Abstract
Objective
We examined the distribution of artemin and its receptor, glial cell line-derived neurotrophic factor family receptor α3 (GFRα3), in the dura mater of rats.
Background
Artemin, a member of the glial cell line-derived neurotrophic factor family, is a vasculature-derived growth factor shown to regulate migration of sympathetic neuroblasts and targeting of sympathetic innervation. The artemin receptor, GFRα3, is present in both sympathetic efferents and a subpopulation of nociceptive afferents. Recent evidence has shown that artemin may contribute to inflammatory hyperalgesia. The extent to which artemin is present in the dural vasculature and its relationship to GFRα3 containing fibers have yet to be investigated.
Methods
We used retrograde labeling, double and triple labeling with immunohistochemistry on the dura mater and trigeminal ganglia of female Sprague-Dawley rats.
Results
Artemin-like immunoreactivity (-LI) was detected in the smooth muscle of dural vasculature. GFRα3-LI was present in nerve fibers that closely associated with tyrosine hydroxylase or calcitonin gene-related peptide (CGRP). CGRP-LI and transient receptor potential ion channel 1 (TRPV1)-LI were present in all GFRα3-positive dural afferents, which constituted 22% of the total population of dural afferents.
Conclusions
These anatomical results support the hypothesis that artemin contributes to dural afferent activity, and possibly migraine pain, through modulation of both primary afferent and sympathetic systems.
Keywords: peptidergic afferent, sympathetic postganglionic neuron, sympathetically mediated pain
Artemin (Artn) is a member of the glial cell line derived neurotrophic factor (GDNF) family of proteins that signals through the receptor complex of ret and its specific GDNF family receptor (GFR)α3.1 Artn is derived from vascular smooth muscle cells and regulates the development of sympathetic postganglionic neurons (SPGN), in particular SPGN innervations of the vasculature.2 Recent evidence suggests that both Artn and GFRα3 expression persists into adulthood in vascular smooth muscle and SPGNs,3 respectively.
The GFRα3 is also expressed in a subset of primary afferent neurons that appear to function as nociceptors and contribute to inflammatory hyperalgesia. GFRα3-labeled neurons in the dorsal root ganglion (DRG) and trigeminal ganglion (TG) have a small to medium-cell body size, and express the proinflammatory neuropeptide calcitonin gene-related peptide (CGRP) and the transient receptor potential ion channel 1 (TRPV1).4 TRPV1 is activated by heat, protons, and endogenous lipid metabolites, and is critical for the manifestation of inflammatory thermal hyperalgesia.5 Artn application significantly potentiates TRPV1 signaling in small diameter DRG neurons.6 Additionally, peripheral inflammation is associated with an increase in Artn expression and injection of Artn causes thermal hyperalgesia.6,7
Vasodilation of the meningeal vessels is due in part to CGRP release and is considered an important component of the neurogenic inflammation thought to underlie migraine. Vasodilation, plasma extravasation, and mast cell degranulation contribute to the release of proinflammatory substances into the meninges, which can activate meningeal primary afferents ultimately resulting in migraine pain.8 The role of vasodilation in migraine is supported by observations that headaches can be induced by vasodilatory, nitric oxide-generating agents such as nitroglycerin,9 and relieved with vasoconstricting agents such as ergots.10 Artn expression in vasculature and its influence on sensory nociception and sympathetic development led us to hypothesize that Artn may be contributing to migraine pain through both primary afferent and sympathetic systems. The goal of this study was to determine if the underlying anatomy supports this hypothesis.
METHODS
Animals
Female adult Sprague-Dawley rats (150–250 g, aged 8–12 weeks; Harlan, Indianapolis, IN, USA) were used for all experiments. Procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, and performed in accordance with National Institutes of Health guidelines for the use of laboratory animals.
Tissue Preparation
Animals were deeply anesthetized with an intraperitoneal injection of anesthetic cocktail (55 mg/kg ketamine, 5.5 mg/kg xylazine, and 1.1 mg/kg acepromazine), and transcardially perfused with cold 1x phosphate-buffered saline (PBS; pH 7.2) followed by cold 4% paraformaldehyde. TG, superior cervical ganglia (SCG), and dura were collected and postfixed in 4% paraformaldehyde for 1 hour. Dural membranes were processed for immunohistochemistry as free-floating whole-mounts, while TG and SCG were equilibrated in 30% sucrose, frozen in OCT (Tissue Tek, Torrance, CA, USA), sectioned at 16 mm and thaw-mounted on SuperFrost plus slides (Fisher Scientific). One dura was embedded in paraffin and 30 mm cross sections collected and processed for immunohistochemistry. Slides were heated to 50°C on a hotplate, soaked in CitriSolv (Fisher Scientific) for 10 minutes and rehydrated through a series of decreasing ethanol concentrations. Antigen retrieval was obtained by boiling slides for 10 minutes in 0.01 M sodium citrate, 0.05%Tween 20, pH 6.0. After cooling, slides were washed in PBS and processed for immunohistochemistry.
Immunolabeling
Dura whole-mounts were placed in blocking solution (10% normal donkey serum and 0.3% Triton X-100 in PBS) for 30 minutes and incubated in primary antibodies in blocking solution for 2 days at 4°C. TG sections on slides were blocked in 10% normal donkey serum and 0.03% Triton X-100 in PBS, and incubated in primary antibodies overnight at room temperature. The following antibodies were used: goat anti-GFRα3 (R&D Systems; 1:500), goat anti-mouse Artn (R&D Systems; 1:40), rabbit anti-CGRP (Sigma; 1:500) and rabbit anti-tyrosine hydroxylase (Chemicon; 1:500), and rabbit anti-TRPV1 (Alomone Labs: 1:1000). The specificity of the Artn and GFRα3 antibodies has been previously documented (manufacturer’s information11,12). Antibodies were visualized with donkey anti-goat and donkey anti-rabbit secondary antibodies conjugated to cyanine 2 or 3 (Jackson ImmunoResearch, West Grove, PA, USA) in blocking solution at 1:500 for 2 hours. Dura were mounted on glass plus slides and coverslipped using Fluoromount-G (Southern Biotech). Slides were photographed under epifluorescence with a Leica DM4000B upright or confocal microscope (Leica, Wetzlar, Germany). Images were captured using a Leica DFC300FX camera and processed for brightness and contrast with Adobe Photoshop (Adobe Systems, San Jose, CA).
Retrograde Labeling
TG and SCG neurons innervating the dura were retrogradely labeled as described previously.13 Briefly, a 3 × 3 mm craniotomy was made over the superior sagittal sinus, leaving the dura intact. A single drop of 1,1′-dioctadecyl-3,3,3′, 3′-tetramethylindocarbocyanine perchloride (DiI; 170 mg/mL in dimethylsulphoxide diluted 1:10 in saline), or true blue chloride powder (Invitrogen; 1 mg) was applied to the exposed dura. The area was covered by dental dam and an acrylic cap. Postoperatively, animals received intramuscular injections of penicillin G (100,000 U/kg) and buprenorphine (0.03 mg/kg). Animals were sacrificed and ganglia extracted for immunohistochemistry 10–14 days following labeling.
Data Analysis
Staining was considered positive when the immunofluorescence was clearly greater than the signal obtained with no primary antibody. Omission of primary antibody was used as a control for non-specific binding of the secondary antibody. The percentage of GFRα3-positive dural afferents was determined by ~50 DiI-labeled neurons in each of 4 rats (8 total ganglia) that exhibited GFRα3- labeling, and ~100 true blue-labeled neurons in each of 3 rats (6 ganglia).
RESULTS
Artn is Located in Smooth Muscle Cells of Dural Blood Vessels
Co-localization of Artn-like immunoreactivity (-LI) and smooth muscle actin (SMA), a marker for smooth muscle cells, was assessed using double-label immunohistochemistry on paraffin embedded cross sections of the dura. Artn-LI was evident surrounding blood vessels and co-localized with SMA (Fig. 1). These results indicate that Artn is expressed in smooth muscle cells of the dural vasculature.
Fig 1.
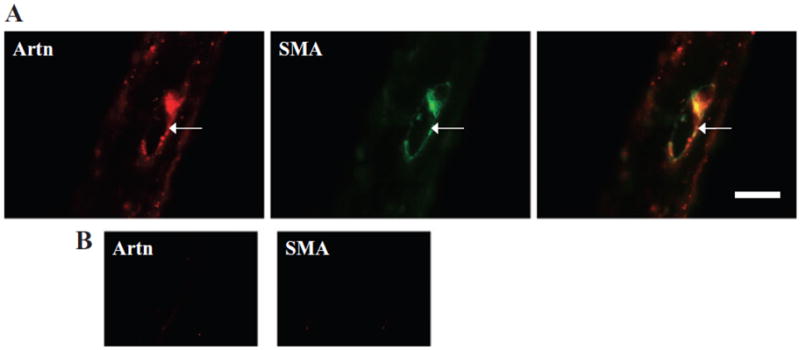
(A) A cross section of the rat dura mater was immunolabeled for artemin (Artn, left panel) and smooth muscle actin (SMA, middle panel). The merged image (right panel) illustrates co-localization. (B) Controls in which the primary antibody was omitted show lack of reactivity. Scale bar, 100 μm.
GFRα3-LI is Present in Neuronal Fibers of the Dura Mater
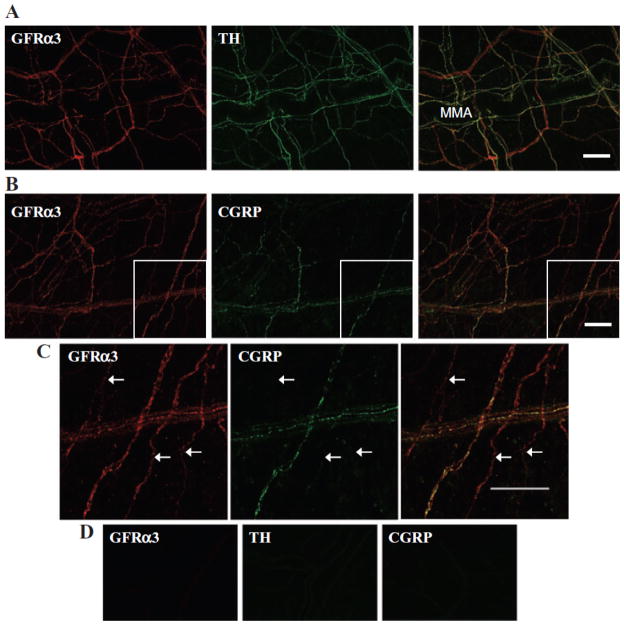
The distribution of GFRα3 throughout the dura mater was also assessed in a whole-mount preparation using immunohistochemistry. GFRα3-LI was present in a network of nerve fibers, in large bundles of axons as well as fine fibers (Fig. 2). Many of these fibers followed the vasculature, with the greatest density concentrated around the middle meningeal artery (MMA). Many fibers also followed the superior and transverse sinuses (not shown). However, some fibers did not follow the vasculature or sinuses, and were observed running perpendicular to the MMA (Fig. 2) and other vessels. GFRα3-LI was not detected in mast cells or the vasculature.
Fig 2.
Double-label immunohistochemistry of whole-mount, adult rat dura mater with anti-glial cell line-derived neurotrophic factor family receptor α3 (GFRα3) and (A) anti-tyrosine hydroxylase (TH) or (B, C) anti-calcitonin gene-related peptide (CGRP), showed GFRα3-like immunoreactivity (-LI) in sympathetic efferents and peptidergic sensory afferents, respectively. (C) Enlarged portion of boxed area in B showing GFRα3-LI fibers that do not show CGRP-LI. (D) Controls in which the primary antibody was omitted show lack of reactivity. Scale bars, 100 μm.
GFRα3-LI is Present in Sympathetic Postganglionic Efferents
Given the developmental link between Artn and sympathetic innervation of the vasculature2 as well as evidence of GFRα3 expression in adult SCG3 we sought to determine whether dural fibers with GFRα3-LI were SPGNs. Although a small subpopulation of TG neurons also display tyrosine hydroxylase reactivity (TH)-LI, dural afferents do not.13 Therefore, TH was used as a marker for SPGN fibers in the dura. TH-LI was present in an extensive network of fibers throughout the dura mater, which closely followed the vasculature (Fig. 2A). Close association of GFRα3-LI and TH-LI in fibers throughout the dura was extensive. Interestingly, while most double-labeled fibers closely followed the dural vasculature, some did not.
GFRα3-LI, TRPV1-LI, and CGRP-LI are Present in Peptidergic Neuronal Afferents
As mentioned above, GFRα3 is present in a subpopulation of peptidergic DRG neurons. To determine whether the same is true in the trigeminal system, we first assessed whether the receptor was co-localized with CGRP in the dura. Of note, while CGRP is also present in a subset of SPGNs in some species, it is not detectable in the SCG of rat.13 As illustrated in Figure 2B, the close association of GFRα3-LI and CGRP-LI was evident in a subpopulation of fibers. To confirm that at least some of the GFRα3-LI dural fibers were sensory afferents, TG neurons innervating the dura mater were back-labeled with DiI or true blue, and subsequently probed for the presence of GFRα3-LI. Approximately 50 DiI-labeled neurons from each of 4 rats and 100 true blue-labeled neurons from each of 3 rats were assessed. To avoid double-counting, we only counted cells in non-adjacent sections when a nucleus was present. While it is possible that true blue and DiI labeled different subpopulations of dural afferents, there was no statistically significant difference (P = .10) in the proportion of dural afferents with GFRα3-LI labeled with each tracer −25.9 ±3.9% (mean ± SEM) vs 17.7 ± 1.4% for DiI and true blue, respectively. Therefore, data obtained with the 2 tracers were pooled as 22.4 ± 3.0% of dural afferents with GFRα3-LI. The TRPV1-LI (Fig. 3) and CGRP-LI (Fig. 4) were seen in 43.2 ± 1.7% and 66.0 ±6.0% of dural afferents, respectively. Twenty-six percent (25.5 ± 2.7%) of dural afferents that were labeled with TRPV1 did not show GFRα3-LI, and 47.6 ±3.6% labeled with CGRP did not show GFRα3-LI. All GFRα3-LI dural afferents were TRPV1- and CGRP positive. These data are summarized in charts shown in Figure 5.
Fig 3.
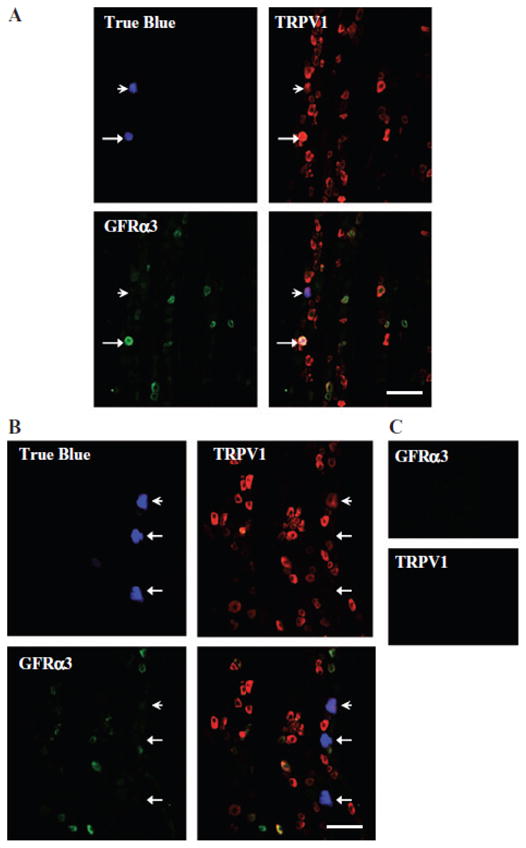
Glial cell line-derived neurotrophic factor family receptor α3 (GFRα3)-like immunoreactivity (-LI) and transient receptor potential ion channel 1 (TRPV1)-LI were seen in 17.7 ± 1.4% and 43.2 ±1.7%, respectively, of dural true blue back-labeled neurons in adult rat trigeminal ganglia (TG). All GFRα3-LI neurons were also TRPV1-LI. Example of dural afferent that was (A) positively labeled for both GFRα3 and TRPV1 (arrow), labeled for only TRPV1 (arrowhead), and (B) negatively labeled (arrows). (C) Controls in which the primary antibody was omitted show lack of reactivity. Scale bar, 100 μm.
Fig 4.
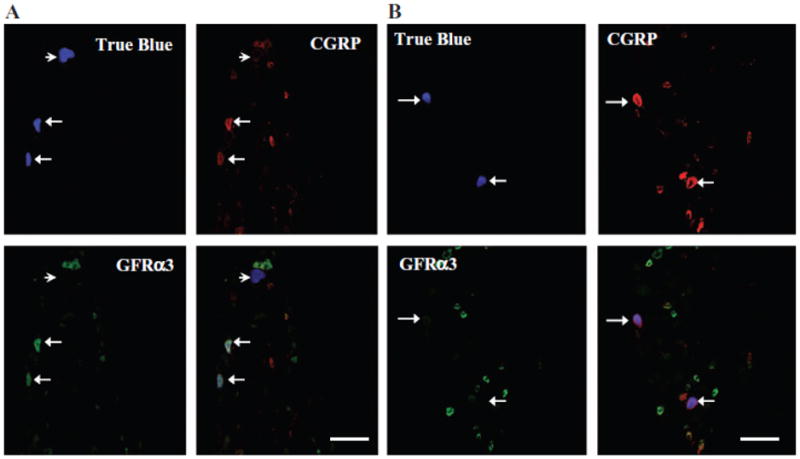
Glial cell line-derived neurotrophic factor family receptor α3 (GFRα3)-like immunoreactivity (-LI) and calcitonin generelated peptide (CGRP)-LI were seen in 18.6 ± 2.6% and 66.0 ± 6.0%, respectively, of dural true blue back-labeled neurons in adult rat trigeminal ganglia (TG). All GFRα3-LI neurons were also CGRP-LI. Example of dural afferents that were (A) positively labeled for both GFRα3 and CGRP (arrows), negatively labeled (arrowhead) and (B) labeled for only CGRP (arrows). Scale bar, 100 μm.
Fig 5.
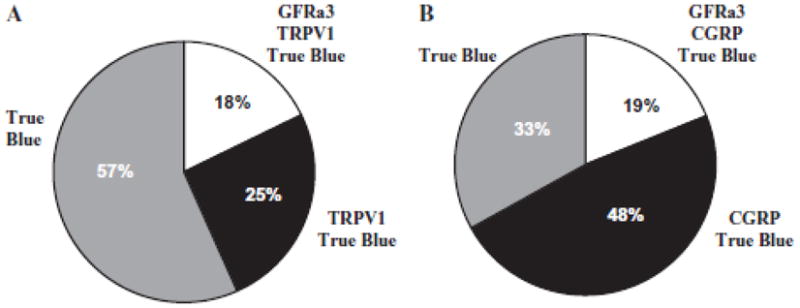
Summary of glial cell line-derived neurotrophic factor family receptor α3 (GFRα3), transient receptor potential ion channel 1 (TRPV1), and calcitonin gene-related peptide (CGRP) in dural afferents. (A) TRPV1-like immunoreactivity (-LI) was seen in 43.2 ± 1.7% of dural afferents, and 17.7 ± 1.4% were labeled for both TRPV1 and GFRα3. (B) CGRP-LI was seen in 66.0 ± 6.0% of dural afferents, and 18.6 ± 2.6% were labeled for both CGRP and GFRα3. All GFRα3-positive dural afferents were CGRP-and TRPV1-positive.
DISCUSSION
The main findings of this study are: (1) Artn-LI was detected in the smooth muscle of adult rat dura mater vasculature; (2) expression of the Artn receptor GFRα3 was detected in afferents that innervate the dura; (3) there was extensive close association of GFRα3-LI and TH-LI in dural fibers; (4) GFRα3 was present in a minority of dural afferents, but all of these afferents were both TRPV1- and CGRP-positive.
While this is the first demonstration of Artn in the dural vasculature, this observation is consistent with other studies reporting Artn expression in vascular structures. For example, Damon et al demonstrated the presence of Artn mRNA and protein in carotid, femoral, and tail arteries of neonatal and adult rats, as well as in cultured vascular smooth muscle cells. In neonatal lacZ Artn+/− mice, Honma et al also found lacZ-LI co-localization with SMA-LI in the superior mesenteric artery. This evidence, in combination with previous data supporting the specificity of the antibody used,11 supports our conclusion that Artn-LI co-localization with SMA-LI in the dural vasculature reflects Artn expression in the smooth muscle.
We suggest that the majority of GFRα3 expression in the dura is present in fibers of SPGNs. This is supported by the comparable labeling patterns of GFRα3-LI and TH-LI in dural fibers (Fig. 2A), and the absence of TH staining in dural afferents.13 Additional evidence comes from data linking Artn/GFRα3 signaling to proper SPGN innervation of the vasculature during development,2 and that the receptor is expressed in the SCG in adults.3 Furthermore, the more limited overlap of GFRα3-LI and CGRP-LI in dural fibers (Fig. 2B, C), in combination with the observation that only 28% of dural afferents with CGRP-LI were double labeled with GFRα3 (Figs. 4 and 5), is also consistent with the suggestion that a minority of GFRα3-LI in the dura was associated with dural afferents. However, because it was not possible to assess GRFα3 levels in SCG, and therefore quantify the extent of co-localization of this receptor in dural efferents, and because staining patterns at the cell body may not be the same as those in the periphery, conclusions about the extent of co-localization in dural fibers are made cautiously.
Interestingly, while only present in a minority of dural afferents, the subpopulation of GFRα3- containing afferents may play a particularly important role in nociceptive processing in the dura. Artn potentiates TRPV1 signaling in DRG neurons, a change that appears to contribute to thermal hyperalgesia associated with inflammation of the hindpaw.6,11 Furthermore, activation of TRPV1, a calcium permeable ion channel, has been shown to promote the release of CGRP from a number of tissues14–16 and contribute to vasodilatation, neurogenic inflammation, and mast cell activation.17,18 Our anatomical results raise the possibility that similar processes occur in the dura mater, underscored by observations that GFRα3 appears to be present in similar subpopulations of nociceptive afferents in the TG and DRG.4
The anatomical results of the present study suggest a model in which Artn contributes to migraine pain indirectly, via the SPGN as well as directly via the sensitization and/or activation of dural afferents. In such a model, triggers for migraine such as stress, exercise, and nitric oxide which are associated with alterations in cerebrovascular tone would result in the release of Artn from vascular smooth muscle. The release of Artn secondary to the modulation of vascular tone would account for the mounting evidence against the vascular hypothesis of migraine that is based on a direct link between the cerebrovasculature and migraine pain. While the impact of Artn on SPGN properties in the adult has yet to be described, the results of the present study highlight the proximity of ligand and receptor. Importantly, there is increasing evidence implicating a critical role for the SPGN in neurogenic inflammation and inflammatory hyperalgesia.19,20 Additional evidence in support of a link between the SPGN and migraine comes from the recent observations that (1) norepinephrine, a neurotransmitter released by sympathetic efferents, increases basal levels of prostaglandin E2 from a rat dural preparation;21 (2) the serotonin 1D receptor, a primary target for the antimigraine triptans, is present in a subpopulation of SPGNs.13 Evidence in support of the link between Artn/GFRα3 signaling in the primary afferent and migraine pain comes from our evidence of the co-localization of GRFa3 with TRPV1 and CGRP in combination with evidence for (1) Artn-induced sensitization of TRPV1;6,22 (2) TRPV1-mediated release of CGRP (discussed above); (3) a critical role for CGRP in migraine pain;23–25 (4) the role for dural afferents in migraine pain.26 While further studies are needed to confirm the details of this model, it does raise the intriguing possibility that Artn/GFRα3 signaling may serve as a novel target for the treatment of migraine.
Acknowledgments
This work supported by NIH grants T32NS007433-10 (LAM), NS41384 (MSG), and NS33730 (KA).
Abbreviations
- Artn
artemin
- CGRP
calcitonin gene-related peptide
- DiI
1,1_-dioctadecyl-3,3,3_,3_-tetramethylindocarbocyanine perchloride
- DRG
dorsal root ganglia
- GDNF
glial cell line-derived neurotrophic factor
- GFRα3
GDNF family receptor a3
- -LI
like immunoreactivity
- MMA
middle meningeal artery
- SMA
smooth muscle actin
- SPGN
sympathetic postganglionic neuron
- TG
trigeminal ganglia
- TH
tyrosine hydroxylase
- TRPV1
transient receptor potential ion channel 1
Footnotes
Conflict of Interest: None
STATEMENT OF AUTHORSHIP
Category 1
(a) Conception and Design
Lisa A. McIlvried; Kathryn Albers; Michael S. Gold
(b) Acquisition of Data
Lisa A. McIlvried
(c) Analysis and Interpretation of Data
Lisa A. McIlvried; Kathryn Albers; Michael S. Gold
Category 2
(a) Drafting the Manuscript
Lisa A. McIlvried
(b) Revising It for Intellectual Content
Lisa A. McIlvried; Kathryn Albers; Michael S. Gold
Category 3
(a) Final Approval of the Completed Manuscript
Lisa A. McIlvried; Kathryn Albers; Michael S. Gold
References
- 1.Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors – Implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 2.Honma Y, Araki T, Gianino S, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 3.Damon DH, Teriele JA, Marko SB. Vascular-derived artemin: A determinant of vascular sympathetic innervation? Am J Physiol. 2007;293:H266–H273. doi: 10.1152/ajpheart.00859.2006. [DOI] [PubMed] [Google Scholar]
- 4.Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]; Headache. :449. [Google Scholar]
- 5.Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 6.Malin SA, Molliver DC, Koerber HR, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56:534–544. doi: 10.1136/gut.2006.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 9.Olesen J, Thomsen LL, Lassen LH, Olesen IJ. The nitric oxide hypothesis of migraine and other vascular headaches. Cephalalgia. 1995;15:94–100. doi: 10.1046/j.1468-2982.1995.015002094.x. [DOI] [PubMed] [Google Scholar]
- 10.Graham JR, Wolff HG. Mechanism of migraine headache and the action of ergotamine tartrate. Arch Neurol Psychiatry. 1938;39:737–763. [Google Scholar]
- 11.Elitt CM, McIlwrath SL, Lawson JJ, et al. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harriott A, Gold M. Serotonin type 1D receptors (5HT(1D)R) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia. 2008;28:933–944. doi: 10.1111/j.1468-2982.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation:A model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 15.Fischer MJ, Reeh PW, Sauer SK. Proton-induced calcitonin gene-related peptide release from rat sciatic nerve axons, in vitro, involving TRPV1. Eur J Neurosci. 2003;18:803–810. doi: 10.1046/j.1460-9568.2003.02811.x. [DOI] [PubMed] [Google Scholar]
- 16.Bowles WR, Flores CM, Jackson DL, Hargreaves KM. beta 2-Adrenoceptor regulation of CGRP release from capsaicin-sensitive neurons. J Dent Res. 2003;82:308–311. doi: 10.1177/154405910308200413. [DOI] [PubMed] [Google Scholar]
- 17.Akerman S, Kaube H, Goadsby PJ. Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol. 2003;140:718–724. doi: 10.1038/sj.bjp.0705486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dux M, Santha P, Jancso G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J Physiol. 2003;552:859–867. doi: 10.1113/jphysiol.2003.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green PG, Basbaum AI, Helms C, Levine JD. Purinergic regulation of bradykinin-induced plasma extravasation and adjuvant-induced arthritis in the rat. Proc Natl Acad Sci USA. 1991;88:4162–4165. doi: 10.1073/pnas.88.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coderre TJ, Basbaum AI, Levine JD. Neural control of vascular permeability: Interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989;62:48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- 21.Ebersberger A, Takac H, Richter F, Schaible HG. Effect of sympathetic and parasympathetic mediators on the release of calcitonin gene-related peptide and prostaglandin E from rat dura mater, in vitro. Cephalalgia. 2006;26:282–289. doi: 10.1111/j.1468-2982.2005.01035.x. [DOI] [PubMed] [Google Scholar]
- 22.Schmutzler BS, Roy S, Hingtgen CM. Glial cell linederived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin generelated peptide from sensory neurons. Neuroscience. 2009;161:148–156. doi: 10.1016/j.neuroscience.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 24.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–918. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 25.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray BS, Wolff HG. Experimental studies on headache. Pain-sensitive structures of the head and their significance in headache. Arch Surg. 1940;41:813– 856. [Google Scholar]