P<0.05).
Effect of Blood Meal on Insemination
Of the sugar-fed females that were allowed to mate with 5-day-old, sugar-fed males in a mesocosm on night 4, a significantly greater percentage (χ21 = 31.8, P<0.05) that took a blood meal on day 3 were inseminated (71%), compared to females that had had only sugar (41%). Likewise, the insemination rate of sugar-deprived females that had taken blood on day 2 and had been allowed to mate on night 3 was significantly greater (56%) than that of females supplemented with sugar (21%) (χ21 = 35.5, P<0.05). All five replicates of both experiments showed the same trend.
DISCUSSION
Our analysis and evaluation of data derived from examining the status of blood feeding, sugar feeding and insemination of females sampled on sequential days after emergence in mesocosms revealed that, when sugar was present in the mesocosm, most females fed on sugar, then mated, and then fed on blood. But, a substantial proportion of females fed on blood first and then mated, bypassing sugar feeding. The significance of this duality of sequences was supported by the model based on transition frequencies. In the absence of sugar, a much larger proportion fed first on blood, then mated. Is it possible then, to determine the timing and necessity of sugar feeding in this species? We hypothesized three distinct early life behaviours: females strongly prefer sugar, strongly prefer blood, or are entirely opportunistic with regards to their initial meal (Table 1). The observed data favour the opportunistic hypothesis. Were females to favour sugar initially (hypothesis 1), we would expect all observed behavioural sequences to start with sugar feeding. The presence of two sequences, one that starts with sugar feeding and one that starts with blood feeding thus does not lend support for hypothesis 1. It also does not appear that sugar feeding is a requirement for successful mating in females, as sugar-deprived, blood-fed females became inseminated at a higher rate.
The results do not support the hypothesis that females favour blood initially, taking sugar only if they fail to obtain a blood meal (hypothesis 2). If they were to do so, one would not expect such a large difference between treatments in the proportion of females feeding on blood. That approximately one quarter of females fed on blood when sugar was available on the night after emergence, and half did so when sugar was not available, lends strong support for hypothesis 3: females are opportunistic with regard to their initial meal choice. Females were willing to feed on blood or sugar initially when both were available, and the absence of environmental sugar resulted in an increased likelihood of feeding on blood.
The appearance of a sugar priority (i.e. when both resources were present, either one might be used, but sugar more often) could be primarily a reflection of environmental access, based on random encounter with the stimuli associated with each food, because blood was available for a relatively brief period each night, but abundant sugar sources were available throughout the night. In nature, such a situation is common for newly eclosed females emerging from habitats distant from human habitations, or in habitations where bed nets insulate humans from mosquitoes a few hours after dark. Blood host availability has been shown to affect the response of mosquitoes (Hancock & Foster 1997) and biting midges (Garcia-Saenz et al., in press) to blood hosts, and it probably affects blood-feeding patterns of mosquitoes (Chaves et al. 2010). The extent to which the host response of A. gambiae depends on the presence and strength of sugar cues remains to be investigated, but it is likely to affect food choice (i.e. the exact values of parameters a21 and a31 may differ).
Wing lengths of 1-day-old females that had taken either a blood or sugar meal first did not differ, indicating that initial meal choice of females is not size dependent. However, mosquitoes used in these experiments were all reared under the same densities and food levels, and most likely were comparable to the large females reported by Takken et al. (1998) and Fernandes & Briegel (2004) that used their first blood meal for vitellogenesis. Our failure to observe any gonotrophic discordance (Briegel & Hörler 1993), a gonoinactive state (‘pregravid’ state, sensu Gillies 1954), or other deviations from the typical blood digestion and egg development patterns in our females (unpublished data) supports this conclusion. Females with shorter wings, indicating crowding or food stress during immature development, may choose their initial meal differently as a result of differing size-dependent benefits of sugar and blood meals (Roitberg et al. 2009).
Finally, we note that studies on fitness consequences of female mosquito diet (feeding on blood, or on blood and sugar) have all been performed in laboratory cages (e.g. Scott et al. 1997; Gary & Foster 2001; Fernandes & Briegel 2004). Lifetime fecundity in these small-scale environments may differ from that in more energetically demanding large-scale surroundings. Life tables derived in more realistic environments may well indicate that females do in fact benefit by including sugar in their diet. Our comparison of the daily survival values derived from cages and mesocosms suggests that the difference in survival on diets with and without sugar is underestimated in cage studies.
Glycogen levels of females in mesocosms with and without access to sugar differed more pronouncedly than did lipid levels, at least until the final day. According to Nayar & Van Handel (1971), only sugars and glycogen are used for flight in mosquitoes, whereas triglycerides contribute to resting metabolism. If glycogen is mainly used to sustain flight, this would support the hypothesis that females take a sugar meal early on to aid them in engaging in mating behaviour and/or host seeking. This fits with the most probable behavioural sequence constructed from the data. However, results of Kaufmann & Briegel (2004) muddle this picture somewhat, showing that, in A. gambiae, carbohydrates contribute significantly to survival, and lipid is mobilized for long-range flight, but hardly at all for survival. Among the late-stage blood-fed and gravid females in our mesocosms, those with sugar had higher reserves of both glycogen and lipid (Figs 7, 8). Individuals having more energy reserves before taking a blood meal may be able to convert a larger part of the meal into eggs. This is supported by Manda et al. (2007), who found that after one blood meal, females with access to sugar had higher fecundity.
Tests of the effect of blood-feeding on mating showed that blood-fed females, whether sugar-fed or not, had higher insemination rates than non-blood-fed females. This unexpected result suggests that blood is a sufficient energetic resource for A. gambiae to locate and join a male swarm. The promoting effect of blood may be behavioural rather than energetic, if the blood meal causes a female to prioritize insemination so that eggs will be fertilized as soon as they mature, as few as 2–3 days later. Alternatively, she may be easier to grasp by swarming males or may be more attractive to them. The plausibility of explanations based on manoeuvrability depends in part on females having control over mating, but the extent to which anopheline females do so (beyond the decision to enter a swarm) is unresolved. The higher insemination rate of blood-fed females fails to support the hypothesis that females should initially feed on sugar to enable mating behaviour itself. In nature, the meal used to facilitate mating may depend more on the proximity of mating arenas relative to adult emergence sites, vegetation and blood hosts than on the flight-enabling effects of a blood or sugar meal.
Gillies (1954) suggested that females typically take a nonvitellogenic (‘pregravid’) blood meal before mating, but among mosquitoes caught in houses, he considered only blood-fed females, discarding unfed (though possibly sugar-fed) females. In a theoretical study, Onyabe et al. (1997) concluded that females should be entirely opportunistic with regard to taking a blood meal before or after mating. A field study on the islands of São Tomé and Príncipe by Charlwood et al. (2003) supports this: A. gambiae females mated either before or after blood feeding in roughly equal proportions. Our results confirm the optional nature of the blood feeding–mate sequence, but in addition indicate that a meal, be it blood or sugar, is not optional and is a normal precondition to mating in this species. The choice between these two resources is, in effect, similar to the decision faced by parasitoids that use hosts for either oviposition or feeding, reflective of a trade-off between current and future reproduction (Heimpel & Rosenheim 1995). Collier et al. (1994) suggested that the decision to host-feed depends on a threshold egg load, and that this threshold in turn depends on the probability of encountering hosts.
How then do mosquitoes resolve the trade-off between increasing expected survival and more immediate reproduction? With regard to foraging for blood hosts, Costantini et al. (1998) suggested that the outcome of the decision-making process relies on the stimulus strength of the chosen host, the stimulus strengths of competing hosts, the internal state of the female and a genetic disposition to respond to the various stimuli (i.e. an intrinsic ranking of host types). That genetic variation underlies this behaviour was elegantly shown by Gillies (1964), who was able to select for host preference in A. gambiae and significantly change females’ preference after a few generations. It is feasible that the degree to which A. gambiae respond to nectar-related or human-related volatiles has a similar genetic basis.
A female’s internal state (e.g. gonotrophic state, size, energetic reserves) probably also influences her response to various stimuli. Houston & McNamara (1985) suggested that the energetic state of animals may allow the incorporation of suboptimal items into a diet if the energetic gain of the preferred food type is highly variable. The prediction is that individuals with high levels of reserves should be risk averse and display a higher degree of opportunism. For mosquitoes, the degree of defensive behaviour of potential blood hosts, with its associated costs, introduces variability in the likelihood of successfully obtaining a meal. This is concomitant with theoretical results, which at first might seem surprising, that female mosquitoes that are close to starvation should seek blood hosts but ignore sugar sources (Roitberg & Friend 1992). This suggests that the use of two behavioural pathways, as found in this study, may be a result of variance in energetic reserves at emergence.
Acknowledgments
Invaluable assistance was provided by Robin Taylor, Robert Aldridge, James Cannon III, Joan Leonard and George Keeney. Comments by Holly Tuten and two anonymous referees greatly improved this manuscript. This study was supported in part by National Institutes of Health grants R21-AI062957 and R01-AI077722.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beier JC. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and A. funestus (Diptera: Culicidae) in western Kenya. Journal of Medical Entomology. 1996;33:613–618. doi: 10.1093/jmedent/33.4.613. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Juliano SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Medical and Veterinary Entomology. 2006;20:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel H, Hörler E. Multiple bloodmeals as reproductive strategy in Anopheles (Diptera: Culicidae) Journal of Medical Entomology. 1993;30:975–985. doi: 10.1093/jmedent/30.6.975. [DOI] [PubMed] [Google Scholar]
- Bull CD, Metcalfe NB, Mangel M. Seasonal matching of foraging to anticipated energy requirements in anorexic juvenile salmon. Proceedings of the Royal Society of London, Series B: Biological Sciences. 1996;263:13–18. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference; a practical information-theoretical approach. second edition Springer; 2002. [Google Scholar]
- Caswell H. Matrix population models. second edition Sinauer; Sunderland, Massachusetts: 2001. [Google Scholar]
- Cator LJ, Arthur BJ, Harrington LC, Hoy RR. Harmonic convergence in the love songs of the dengue vector mosquito. Science. 2009;323:1077–1079. doi: 10.1126/science.1166541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, Petrarca V, do Rosario VE. ‘A mate or a meal’: pre-gravid behaviour of female Anopheles gambiae from the islands of São Tomé and Príncipe, West Africa. Malaria Journal. 2003;2:9. doi: 10.1186/1475-2875-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves LF, Harrington LC, Keogh CL, Nguyen AM, Kitron UD. Blood feeding patterns of mosquitoes: random or structured? Frontiers in Zoology. 2010;7:3. doi: 10.1186/1742-9994-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Vol. 2: Sensory Reception and Behaviour. CABI; Wallingford: 1999. [Google Scholar]
- Collier TR, Murdoch WW, Nisbet RM. Egg load and the decision to host-feed in the parasitoid, Aphytis melinus. Journal of Animal Ecology. 1994;63:299–306. [Google Scholar]
- Costantini C, Sagnon N, Torre A. Della, Diallo M, Brady J, Gibson G, Coluzzi M. Odor-mediated host preferences of West-African mosquitoes, with particular reference to malaria vectors. American Journal of Tropical Medicine and Hygiene. 1998;58:56–63. doi: 10.4269/ajtmh.1998.58.56. [DOI] [PubMed] [Google Scholar]
- Fernandes L, Briegel H. Reproductive physiology of Anopheles gambiae and Anopheles atroparvus. Journal of Vector Ecology. 2004;30:11–26. [PubMed] [Google Scholar]
- Finstad AG, Berg OK, Forseth T, Ugedal O, Næsje TF. Adaptive winter survival strategies: defended energy levels in juvenile Atlantic salmon along a latitudinal gradient. Proceedings of the Royal Society B. 2010;277:1113–1120. doi: 10.1098/rspb.2009.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WA, Takken W. Nectar-related vs. human-related volatiles: behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding. Bulletin of Entomological Research. 2004;94:145–157. doi: 10.1079/ber2003288. [DOI] [PubMed] [Google Scholar]
- Garcia-Saenz A, McCarter P, Baylis M. The influence of host number on the attraction of biting midges, Culicoides spp., to light traps. Medical and Veterinary Entomology. doi: 10.1111/j.1365-2915.2010.00904.x. In press. doi: 10.1111/j.1365-2915.2010.00904.x. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Medical and Veterinary Entomology. 2004;18:102–107. doi: 10.1111/j.0269-283X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Diel timing and frequency of sugar feeding in the mosquito Anopheles gambiae, depending on sex, gonotrophic state and resource availability. Medical and Veterinary Entomology. 2006;20:308–316. doi: 10.1111/j.1365-2915.2006.00638.x. [DOI] [PubMed] [Google Scholar]
- Gary RE, Cannon JW, Foster WA. Effect of sugar on male Anopheles gambiae Giles (Diptera: Culicidae) mating performance, as modified by temperature, space, and body size. Parasites and Vectors. 2009;2:19. doi: 10.1186/1756-3305-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle LK, Gosler AC. Fat reserves and perceived predation risk in the great tit, Parus major. Proceedings of the Royal Society of London, Series B. 2001;268:487–491. doi: 10.1098/rspb.2000.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, Russell I. Flying in tune: sexual recognition in mosquitoes. Current Biology. 2006;16:1311–1316. doi: 10.1016/j.cub.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Gillies MT. The recognition of age-groups within populations of Anopheles gambiae by the pre-gravid rate and the sporozoite rate. Annals of Tropical Medicine and Parasitology. 1954;48:58–74. doi: 10.1080/00034983.1954.11685599. [DOI] [PubMed] [Google Scholar]
- Gillies MT. Selection for host preference in Anopheles gambiae. Nature. 1964;203:852–854. doi: 10.1038/203852a0. [DOI] [PubMed] [Google Scholar]
- Hancock RG, Foster WA. Larval and adult nutrition effects on blood/nectar choice of Culex nigripalpus mosquitoes. Medical and Veterinary Entomology. 1997;11:112–122. doi: 10.1111/j.1365-2915.1997.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? Journal of Medical Entomology. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Heimpel GE, Rosenheim JA. Dynamic host feeding by the parasitoid Aphytis melinus : the balance between current and future reproduction. Journal of Animal Ecology. 1995;64:153–167. [Google Scholar]
- Houston AI, McNamara JM. The choice of two prey types that minimises the probability of starvation. Behavioral Ecology and Sociobiology. 1985;17:135–141. [Google Scholar]
- Houston AI, McNamara JM. Models of Adaptive Behaviour. An Approach Based on State. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- Impoinvil DE, Kongere JO, Foster WA, Njiru BN, Killeen GF, Githure JI, Beier JC, Hassanali A, Knols BJG. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Medical and Veterinary Entomology. 2004;18:108–115. doi: 10.1111/j.0269-283X.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Briegel H. Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. Journal of Vector Ecology. 2004;29:140–153. [PubMed] [Google Scholar]
- Ma BO, Roitberg BD. The role of resource availability and state-dependence in the foraging strategy of blood-feeding mosquitoes. Evolutionary Ecology Research. 2008;10:1111–1130. [Google Scholar]
- Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, Hassanali A. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malaria Journal. 2007;6:113. doi: 10.1186/1475-2875-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy WM, Foster WA. Antagonistic effects of energy status on meal size and egg-batch size of Aedes aegypti (Diptera; Culicidae) Journal of Vector Ecology. 2004;29:84–93. [PubMed] [Google Scholar]
- Nayar JK, Sauerman DM. The effects of nutrition on survival and fecundity in Florida mosquitoes part 2: utilization of a blood meal for survival. Journal of Medical Entomology. 1975;12:99–103. doi: 10.1093/jmedent/12.1.99. [DOI] [PubMed] [Google Scholar]
- Nayar JK, Van Handel E. The fuel for sustained mosquito flight. Journal of Insect Physiology. 1971;17:471–481. doi: 10.1016/0022-1910(71)90095-3. [DOI] [PubMed] [Google Scholar]
- Onyabe DY, Roitberg BD, Friend WG. Feeding and mating in Anopheles (Diptera: Culicidae): theoretical modeling approach. Journal of Medical Entomology. 1997;34:644–650. doi: 10.1093/jmedent/34.6.644. [DOI] [PubMed] [Google Scholar]
- Pennetier C, Warren B, Dabire K. Roch, Russell I, Gibson G. ‘Singing on the wing’ as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Current Biology. 2010;20:131–136. doi: 10.1016/j.cub.2009.11.040. [DOI] [PubMed] [Google Scholar]
- Roitberg BD, Friend WG. A general theory for host seeking decisions in mosquitoes. Bulletin of Mathematical Biology. 1992;54:401–412. doi: 10.1007/BF02464840. [DOI] [PubMed] [Google Scholar]
- Roitberg BD, Keiser S, Hoffmeister T. State-dependent attacks in a mosquito. Physiological Entomology. 2009;35:46–51. [Google Scholar]
- Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. American Journal of Tropical Medicine and Hygiene. 1997;57:235–239. doi: 10.4269/ajtmh.1997.57.235. [DOI] [PubMed] [Google Scholar]
- Stone CM, Taylor RM, Foster WA. An effective indoor mesocosm for studying populations of Anopheles gambiae in temperate climates. Journal of the American Mosquito Control Association. 2009a;25:514–516. doi: 10.2987/08-5885.1. [DOI] [PubMed] [Google Scholar]
- Stone CM, Taylor RM, Roitberg BD, Foster WA. Sugar deprivation reduces insemination of Anopheles gambiae (Diptera: Culicidae), despite daily recruitment of adults, and predicts decline in model populations. Journal of Medical Entomology. 2009b;46:1327–1337. doi: 10.1603/033.046.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straif SC, Beier JC. Effects of sugar availability on the blood feeding behaviour of Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 1996;33:608–612. doi: 10.1093/jmedent/33.4.608. [DOI] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. Journal of Medical Entomology. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Van Handel E. The obese mosquito. Journal of Physiology. 1965;161:478–486. doi: 10.1113/jphysiol.1965.sp007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel E. Rapid determination of glycogen and sugars in mosquitoes. Journal of the American Mosquito Control Association. 1985;1:299–301. [PubMed] [Google Scholar]
- Van Handel E, Day JF. Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field-collected Aedes vexans. Journal of the American Mosquito Control Association. 1988;4:549–550. [PubMed] [Google Scholar]
- Warren B, Gibson G, Russell I. Sex recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Current Biology. 2009;19:485–491. doi: 10.1016/j.cub.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Witter MS, Cuthill IC. The ecological costs of avian fat storage. Philosophical Transactions of the Royal Society of London, Series B. 1993;340:73–92. doi: 10.1098/rstb.1993.0050. [DOI] [PubMed] [Google Scholar]
- Yuval B. Mating systems of blood-feeding flies. Annual Review of Entomology. 2006;51:413–440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]