Abstract
Differences in cellular competence offer explanation of differences in the healing capacity of tissues of various ages and conditions. The homeobox family of genes plays key roles in governing cellular competence. Of these, we hypothesize that Msx2 is a strong candidate regulator of competence in skin wound healing because it is expressed in the skin during fetal development at stage of scarless healing, affects postnatal digit regeneration, and is re-expressed transiently during postnatal skin wound repair. To address if Msx2 affects cellular competence in injury repair, three millimeter full thickness excisional wounds were created on the back of C.Cg-Msx2tm1Rilm/Mmcd (Msx2 null) mice and the healing pattern was compared with that of the WT mice. Results show that Msx2 null mice exhibited faster wound closure with accelerated re-epithelialization plus earlier appearance of keratin markers for differentiation and increased level of smooth muscle actin and tenascin in the granulation tissue. In vitro, keratinocytes of Msx2 null mice exhibit increased cell migration and the fibroblasts stronger collagen gel contraction. Thus, our results suggest that Msx2 regulates cellular competence of keratinocytes and fibroblasts in skin injury repair.
Keywords: Skin wound healing, regeneration, re-epithelialization, keratinocyte migration, fibroblast contraction collagen gel, homeobox gene, Msx2 mouse model
INTRODUCTION
Cellular competence is defined as the ability of the target tissue to respond to stimuli. A good example of cellular competence lies within wound healing. Upon injury to adult skin, tissue strives to regain homeostasis by wound repair. In the acute phase, a fibrin blood clot forms to stop bleeding and acts as a wound barrier and provisional matrix for the following events of wound healing to occur. Inflammation quickly ensues, signaling specific responses to the damaged area and for removal of tissue debris. Cytokines and growth factors are secreted to aid in the wound healing process. Within hours, re-epithelialization begins with epidermal cell proliferation and migration followed by extended stages of fibroplasia, angiogenesis, wound contraction, extracellular matrix (ECM) remodeling, and scar maturation 1, 2. This wound repair scenario is most often the case in adult mammals without the regeneration of epidermal appendages such as hair or glands.
Injury of fetal skin in vertebrate animals during certain gestational stages can heal in a regenerative fashion without scar formation. In mice and rats, for instance, fetal skin injuries can heal without scars between embryonic day 14 to 16 3, 4, 5. This age-dependent repair phenomenon is also observed during postnatal digit repair of mice 6. Interestingly, the regenerative capacity seems to be influenced by homeobox genes and/or molecules of the TGF-beta superfamily. Homeobox genes are a family of transcriptional regulators that contain a common sequence element of 180 base pairs known as the homeobox region, which encodes a DNA binding homeodomain, comprised of 60 amino acids7. Homeobox genes exhibit regional specificity and are expressed in nested patterns throughout the body of a developing organism, critical in pattern formation during embryonic morphogenesis8. Thus they are also known as master regulators of pattern formation and have been discovered in a multitude of organisms from earthworms to humans. Homeobox genes are categorized into two classes: hox genes and non-hox genes. Hox genes are clustered in four genomic loci (A, B, C, and D) while non-Hox genes are dispersed throughout the genome9. Accordingly, Msx1 homeobox gene and its upstream partner, Bmp4 influence digit tip regeneration 6, 10, 11, while the ratio between TGF-beta1, beta2, and beta3 affect the healing capacity of skin injury 4, 12 . Perhaps the most dramatic examples of adult regeneration are in amphibian limb regeneration 13 and tail regeneration in adult lizards 14. The different responses in wound healing between adult and fetal skin may depend on the “competence” of the responding tissues and cells, in such that fetal or younger tissues have the ability to regenerate better than aged tissue 15. Therefore, while the injury may be the same, the outcome can vastly differ depending on the competence of the cells which is influenced by a multitude of aspects ranging from animal species, age, and anatomical structure and location of the injury. Besides Msx, Hoxd3, Hoxd8, Hoxd10, Hoxb4, Prx2, Pou2f3 have also been documented to vary the level of expression and/or modulate cellular behavior during wound healing and, in most cases, they are differentially expressed from embryonic stages of development to adulthood 9, 16-19. Here, we have chosen to study the role of Msx2 homeobox transcription factor in cellular competence during skin wound healing because Msx2 is implicated in regeneration of vertebrate digit organ regeneration 10 and it is differentially expressed in the dermis and epidermis between fetal and adult skin in which its expression coincides with the stage of fetal scarless skin repair 6, 9, 20. We report herein the expression of Msx2 during normal skin wound healing and the effects of Msx2 on skin wound healing using Msx2 null mice.
MATERIAL AND METHODS
Production and Genotyping of Msx2 Deficient Mice
Animal utilization and care were approved by the Institutional Animal Care and Utilization Committee (IACUC) of University of Southern California. Msx2 deficient mice (Msx2tm1Rilm/Mmcd), or Msx2 null, were generated by inserting the neo cassette into the Nde1 site, 5′ of the Msx2 homeobox region in exon 2 as previously described 21. The following primers were used for amplification: Sxg2 (neo; 5′-tctggacgaagagctagg-3′), Sxg4 (5′-ccctctctgtcctctaggac-3′), Sxg5 (5′-gcctgagggcagcataggct-3′). Allele products were amplified using primer 2/5 for mutant (650bp) and primer 4/5 for wildtype (350bp). The amplification was carried out in the following manner: 94°C for 3 minutes (1 cycle); 94°C for 1 minute, 61°C for 1 minute, 72°C for 1.5 minute (40 cycles); and 72°C for 10 minute.
220bpMsx2-lacZ transgenic mice
220bpMsx2-lacZ transgenic mice were produced by fusing Msx2 promoter fragment with the hsp68 minimal promoter and a lacZ reporter. The 220 bp fragment used is located between −3521 and −3310 bp upstream of the Msx2 translation start site 22. Expression of Msx2 was monitored by staining for β-galactosidase activity. Frozen sections or whole skin pieces were incubated overnight at 4°C in X-Gal staining solution (2mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 0.1% 4-chloro-5-bromo-3-indolyl β-galactosidase (X-Gal) (Research Organics, Cleveland, OH), and 5mM potassium ferrocyanide in phosphate-buffered saline (PBS)).
Full thickness excision wound
Animal utilization, care, wounding and post wounding care were approved by the Institutional Animal Care and Utilization Committee (IACUC) of University of Southern California. Four week old Msx2 null mice and their littermate controls were used in the experiments. Mice were anesthetized by intraperitoneal injections of Xylazine (20ng/ml) 5mg/kg and Ketamine (100mg/ml) 50mg/kg and shaved. Sterile surgical technique was used following a cleansing of the dorsal skin with betadine scrub and a wash with 70% alcohol. To minimize the influence of hair follicles on wound healing, wounds were created on these mice when their hairs were undergoing exogen, the resting phase of the hair cycle. Four 3mm excisional wounds were made with dermal punch biopsies on the dorsum of each mouse. Mice were housed separately after wounding, and acetaminophen 100mg/ml was placed in the drinking water for pain relief. Wound sites were monitored daily for signs of infection/healing with photo-documentation, which was performed with a digital camera starting at day zero and everyday thereafter for 15 days.
In situ hybridization
Specimens were harvested at different Post Wound Days (PWD one, three, five, seven, and fourteen), in the manner as stated above. Samples were dehydrated through a series of ethanol and placed into paraffin blocks for 7mm cryo-sections. The sections were then fixed and rehydrated in methanol according to the standard protocol. All solutions used for the procedure were treated with diethyl pyrocarbonate to deactivate RNase. To detect the RNA expression, the tissue was hybridized with digoxigenin-labeled probes and detected using anti-digoxigenin antibody conjugated with alkaline phosphatase (Roche Basel, Switzerland).
Immunohistochemistry analysis
Wound specimens were fixed in 4% paraformaldehyde for 24 hours at 4°C followed by dehydration in a series of ethanol and paraffin embedding. Sections were made by orienting and cutting through the center of the wound bed and were re-hydrated through a series of ethanol series and then distilled water. Antigen retrieval was performed by boiling sections in citrate buffer (pH 6.0) for 20 minutes and cooling (20 minutes). Immunohistochemistry was performed by incubating sections in 10% serum blocking solution (30 minutes) and followed by incubating with primary antibody, overnight at room temperature. After washing samples in PBS, samples were incubated with secondary antibody conjugated with HRP-conjugated antibody (1 hour, room temperature). After washing with PBS, bound secondary antibody was detected using diaminobenzidine peroxidase substrate (Sigma FASTDAB tablets). The source and dilution of primary antibodies were: Keratin 14 (Krt 14) (Berkeley Antibody Company) 1:400 dilution; Keratin 10 (Krt 10) (Sigma) 1:200 dilution; α-smooth muscle actin (SMA) (Chemicon) 1:200 dilution; β1-Integrin (Santa Cruz) 1:200 dilution; and rabbit anti-tenascin C (CT) was raised in the laboratory of Dr. C-M. Chuong, 1:500 dilution.
Primary keratinocyte and fibroblast cultures
Newborn mice were euthanized (protocol approved by IACUC of University of Southern California), soaked in Betadine for 5 minutes, rinsed twice in 70% alcohol, and then sterile water. Using a sterile technique, the mouse skin was peeled off, stretched out with the dermis faced down in a 100 mm culture dish, and floated on the surface of 0.25% trypsin at 4°C for 18 hours. Epidermis was then separated from the dermis, minced, and treated/digested with Dispase (Sigma) in keratinocytes growth medium (KGM, Gibco) supplemented with Ca2+ (1.4mM) for 1 hour. The undigested cornified fragments were removed by filtering the cell suspension through a sterile, 100 m mesh nylon gauze. The cells were then collected by centrifugation, resuspended in fresh KGM (0.3 mM Ca2+) supplemented with 10% FBS, and plated at a density of 3 × 105/cm2 on type I collagen coated six-well plates. After 24 hours, attached keratinocytes were rinsed with PBS, and incubated with fresh KGM (0.05mM Ca2+).
To isolate fibroblasts, dermis was minced, placed in the DMEM containing dispase II (0.5%, Boeheringer Mannheim) and collagenase II (1000 U/ml, Worthington Biochemical), and incubated at 37°C with gentle shaking for 2 hours. The preparation was subsequently filtered through a 100 μm mesh nylon gauze to collect the cells, which was centrifuged and the cell pellet was suspended in 20% FCS-DMEM, centrifuged again, and re-suspended in fresh 20% FCS-DMEM. The final cell suspension was plated at a concentration of 1 × 106 cells/10 ml in 100mm tissue culture plate. The un-attached cells were removed by changing the culture medium into 10%FCS-DMEM.
Keratinocyte motility assay
Keratinocytes (≤ 2 passages) were harvested, resupended in KGM and subjected to cell migration assay on coverslip surface covered with colloidal gold particles coated with collagen 23, 24. The coverslips were prepared by dipping into 1% freshly prepared BSA/PBS and 100% ethanol sequentially for 5 seconds each, and placed into 12-well tissue culture plates to dry. Gold salt solution (9% of gold salt, 52% water, and 30% Na2CO3) was brought to boiling temperature, removed from heat source, and added drop-wise an equal volume of freshly prepared formaldehyde (0.1%). The mixture (purple-brown in color) was immediately added into the 12-well plates containing BSA-coated coverslips (3.5ml per well). Plates were covered and left undisturbed for 24 hours at room temperature. Subsequently the coverslips were rinsed with Hank’s buffered salt solution (HBSS) and coated with collagen (1ml HBSS containing 5mM Ca2+ and 30μg/ml type I collagen) at 37°C for 2 hours. Four thousand cells in 200 ul either serum-free KGM or KGM plus recombinant epidermal growth factor (50 ng/ml, Gibco) were added onto each coverslips and incubated for 24hours at 37°C. Following incubation, medium was removed and cells were fixed in 0.1% formaldehyde in PBS. Cells migrate by phagocytosing or displacing the gold particles, which results in areas devoid of gold particles (migration tracks). The migration track was examined under dark field optics and photographed. Fifteen randomly selected and non-overlapping fields under each experimental condition were photographed and analyzed digitally to calculate migration index (MI). The MI = 100 % x (area size of migration track / the total area viewed).
Collagen gel contraction assay
Fibroblasts contraction of collagen gel (attached format) was performed using the procedure described previously 4. Gel contraction by fibroblasts was measured every 30 minutes and the extent of collagen gel contraction was expressed as a percentage of original gel thickness.
RESULTS
Msx2 is transiently induced during skin wound healing
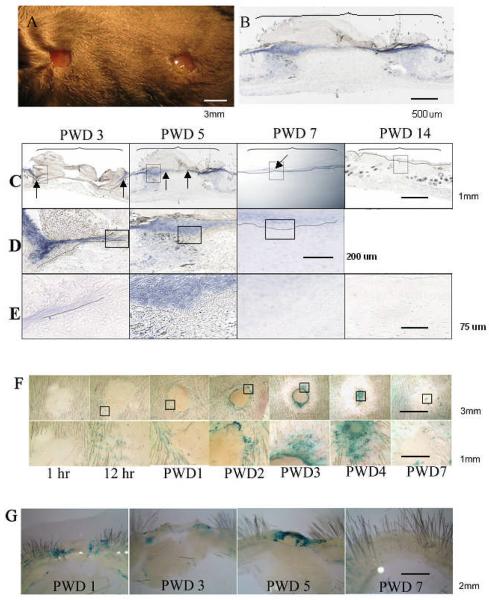
In the unwounded skin Msx2 can only be detected in hair follicles of mice 25. To study if Msx2 is expressed in the adult skin following wounding, we used two approaches: 1) in situ hybridization for Msx2 mRNA expression in skin wounds of wild type (WT) mice; 2) β-galactosidase staining for protein expression of skin wounds from 220bpMsx2-lacZ transgenic mice 22. Accordingly, mice were wounded via punch biopsies creating excisional wounds of 3mm diameter in size (Fig. 1A). In situ hybridization was used to detect the levels of Msx2 mRNA in skin wounds of WT mice (n = 3). A low magnification view of the wound bed at post wounding day (PWD) 5 is shown in Fig. 1B. Cross sections of the wounds from different dates with increasing magnifications are shown in Fig. C through E. The results show that the expression of Msx2 mRNA is induced in the skin of WT mice during wound healing (Fig. 1B-1E). At PWD 3, Msx2 is detected in the epidermis flanking the wound edges (blue color and arrows). The staining can be seen in multiple layers of the epidermis (Fig. 1C, D, and E: PWD 3). By PWD 5, Msx2 is detected in both the epidermis flanking the wound and areas in the wound bed (arrows) (Fig. 1C, D, and E: PWD 5). By PWD 7, the newly formed epidermis has completely covered the wound bed and Msx2 is detected only at the differentiated layers of the newly formed epidermis (Fig. 1C, D, and E: PWD 7). By PWD 14, Msx2 is present only in hair follicles and is no longer detectable in the epidermis (Fig. 1C: PWD 7) or the granulation tissue/dermis (Fig. 1E: PWD 7).
Figure 1. Msx2 mRNA is transiently induced in the epidermis during skin wound healing.
(A): An example of a WT mouse with two excisional wounds, 3mm in diameter, created by punch biopsies. (B) through (E): Levels of Msx2 mRNA in the cross sections of skin wounds detected by in situ hybridization. (B): A low magnification view of the wound bed (bracketed area) at PWD 5. (C) through (E): Successive magnifications of the wounds (framed areas) from different dates, i.e., PWD 3, PWD 5, PWD 7, and PWD 14. Dotted line indicates where the basement membrane is. In situ whole mounts (F) and cross section views (G) of a healing wound created on the dorsum of 220bpMsx2-lacZ transgenic mouse. The expression of Msx2 was detected by β-galatosidase staining. The framed areas in the upper panels of (F) are enlarged in the lower panels. See “Results” for detailed description.
Similar wounds were created on the dorsum of 220bpMsx2-lacZ transgenic mice to further study Msx2 expression by β-galactosidase staining (Fig. 1F, in situ whole mount; Fig. 1G, cross section views). There was no detectable expression of Msx2 one hour post-wounding (Fig. 1F: 1 hr). However, by 12 hours, distinct staining can be seen in the hair follicles surrounding the wound bed (Fig. 1F: 12 hr). The staining was intensified along the edge of the wound from PWD 1 to PWD3 (Fig. 1F: PWD1, PWD2, PWD3). At PWD 4 as the wound proceeded to heal, evident by the reduced ring size that marked the advancing front of the epidermal cells, there was increasing staining of beta-galactosidase in the wound bed (Fig. 1F: PWD4). The staining was drastically decreased by PWD7, as the wound seemed to be completely surfaced by the epidermal cells (Fig. 1F: PWD7). Sectional areas of the wounds (Fig. 1G) showed that the expression of β-galactosidase staining is largely localized to the epidermis of the wound (Fig. 1G, PWD 1 to PWD 5). Consistent with the results of the whole mount studies (Fig. 1F), by PWD 7 the β-galactosidase staining has largely disappeared from the wound bed (Fig. 1 G: PWD 7). Collectively, these results demonstrate that Msx2 is expressed in the hair follicles of unwounded mouse skin, and is transiently up-regulated in the migrating and/or proliferating epidermal cells during the healing of adult mouse skin wounds.
Msx2 null mice exhibit a faster rate of skin wound closure
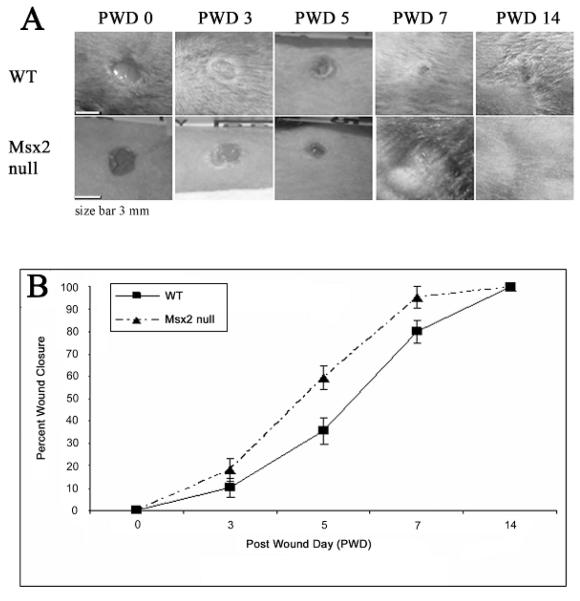
Three millimeter diameter full thickness excisional wounds were created on both Msx2 null (C.Cg-Msx2tm1Rilm/Mmcd) and wild type C57Bl/6 littermate control mice (n ≥ 3) to study the effect of Msx2 on skin wound healing. At different post wounding dates, photographs were taken (Fig. 2A), the size of opening/wound edge measured, and the percent of wound closure calculated (the percentage of the remaining open wound over the original wound size) (Fig. 2B). The results show that the skin wounds of Msx2 null mice close considerable faster than the WT mice (PWD 7 vs. PWD 14). The largest difference in wound closure occurred between PWD 5 and PWD 7 (Fig. 2B).
Figure 2. Msx2 null mice exhibit an accelerated rate of wound closure.
(A): Punch biopsy wounds of 3 mm diameter in size were created on the back of WT or Msx2 null mouse skin and digital photos were taken at time of wounding (PWD 0), PWD 3, PWD 5, PWD 7 and PWD 14. (B): The graph depicts percent of wound closure over PWD 3, PWD 5, PWD 7 and PWD 14 (n = 3).
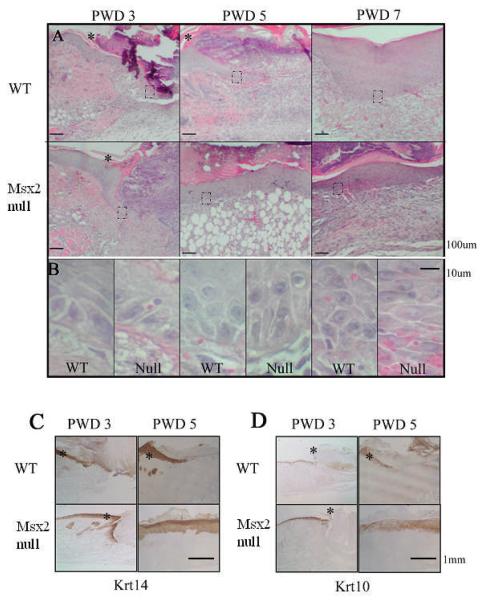
Since wound closure could be contributed by re-epithelialization and/or enhanced wound contraction, we examined the wounds at the histological level for markers of keratinocytes in re-epithelialization (Fig. 3) and of fibroblast differentiation in the granulation tissue (Fig. 4). In WT samples, the epithelial tongue extends toward the wound bed at PWD 3 and PWD 5 (Fig. 3A, WT: PWD3 and PWD5). Higher magnification of the epithelial tongue region shows these keratinocytes are polygonal in shape, and only begin to form multiple layers at PWD 7 (Fig. 3B, WT: PWD3 and PWD5). In contrast, in Msx2 null mice, the epithelial tongue is present by PWD 3 and the newly formed epidermis completely covers the wound by PWD 5 (Fig 3A, Msx2 null: PWD3 and PWD5). A fully stratified keratinocyte layer is seen by PWD 7 (Fig. 3A, Msx2 null: PWD7). At a higher magnification keratinocytes of Msx2 null mice appear to be more elongated at PWD 3 and PWD 5 (Fig. 3B, Null: PWD3 and PWD5). At PWD 7, these keratinocytes have formed stratified layer with horizontally arranged stratum spinosum (Fig. 3B, Null: PWD7). Therefore, differences exist in both the speed of re-epithelialization as well as the morphology of keratinocytes between WT and Msx2 null mice. The discrepancy in the timing of closure between the whole mount (Fig. 2) and histological sections (Fig. 3) can be explained by the different criteria used in defining wound closure. The whole mount wounds (Fig. 2) were studied by visual examination of the margins of the wound and measuring the healing area including the scab, while histological sections were done by vertical sectioning through the center of the wound and examining the sections under a microscope (Fig. 3). Therefore, keratinocytes have already completely surfaced the wound underneath the scab at PWD 5 for Msx2 null mice.
Figure 3. Msx2 null wounds show faster re-epithelialization.
Hemotoxylin and eosin staining was performed on samples of WT and Msx2 null wounds (A). Magnified views of the framed areas (dotted boxes) are shown in (B). Asterisks in (A) demarcate the edge of the wound and the wound is to the right of the asterisk. Immunohistochemistry was performed on wound areas at PWD 5 and PWD 7 for Krt14 (C) or Krt10 (D) staining. See “Results” for detailed description.
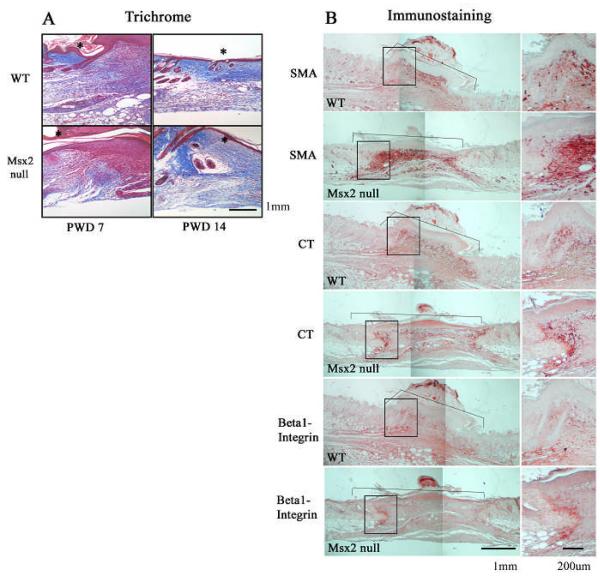
Figure 4. Msx2 null wounds show higher levels in the markers of fibroblast differentiation.
Trichrome staining was performed at PWD 7 and PWD 14 (A). The edge of the wound is marked by an asterisk. The wound is to the right to the asterisk. Immunostaining for smooth muscle actin (SMA), tenascin C (CT), and β1- Integrin at PWD 7 in WT and Msx2 null skin wounds (B). Wound area is demarcated by a bracket. The boxed areas of the wound are magnified (the right hand panels). See “Results” for detailed description.
Due to the difference in keratinocyte re-epithelialization between WT and Msx2 null mice, we further examined these sections with markers of keratinocyte differentiation (Fig. 3C and 3D) (n = 3). Samples were taken at PWD 3 and PWD 5 and processed for the following molecular markers: Krt14 for basal cells (Fig. 3C) and Krt10 for supra-basal layer of cells (Fig. 3D). In the WT mouse, keratinocytes stained positive for Krt14 at PWD 3 at the epithelial tongue, which stained weakly for Krt10 (Fig.3C, WT: PWD 3; Fig.3D, WT: PWD 3). As expected, no staining for either marker could be seen in the denuded region of the wound bed at PWD 3. At PWD 5, the epithelial tongue advanced into the wound bed and was staining positive for both Krt14 and Krt10 (Fig.3C, WT: PWD 5; Fig.3D, WT: PWD 5). In contrast, for Msx2 null mice, Krt14 was present on the epithelial tongue at PWD 3 and on all layers of the newly formed epidermis at PWD 5 that covered the wound bed (Fig. 3C, Msx2 null: PWD 3 and PWD 5). Moreover, the epidermis was staining positive for Krt10 at PWD 3 (normal epidermis flanking the wound) and PWD 5 (newly formed epidermis) (Fig. 3D). These results indicate that a faster re-epithelialization and keratinocyte differentiation occurred in the absence of Msx2 during mouse skin wound healing.
Fibroblast differentiation was studied using immunohistochemistry for collagen (Masson’s trichrome), tenascin C (CT), integrin β1, and myofibroblasts (α smooth muscle actin, SMA). A set of images representing typical results of the study is presented in Fig. 4A and 4B. First of all, among the three each of WT and Msx2 null mice studied, a greater amount of granulation tissue seems to be present in the wounds of Msx2 null mice at PWD 14 (Fig. 4A, PWD 14). No significant difference, otherwise, was observed in the orientation or thickness of collagen fibers between WT and Msx2 null mouse wounds (Fig. 4A). In contrast, the content of SMA was much more intense in the granulation tissue at the margin of the wound in Msx2 null mice at PWD 7 (Fig. 4B). Moreover, there was an increase of CT at the same region in the granulation tissue of Msx2 null mouse wounds (Fig. 4B). The distribution of β1 Integrin, which mediates cell-extracellular matrix interactions, seemed to be different in the granulation tissue between WT and Msx2 null mouse wounds, which the latter was more localized at the margin of the wound (Fig. 4B).
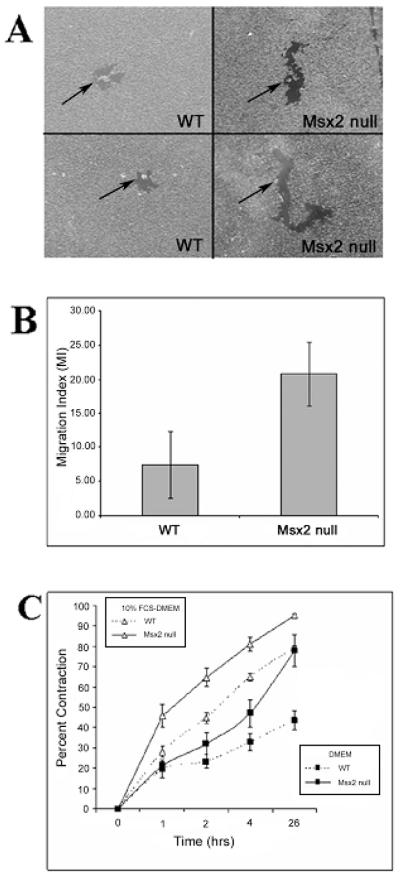
In vitro analyses of keratinocyte migration and fibroblast contraction of collagen gels
The faster rate in re-epithelialization by keratinocytes in skin wounds of Msx2 null mice may attribute to either an increase capacity in keratinocytes proliferation or migration or both. We performed PCNA (Proliferating Cell Nuclear Antigen) staining and detected no difference in the staining/keratinocyte proliferation between Msx2 null and WT mice (data not shown). To test whether the faster re-epithelialization in Msx2 null mice was due to a faster rate of keratinocyte migration, we used an in vitro colloidal gold assay to examine the migratory ability of keratinocytes on a collagen type I substrate 26(Fig. 5A). The results showed that Msx2 null keratinocytes migrate about twice as fast as WT keratinocytes over the course of 24 hours (n = 3) (Fig. 5B). A typical image of the assay result is shown in Fig. 5A. Therefore, an increased rate in cell migration by keratinocytes may have contributed to the faster rate of re-epithelialization in the skin wounds of Msx2 null mice. A three dimensional collagen gel contraction model was used to study if fibroblasts from skin of Msx2 null mice differ from the WT mice in contraction of the collagen matrix, which may also contribute to wound closure. The first passage of dermal fibroblasts isolated from the skin of WT or Msx2 null mice were used and the assay was performed under serum free (DMEM only) or serum-containing (10% FCS-DMEM) conditions (n = 3). Results showed that in DMEM Msx2 null fibroblasts displayed a significantly greater degree of gel contraction than WT fibroblasts (Fig. 5C). Furthermore, serum stimulated collagen gel contraction by both WT and Msx2 null mouse fibroblasts and under the condition Msx2 null fibroblasts still displayed a greater degree of gel contraction than WT fibroblasts (Fig. 5C).
Figure 5. In vitro, Msx2 null keratinocytes showed increased migration and Msx2 null fibroblasts display greater ability in collagen gel contraction.
(A): Keratinocytes isolated from Msx2 null and WT newborn pups were plated onto gold salt coated coverslips and allowed to migrate for 24 hours (Materials and Methods). (B): The migration index (MI) (Materials and Methods) is being compared between Msx2 null and WT keratinocytes. (C): Collagen gel contraction by WT and Msx2 null fibroblasts in both DMEM and 10% FCS-DMEM. Triplicate samples were used for each genotype in each assay and each assay was repeated at least 3 times.
DISCUSSION
Msx2 is an important transcription factor that regulates the development of various organs. In humans, Msx2 gene localizes to chromosome 5 and a mutation in the homeodomain causes craniosynostosis, the premature fusion of calvarial sutures and a common developmental anomaly that causes abnormal skull shape 27. In developing human fetal skin, Msx2 expression patterns were detected throughout the epidermis and dermis, with the greatest level of expression in the suprabasal epithelial layer. In the adult human skin, Msx2 was confined to the basal and suprabasal layers of the epidermis, with the strongest expression in the hair follicles 9 and was required, with Foxn1, to maintain Notch1 expression in the hair follicle matrix 25, 28. In this study, we found that Msx2 is transiently up-regulated during healing of the excisional wounds of normal mouse skin and its expression coincides with the migratory pattern of epithelial cells. In addition, Msx2 null mice demonstrate faster re-epithelialization and wound closure in comparison to their WT littermate controls. When wound healing is compared at the cellular level, differences in keratinocyte differentiation is noted. At the microscopic level, collagen, SMA, and tenacin C are elevated in granulation tissue of Msx2 null mice compared to that of same stage WT controls. In vitro assays reveal a faster keratinocyte migration by Msx2 null keratinocytes and an increased ability in collagen matrix contraction by Msx2 null fibroblasts. Therefore, the results suggest that Msx2 regulate cellular competence of keratinocytes and fibroblasts in skin injury repair.
Injury repair is one of the most complex biological processes. After an injury, multiple biological pathways immediately become activated and are synchronized to respond. In the postnatal stage, the process commonly leads to a non-functioning mass of fibrotic tissue known as a scar. By contrast, early in gestation, injured fetal tissues can heal in a regenerative fashion without scar formation 1. These differences in response to the same stimuli may be explained by the developmentally regulated general make up of the participating soluble and cellular and extracellular matrix components 15. More importantly, however, is the intrinsic difference in the composition of sub-cellular ingredients that govern cellular response or competence. The competence is molecularly based on the makeup of different cell membrane receptors, intracellular signaling molecules, transcription factors and chromatin modifications. The homeobox transcription factors such as Msx1 and Msx2 are candidates of such molecules in which they differentially expressed by cells of different developmental stages and in a variety of locations involved typically in epithelial-mesenchymal interactions during organ morphogenesis 27, 29, 30. Furthermore, Msx2 is expressed in developing epidermis and hair follicles in mouse fetus while limited to the hair follicle in adult skin 31. As shown in this study, Msx2 is transiently induced in the epidermal cells upon wounding, beginning at PWD 1, reaching higher level by PWD 3-5, dissipating by PWD 7, and is completely absent from the epidermis by PWD 14 (Fig. 1). Interestingly, the expression seems to start from the infundibulum region of the hair follicles surrounding the wound, and appears to migrate towards the wound as shown by the image of a centripetally arranged blue tracks. This is reminiscent of similar tracks observed in the wounding of Tg(Krt15-cre/PGR)22Cot;R26R 32, 33. It is also consistent with the thought that some cells derived from infundibulum regions of hair follicles emigrate to the epidermis 34, 35. While the results of these previous investigations suggest that cells from hair follicles migrate out to aid in the process of re-epitheliazation during skin wound repair, Ito et al. 32, 33 observed that the bulge derived cells are there only for a few weeks, and eventually disappear from the epidermis. On the other hand, Morgan et al.34, 35, using ShhGFPcre;R26R mice, showed that some cells in the upper follicles indeed stay in the epidermis. Collectively, these studies point out to the possible multiple origins of cells in the re-epitheliazation process, i.e., from the proliferation of the transiently amplifying cells in the basal layer, activated stem cell in the basal layer, de-differentiation of suprabasal keratinocytes, both follicle bulge and non-bulge cells, or the combination of some of these possibilities 36. Based on the pattern of Msx2 expression in our study, we speculate that the Msx2 positive epidermal cells from the infundibulum of hair follicles are activated, migrate to the wound, and participate in re-epithelialization (Fig.1).
A balanced homeostasis during the healing process is required among proliferation, differentiation, apoptosis and migration to achieve the formation of a new epithelium that covers the wound. Since the re-conditioning of cell behavior from physiological homeostasis to active re-epithelialization requires epigenetic changes that involves homeobox genes, the accelerated closure of excision, full thickness wound in the dorsal skin of Msx2 null mice (Fig. 2) may be contributed by an increase in keratinocyte migration and differentiation (Fig. 3). Our in vitro migration assay supports the altered/increased migratory phenotype of Msx2 null keratinocytes (Fig. 5A and B). Consistently, past findings from our laboratory have shown that an overexpression of Msx2 seems to have an opposing effect on keratinocytes differentiation with increasing cell proliferation in the suprabasal keratinocytes 8, 31, 37. Interestingly, mice overexpressing Msx2 also exhibit a thicker epidermis accompanied with more hair follicles and hypercellularity, while a lack of Msx2 led to a thinner dermis and ‘cyclic alopecia’, or an asynchronous cycling of hair follicles, and defective hair shaft differentiation 25, 31. Therefore, in the current study we conclude that, when faced with the same wound stimuli, keratinocytes without Msx2 may undergo accelerated differentiation; while keratinocytes with Msx2 stay longer in the morphogenetically malleable progenitor stage and allow time for regeneration.
Also in the current study, unlike the epidermal cells, Msx2 is not induced in fibroblasts during skin wound healing (Fig. 1). Nonetheless, for the Msx2 null mice we observe an increase of SMA in the wound fibroblasts and tenacin C in the extra-cellular matrix of granulation tissue (Fig. 4). It is likely that the effect may be mediated by some factors (co-modulators or targets of Msx2) secreted by the epidermis, highlighting the role of Msx genes in epithelial-mesenchymal interactions 38. Therefore, the absence of Msx2 may alter the microenvironment around the wound and cause a change in the overall pattern of wound healing. As more functional studies are carried out with different homeobox genes, we should gain more insight on the molecular basis of cell competence in response to injuries. For instance, some of the more recent studies have shown that Hoxd3 enhances cell motility, increase the expression of integrin α5β1 and αvβ3 but decrease that of E-cadherin in normal mouse wounds 39; while under diabetic situation, Hoxd3 can accelerate wound healing by inducing collagen synthesis 40. Hoxb4, on the other hand, increases cell proliferation and reduces the expression of α2 integrin 41. Mice deficient of Prx2, a homeobox gene preferentially expressed in mesenchyme, exhibit altered healing of fetal wounds but not post-natal skin wounds 9, 42. Pou2f3 inhibits keratinocytes differentiation induced by wounding 43. Since wound repair recapitulates embryonic morphogenesis, Hox genes are clearly a significant player in controlling cellular competency in post-natal tissue injury repair. Certainly more in depth and cohesive investigations are required to gain insights in the difference among healing of individuals of various age group and disease conditions.
In summary, consistent with the notion suggested in earlier correlated studies 9, this study presents an example in which the presence or absence of a homeobox gene can affect the course of wound healing by altering the timing of proliferation and differentiation. The study also pointed out that Msx2 expression may be important for the homeostasis of proliferation and maturation process of basal keratinocytes in the inter-follicular epidermis and subsequently mesenchymal activities in the healing process. The understanding of these intrinsic cellular differences is fundamental to the eventual development of novel therapeutics for wound healing by modulating molecules constituting cellular competence.
Acknowledgement
This work is supported by grants from NIAMS (CMC) and NIGMS (TLT). We thank Dr. Robert Maxson and Dr. Yi-Hsin Liu for providing 220bpMsx2-lacZ and Msx2 null mice. We thank Dr. Wei Li for help in gold particle keratinocyte migration.
Abbreviations
- Msx2 null
C. Cg-Msx2tm1Rilm/Mmcd
- WT
wild type
- PWD
post wounding date
REFERENCES
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Dudas M, Wysocki A, Gelpi B, Tuan TL. Memory encoded throughout our bodies: molecular and cellular basis of tissue regeneration. Pediatr Res. 2008;63(5):502–12. doi: 10.1203/PDR.0b013e31816a7453. [DOI] [PubMed] [Google Scholar]
- 3.Huang EY, Wu H, Island ER, Chong SS, Warburton D, Anderson KD, Tuan TL. Differential expression of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 in early and late gestational mouse skin and skin wounds. Wound Repair Regen. 2002;10(6):387–96. doi: 10.1046/j.1524-475x.2002.t01-1-10608.x. [DOI] [PubMed] [Google Scholar]
- 4.Li WY, Huang EY, Dudas M, Kaartinen V, Warburton D, Tuan TL. Transforming growth factor-beta3 affects plasminogen activator inhibitor-1 expression in fetal mice and modulates fibroblast-mediated collagen gel contraction. Wound Repair Regen. 2006;14(5):516–25. doi: 10.1111/j.1743-6109.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- 5.Soo C, Hu FY, Zhang X, Wang Y, Beanes SR, Lorenz HP, Hedrick MH, Mackool RJ, Plaas A, Kim SJ, Longaker MT, Freymiller E, Ting K. Differential expression of fibromodulin, a transforming growth factor-beta modulator, in fetal skin development and scarless repair. Am J Pathol. 2000;157(2):423–33. doi: 10.1016/s0002-9440(10)64555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han M, Yang X, Farrington JE, Muneoka K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development. 2003;130(21):5123–32. doi: 10.1242/dev.00710. [DOI] [PubMed] [Google Scholar]
- 7.Mark M, Rijli FM, Chambon P. Homeobox genes in embryogenesis and pathogenesis. Pediatr Res. 1997;42(4):421–9. doi: 10.1203/00006450-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Wang DG, Britten RJ, Davidson EH. Maternal and embryonic provenance of a sea urchin embryo transcription factor, SpZ12-1. Mol Mar Biol Biotechnol. 1995;4(2):148–53. [PubMed] [Google Scholar]
- 9.Stelnicki EJ, Kömüves LG, Holmes D, Clavin W, Harrison MR, Adzick NS, Largman C. The human homeobox genes MSX-1, MSX-2, and MOX-1 are differentially expressed in the dermis and epidermis in fetal and adult skin. Differentiation. 1997;62(1):33–41. doi: 10.1046/j.1432-0436.1997.6210033.x. [DOI] [PubMed] [Google Scholar]
- 10.Reginelli AD, Wang YQ, Sassoon D, Muneoka K. Digit tip regeneration correlates with regions of Msx1 (Hox 7) expression in fetal and newborn mice. Development. 1995;121(4):1065–76. doi: 10.1242/dev.121.4.1065. [DOI] [PubMed] [Google Scholar]
- 11.Han M, Yang X, Lee J, Allan CH, Muneoka K. Development and regeneration of the neonatal digit tip in mice. Dev Biol. 2008;315(1):125–35. doi: 10.1016/j.ydbio.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 13.Gardiner DM, Endo T, Bryant SV. The molecular basis of amphibian limb regeneration: integrating the old with the new. Semin Cell Dev Biol. 2002;13(5):345–52. doi: 10.1016/s1084952102000903. [DOI] [PubMed] [Google Scholar]
- 14.Clause AR, Capaldi EA. Caudal autotomy and regeneration in lizards. J Exp Zoolog A Comp Exp Biol. 2006;305(12):965–73. doi: 10.1002/jez.a.346. [DOI] [PubMed] [Google Scholar]
- 15.Colwell AS, Longaker MT, Lorenz HP. Fetal wound healing. Front Biosci. 2003;8:s1240–8. doi: 10.2741/1183. [DOI] [PubMed] [Google Scholar]
- 16.Uyeno LA, Newman-Keagle JA, Cheung I, Hunt TK, Young DM, Boudreau N. Hox D3 expression in normal and impaired wound healing. J Surg Res. 2001;100(1):46–56. doi: 10.1006/jsre.2001.6174. [DOI] [PubMed] [Google Scholar]
- 17.Boudreau NJ, Varner JA. The homeobox transcription factor Hox D3 promotes integrin alpha5beta1 expression and function during angiogenesis. J Biol Chem. 2004;279(6):4862–8. doi: 10.1074/jbc.M305190200. [DOI] [PubMed] [Google Scholar]
- 18.Jain K, Sykes V, Kordula T, Lanning D. Homeobox genes Hoxd3 and Hoxd8 are differentially expressed in fetal mouse excisional wounds. J Surg Res. 2008;148(1):45–8. doi: 10.1016/j.jss.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Mack JA, Abramson SR, Ben Y, Coffin JC, Rothrock JK, Maytin EV, Hascall VC, Largman C, Stelnicki EJ. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. FASEB J. 2003;17(10):1352–4. doi: 10.1096/fj.02-0959fje. [DOI] [PubMed] [Google Scholar]
- 20.Carlson MR, Bryant SV, Gardiner DM. Expression of Msx-2 during development, regeneration, and wound healing in axolotl limbs. J Exp Zool. 1998;282(6):715–23. doi: 10.1002/(sici)1097-010x(19981215)282:6<715::aid-jez7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 21.Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24(4):391–5. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 22.Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, Dobias SL, Yi SE, Lyons K, Bell JR, Arora K, Warrior R, Maxson R. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131(20):5153–65. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht-Buehler G. The phagokinetic tracks of 3T3 cells. Cell. 1977;11(2):395–404. doi: 10.1016/0092-8674(77)90057-5. [DOI] [PubMed] [Google Scholar]
- 24.Woodley DT, Bachmann PM, O’Keefe EJ. Laminin inhibits human keratinocyte migration. J Cell Physiol. 1988;136(1):140–6. doi: 10.1002/jcp.1041360118. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, Liu YH, Müller-Röver S, Peters H, Sundberg JP, Maxson R, Maas RL, Chuong CM. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130(2):379–89. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown C, Stenn KS, Falk RJ, Woodley DT, O’Keefe EJ. Vitronectin: effects on keratinocyte motility and inhibition of collagen-induced motility. J Invest Dermatol. 1991;96(5):724–8. doi: 10.1111/1523-1747.ep12470960. [DOI] [PubMed] [Google Scholar]
- 27.Jabs EW, Müller U, Li X, Ma L, Luo W, Haworth IS, Klisak I, Sparkes R, Warman ML, Mulliken JB, et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993;75(3):443–50. doi: 10.1016/0092-8674(93)90379-5. [DOI] [PubMed] [Google Scholar]
- 28.Cai J, Lee J, Kopan R, Ma L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Dev Biol. 2009 Feb 15;326(2):420–30. doi: 10.1016/j.ydbio.2008.11.021. Epub 2008 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jowett AK, Vainio S, Ferguson MW, Sharpe PT, Thesleff I. Epithelial-mesenchymal interactions are required for msx 1 and msx 2 gene expression in the developing murine molar tooth. Development. 1993;117(2):461–70. doi: 10.1242/dev.117.2.461. [DOI] [PubMed] [Google Scholar]
- 30.Monaghan AP, Davidson DR, Sime C, Graham E, Baldock R, Bhattacharya SS, Hill RE. The Msh-like homeobox genes define domains in the developing vertebrate eye. Development. 1991;112(4):1053–61. doi: 10.1242/dev.112.4.1053. [DOI] [PubMed] [Google Scholar]
- 31.Jiang TX, Liu YH, Widelitz RB, Kundu RK, Maxson RE, Chuong CM. Epidermal dysplasia and abnormal hair follicles in transgenic mice overexpressing homeobox gene MSX-2. J Invest Dermatol. 1999;113(2):230–7. doi: 10.1046/j.1523-1747.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–4. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 33.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316–20. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 34.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9(6):855–61. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21(7):1358–66. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 36.Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005;102(41):14677–82. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuong CM. Regenerative biology: new hair from healing wounds. Nature. 2007;447(7142):265–6. doi: 10.1038/447265a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maas R, Bei M. The genetic control of early tooth development. Crit Rev Oral Biol Med. 1997;8(1):4–39. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- 39.Ohta H, Hamada J, Tada M, Aoyama T, Furuuchi K, Takahashi Y, Totsuka Y, Moriuchi T. HOXD3-overexpression increases integrin alpha v beta 3 expression and deprives E-cadherin while it enhances cell motility in A549 cells. Clin Exp Metastasis. 2006;23(7-8):381–90. doi: 10.1007/s10585-006-9047-5. [DOI] [PubMed] [Google Scholar]
- 40.Hansen SL, Young DM, Boudreau NJ. HoxD3 expression and collagen synthesis in diabetic fibroblasts. Wound Repair Regen. 2003;11(6):474–80. doi: 10.1046/j.1524-475x.2003.11615.x. [DOI] [PubMed] [Google Scholar]
- 41.Kömüves LG, Michael E, Arbeit JM, Ma XK, Kwong A, Stelnicki E, Rozenfeld S, Morimune M, Yu QC, Largman C. HOXB4 homeodomain protein is expressed in developing epidermis and skin disorders and modulates keratinocyte proliferation. Dev Dyn. 2002;224(1):58–68. doi: 10.1002/dvdy.10085. [DOI] [PubMed] [Google Scholar]
- 42.White P, Thomas DW, Fong S, Stelnicki E, Meijlink F, Largman C, Stephens P. Deletion of the homeobox gene PRX-2 affects fetal but not adult fibroblast wound healing responses. J Invest Dermatol. 2003;120(1):135–44. doi: 10.1046/j.1523-1747.2003.12015.x. [DOI] [PubMed] [Google Scholar]
- 43.Andersen B, Weinberg WC, Rennekampff O, McEvilly RJ, Bermingham JR, Jr, Hooshmand F, Vasilyev V, Hansbrough JF, Pittelkow MR, Yuspa SH, Rosenfeld MG. Functions of the POU domain genes Skn-1a/i and Tst-1/Oct-6/SCIP in epidermal differentiation. Genes Dev. 1997;11(14):1873–84. doi: 10.1101/gad.11.14.1873. [DOI] [PubMed] [Google Scholar]