Abstract
Objective
To test whether estrogen receptor polymorphisms modify the effects of postmenopausal hormone therapy on biomarkers and on risk of coronary heart disease events, stroke, or venous thrombo-embolism.
Methods and Results
The design was a nested case-control study in the Women’s Health Initiative trials of postmenopausal hormone therapy. The study included all cases in the first 4 years: coronary heart disease, 359; stroke, 248; venous thrombo-embolism, 217). Six estrogen receptor-αand one estrogen receptor-β polymorphisms were genotyped; 8 biomarkers known to be affected by hormone therapy were measured at baseline and one year after randomization. The polymorphisms were not associated with risk of vascular events, and did not modify the increased risks of coronary heart disease, stroke, or venous thrombo-embolism due to hormone therapy. However, a reduced response of plasmin-antiplasmin (PAP) to hormone therapy was noted for ESR1 IVS1-354 (interaction P<0.0001, corrected for multiple comparisons P=0.014) and ESR1 IVS1-1415 (interaction P<0.0001, corrected P= 0.014).
Conclusions
Estrogen receptor polymorphisms reduce the effect of postmenopausal hormone therapy on PAP, a marker of coagulation and fibrinolysis. However screening for ER polymorphisms to identify women at less risk of adverse cardiovascular outcomes is not likely to be useful for making HT treatment decisions.
Keywords: coronary heart disease, stroke, venous thromboembolism, estrogen, estrogen receptor, genetics, single nucleotide polymorphisms
Introduction
Results from the Women’s Health Initiative (WHI) and other clinical trials of postmenopausal hormone therapy (HT) for prevention of cardiovascular underline the complexity of the effects of HT. The WHI trials of estrogen (E-alone) and estrogen plus progestin (E+P) found increased risks of stroke and venous thrombo-embolism (VTE), and in the trial of E+P also an increased risk of coronary heart disease (CHD); in both WHI trials the risk of CHD and VTE appeared to be higher in the first few years after randomization.1–8 The effects of HT on CHD may depend on baseline lipoprotein levels and the effects on stroke may depend on plasmin-antiplasmin (PAP) levels.9–12 It is not known whether genetic factors modulate clinical cardiovascular responses to HT. However, some studies have suggested that women with the minor allele of IVS1-401 (rs 2234693) of the estrogen receptor-α (ERα, estrogen receptor-1, ESR1) gene have a twofold greater response to HT in some domains of estrogen action such as blood levels of high-density lipoprotein (HDL) cholesterol and E-selectin but not of C-reactive protein (CRP).13–15 Sequence variations of the ERα gene or its haplotypes have been reported to be associated with atherosclerosis16–18 and clinical cardiovascular disease or risk factors in some19–22 but not all studies.23–26 Sequence variations in the ERβ (ESR2) gene were associated with extent of atherosclerosis and coronary calcification in an autopsy study, and with clinical cardiovascular disease in women but not men.27–28 Polymorphism of IVS1-401 (rs 2234693) has been associated with VTE independent of other risk factors such as use of HT,29,30 but the influence of ER-α and ER-β sequence variants on HT- associated risks for clinical arterial cardiovascular events or with venous thrombo-embolism has not been tested previously in a clinical trial setting.
In this study we tested whether several ER-α polymorphisms and one ER-β polymorphism interacted with treatment assignment on risk of CHD, stroke, and VTE in a nested case-control sample from the WHI E+P and E-alone trials, and whether the polymorphisms modify selected biomarker responses to HT.
Methods
Details of the design, recruitment, randomization, data collection, intervention, and outcomes ascertainment procedures in the WHI HT trials, including CONSORT diagrams, have been published previously.1,5
Study population and interventions
The WHI hormone trials enrolled 27 347 postmenopausal women aged 50–79 from 1993 to 1998 at 40 US clinical centers based on hysterectomy status: 16 608 without hysterectomy in a trial of E+P; 10 739 with hysterectomy in a trial of E-alone. The study was approved by the human subjects review committee at each participating institution, and all participants provided written informed consent. Participants were randomly assigned to take a single daily tablet containing a placebo or active medication: women without hysterectomy took 0.625 mg conjugated equine estrogens plus 2.5 mg medroxy-progesterone (Prempro) or placebo, and women with hysterectomy took 0.625 mg conjugated equine estrogen (Premarin) or placebo.
All centrally-adjudicated cases of CHD, stroke, and venous thromboembolism (VTE) occurring during the first 4 years of follow up were included in biomarker and genotyping studies. Procedures for ascertaining and adjudicating clinical outcomes have been published. 4, 8, 10, 12 Controls were matched on age, randomization date, hysterectomy status, and prevalent cardiovascular disease at baseline. Matching on prevalent disease was specific to the case type, so that cases of CHD were matched on prevalent myocardial infarction, cases of stroke on prevalent stroke, and cases of VTE on prevalent VTE. All controls for the three case types were used, after excluding any with incident CHD, stroke, or VTE. The study included 792 cases (CHD=359, stroke=248, VTE=217) and 817 controls. Thirty-two participants experienced more than one type of event. Analyses of year one biomarker data involved cases who experienced their clinical event after the year one visit and corresponding controls; these analyses included 561 women on active treatment and 439 on placebo. Baseline characteristics of the cases and controls included in these analyses, and the associations of baseline characteristics with CHD events, ischemic stroke, and VTE have been published previously.4, 8, 10, 12
Genetic and biomarker analysis
Blood samples were collected from all participants at baseline and 1 year and stored at −70° Celsius using standardized procedures. Analyses were run in single batches including both cases and controls and 10% blind duplicates within 8 years of collection. Biomarkers measured in the case-control studies were hypothesized to modify or mediate HT treatment effects; those included in the current analyses were those previously shown to respond to HT.10,12 Lipid profiles were analyzed in EDTA plasma with high density lipoprotein (HDL) precipitation by heparin manganese (Dade-Behring, Deerfield Illinois, United States). E-selectin, matrix metalloproteinase-9 (MMP-9), and homocysteine were measured at Medical Research Laboratories (Highland Heights, Kentucky, United States). C-reactive protein (N-High Sensitivity CRP, Dade-Behring, Deerfield, Illinois, United States), fibrinogen (clot rate assay: Diagnostica Stago, Parsippany, New Jersey, United States), plasminogen activator inhibitor-1 antigen (PAI-1) and plasmin-antiplasmin complex (PAP, both by in-house immunoassay), and fibrin D-dimer (immunoturbidometric assay, Liatest D-Di) were measured at the Laboratory for Clinical Biochemistry Research, University of Vermont (Burlington, Vermont, United States). Genetic polymorphisms frequently reported in the literature13–30 or identified from prior sequencing13 were assayed on DNA extracted from buffy coat samples at Wake Forest University, Winston Salem, NC Center for Human Genomics using PCR-based methods described previously.13 Six ERα polymorphisms were studied: ESR1 IVS1-397T>C also known as −401 and –PvuII (rs2234693), ESR1 Exon 1+30T>C (rs2077647), ESR1 351A>G also known as −345 and –XbaI (rs9340799), ESR1 IVS1-1415A>G (rs9322331), ESR1 IVS1-1505G>A (rs4870056), and ESR1 1989T>C (rs2071454). A single polymorphism for ERβ was studied: ESR2 1730A>G (rs4986938).
Statistical Design and Analysis
In preliminary genetic analyses the genotype/allele frequencies for all markers were calculated in race/ethnicity strata, and each race stratum was tested separately for Hardy-Weinberg equilibrium. Additive, dominant, and recessive models were examined initially to determine which genetic model best describes the data. Pairwise linkage disequilibrium was examined as described by Devlin and Risch.31 The association of ER polymorphisms with CHD, stroke, and VTE, and their interaction with treatment assignment on these outcomes, were described using logistic regression models linear in the number of minor SNP alleles, while adjusting for covariates known to be associated with the outcomes of interest (age, race/ethnicity, log body mass index, log waist/hip ratio, treated diabetes, smoking status, alcohol consumption, physical activity, history of cardiovascular disease, left ventricular hypertrophy on electrocardiogram, systolic blood pressure, history of hypertension, aspirin use, statin use, history of high cholesterol requiring pills, and hormone use at baseline). Covariates were log-transformed if they showed a skewed distribution or if log-transformation improved model fit. Secondary analyses examined the effects of HT on clinical outcomes by ER polymorphisms within and after the first 2 years following randomization, and other secondary analyses were stratified by age or time since menopause. To assess whether the effect of HT on one-year change in biomarker levels differ by ER polymorphism, linear regression models were used with individual difference in Year 1 and baseline biomarker levels as the outcome; these models included the main effect for treatment assignment (E-alone placebo, E-alone, E+P placebo, E+P), ER polymorphism (coded as continuous according to number of minor SNP alleles), and their interactions, while adjusting for the same covariates described above. All the analyses were repeated for Whites only to investigate possible population stratification.
Multiple testing was acknowledged using a permutation test: first, the marginal p-values were calculated on the original data set for a group of related hypothesis; then data were permuted randomly 1000 times, and p-values were calculated on each permuted data set for the same group of hypothesis but only the smallest p-value was retained; lastly the permutation p-value was calculated by counting the times the p-values retained after each permutation was smaller than the one obtained in the original data set, and dividing that value by the number of permutations.32,33 Statistical analyses were performed using SAS statistical software (version 9.2; SAS Institute Inc, Cary, North Carolina) and R (version 2.11.0; the R Foundation for Statistical Computing).
Results
Results of analyses including Whites only were similar to those for the entire sample, and are not shown. Table 1 shows significance levels for association of the 7 ER SNPs with incidence of CHD events, stroke, and VTE. None of the SNPs show evidence of association with these outcomes in this study population. In secondary analyses the association of ESR1 Exon 1+30 with CHD varied by years since menopause, with per-allele ORs of 0.51, 0.87, and 1.50 in women <10, 10–19, and >20 years since menopause respectively (corrected three-way interaction p=0.026); similar analyses by age yielded a non-significant p=0.064. Table 2 shows significance levels for interaction of each of the SNPs with treatment assignment on the odds ratios for CHD, stroke, and VTE. None of the SNPs appeared to modify the effect of HT on these disease outcomes. In secondary analyses there were no significant interactions of these SNPs and treatment assignment on risk of CHD, stroke, and VTE during the first 2 years after randomization, or during the subsequent 2 years (data not shown). Similarly, there were no three-way interactions by age or years since menopause. Analyses of the individual trials did not yield any significant results.
Table 1.
Association of Estrogen Receptor SNPs with Coronary Heart Disease, Stroke, and Venous Thrombo-embolism
| Polymorphism | SNP ID | Minor/major allele | Minor allele Frequency% (n)1 | CHD (359 cases) | Stroke (248 cases) | VTE (217 cases) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per-minor-allele OR (95%CI)2 | Marginal P3 | Corrected P4 | Per-minor-allele OR (95%CI)2 | Marginal P3 | Corrected P4 | Per-minor-allele OR (95%CI)2 | Marginal P3 | Corrected P4 | ||||
| ESR1 IVS1-401 | rs2234693 | C/T | 46.6% (362) | 1.12(0.89,1.41) | 0.343 | 0.991 | 0.86(0.67,1.11) | 0.247 | 0.963 | 0.91(0.70,1.19) | 0.508 | 1.000 |
| ESR1 Exon 1+30 | rs2077647 | C/T | 47.7% (368) | 1.06(0.85,1.33) | 0.601 | 1.000 | 0.85(0.66,1.09) | 0.207 | 0.923 | 1.01(0.79,1.31) | 0.921 | 1.000 |
| ESR1 IVS1-354 | rs9340799 | G/A | 34.5% (268) | 0.97(0.76,1.24) | 0.804 | 1.000 | 0.93(0.71,1.22) | 0.591 | 1.000 | 0.85(0.64,1.12) | 0.251 | 0.964 |
| ESR1 IVS1-1415 | rs9322331 | T/C | 31.1% (242) | 0.98(0.76,1.26) | 0.889 | 1.000 | 0.84(0.63,1.12) | 0.239 | 0.959 | 0.78(0.58,1.05) | 0.098 | 0.712 |
| ESR1 IVS1-1505 | rs4870056 | A/G | 45.7% (355) | 1.10(0.87,1.39) | 0.428 | 0.997 | 0.88(0.68,1.13) | 0.310 | 0.986 | 0.90(0.69,1.17) | 0.438 | 0.997 |
| ESR1 IVS1-1989 | rs2071454 | G/T | 14.2% (106) | 1.16(0.83,1.63) | 0.379 | 0.997 | 1.01(0.69,1.47) | 0.964 | 1.000 | 1.03(0.69,1.54) | 0.885 | 1.000 |
| ESR2 1730 | rs4986938 | T/C | 38.0% (294) | 0.95(0.75,1.21) | 0.687 | 1.000 | 0.86(0.65,1.12) | 0.263 | 0.967 | 1.08(0.82,1.43) | 0.581 | 1.000 |
MAF in pooled controls (n=817)
Odds ratio estimate from logistic regression model adjusted for treatment assignment (CEE, CEE placebo, CEE+MPA, CEE+MPA placebo), age, race/ethnicity, log body mass index, log waist/hip ratio, treated diabetes, smoking status, alcohol use, physical activity, history of cardiovascular disease, left ventricular hypertrophy on electrocardiogram, systolic blood pressure, history of hypertension, aspirin use, statin use, history of high cholesterol requiring pills, and hormone use at baseline.
Marginal p-value was based on one-degree of freedom test of association between clinical outcome and polymorphisms in the abovementioned logistic model.
Multiple comparison permutation adjusted p-values, based on 1,000 permutations. The responses were permuted at each iteration.
Table 2.
Coronary Heart Disease, Stroke, and Venous Thromboembolism Odds Ratio Estimates for Postmenopausal Hormone Therapy versus Placebo according to Number of Minor Alleles of Estrogen Receptor SNPs
| Polymorphism | Odds ratio estimate (95% confidence interval)1 | Marginal p2 | Corrected p3 | ||
|---|---|---|---|---|---|
| Number of minor SNP alleles | |||||
| 0 | 1 | 2 | |||
| Coronary heart disease | |||||
| ESR1 IVS1-401 | 2.06(1.06,4.01) | 0.92(0.59,1.42) | 1.28(0.63,2.58) | 0.300 | 0.993 |
| ESR1 Exon 1+30 | 1.64(0.87,3.07) | 0.96(0.61,1.53) | 1.24(0.63,2.43) | 0.511 | 1.0000 |
| ESR1 IVS1-354 | 1.60(0.97,2.64) | 0.90(0.56,1.45) | 1.20(0.46,3.16) | 0.261 | 0.917 |
| ESR1 IVS1-1415 | 1.26(0.79,2.02) | 1.15(0.70,1.87) | 1.16(0.41,3.31) | 0.825 | 1.000 |
| ESR1 IVS1-1505 | 1.95(1.02,3.72) | 0.94(0.60,1.46) | 1.18(0.58,2.41) | 0.262 | 0.979 |
| ESR1 IVS1-1989 | 1.19(0.81,1.74) | 1.01(0.52,1.96) | 3.40(0.42,27.3) | 0.826 | 1.000 |
| ESR2 1730 | 1.52(0.89,2.59) | 1.12(0.71,1.77) | 0.91(0.37,2.22) | 0.273 | 0.999 |
| Stroke | |||||
| ESR1 IVS1-401 | 1.62(0.86,3.05) | 0.82(0.49,1.38) | 2.89(1.21,6.89) | 0.541 | 1.000 |
| ESR1 Exon 1+30 | 1.49(0.79,2.80) | 1.01(0.60,1.69) | 2.36(1.00,5.56) | 0.563 | 1.000 |
| ESR1 IVS1-354 | 1.72(1.01,2.95) | 0.84(0.49,1.43) | 3.00(0.84,10.7) | 0.680 | 1.000 |
| ESR1 IVS1-1415 | 1.60(0.97,2.64) | 0.94(0.54,1.65) | 2.13(0.54,8.33) | 0.602 | 1.000 |
| ESR1 IVS1-1505 | 1.48(0.79,2.76) | 0.86(0.51,1.45) | 3.31(1.34,8.15) | 0.335 | 0.995 |
| ESR1 IVS1-1989 | 1.12(0.74,1.70) | 1.66(0.74,3.76) | 5.95(0.71,49.9) | 0.107 | 0.835 |
| ESR2 1730 | 2.20(1.25,3.87) | 0.99(0.59,1.67) | 0.71(0.26,1.97) | 0.019 | 0.103 |
| Venous thrombo-embolism | |||||
| ESR1 IVS1-401 | 2.97(1.43,6.14) | 2.08(1.22,3.54) | 1.75(0.77,3.95) | 0.330 | 1.000 |
| ESR1 Exon 1+30 | 2.74(1.33,5.68) | 2.24(1.28,3.91) | 1.65(0.78,3.46) | 0.330 | 1.000 |
| ESR1 IVS1-354 | 4.09(2.23,7.52) | 1.27(0.74,2.19) | 2.06(0.62,6.86) | 0.033 | 0.661 |
| ESR1 IVS1-1415 | 3.03(1.75,5.26) | 1.60(0.90,2.83) | 1.64(0.42,6.42) | 0.143 | 0.930 |
| ESR1 IVS1-1505 | 3.18(1.55,6.53) | 1.95(1.14,3.34) | 1.68(0.74,3.85) | 0.229 | 1.000 |
| ESR1 IVS1-1989 | 2.11(1.34,3.34) | 2.40(1.04,5.55) | 5.79(0.48,69.8) | 0.684 | 1.000 |
| ESR2 1730 | 3.67(1.83,7.39) | 1.73(1.04,2.88) | 1.91(0.62,5.85) | 0.160 | 0.693 |
Obtained from a logistic regression model adjusted for hysterectomy status, age, race/ethnicity, body mass index, waist/hip ratio, treated diabetes, smoking status, alcohol use, physical activity, history of cardiovascular disease, left ventricular hypertrophy on electrocardiogram, systolic blood pressure, history of hypertension, aspirin use, statin use, history of high cholesterol requiring pills, and hormone use at baseline.
One degree of freedom test of no interaction between treatment assignment (active vs placebo) and polymorphism linear in the number of minor SNP alleles, adjusted for covariates as above.
Multiple comparison permutation adjusted p-values, based on 1,000 permutations. The SNP by treatment interaction was permuted at each iteration.
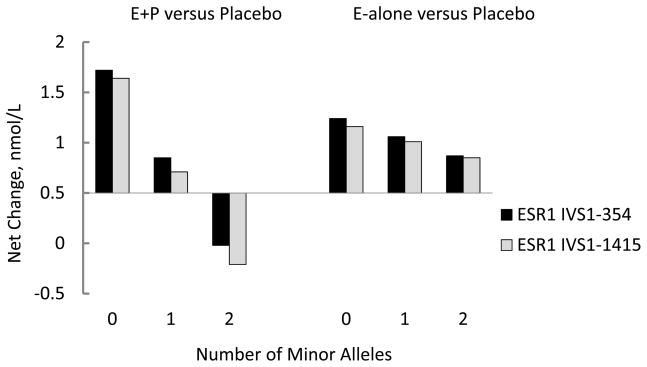
Baseline biomarker levels were not associated significantly with the tested SNPs (data not shown). However, change of PAP levels on HT compared to placebo varied according to the number of minor alleles of two ERα SNPs. ESR1 IVS1-354 and ESR1 IVS1-1415 were significantly (interaction P<0.0001, corrected P=0.014 for each) and inversely associated with change in levels of PAP (Figure 1). The effect modification was consistent across the two trials, but appeared to be more marked in the E+P trial. These 2 SNPs are in strong linkage disequilibrium (D′=0.9998) with a correlation coefficient of 0.8491 (Table 3). The effect of HT on the remaining biomarkers did not appear to be modified by any of the SNPs tested (Supplementary Table 1). However, there were three-way interactions of age or years since menopause, ESR1 Exon 1+30, and treatment on change in fibrinogen (corrected p=0.021 and 0.003, respectively), and of years since menopause, ESR2 1730G, and treatment on change in fibrinogen (p=0.02).
Figure 1.
Postmenopausal hormone therapy effects on change in biomarker levels according to number of minor alleles of ERα SNPs. Net change calculated as change from baseline to year one in active treatment arm -change from baseline to year one in placebo arm, estimated from regression models that are linear in the number of minor SNP alleles. Models adjusted for potential confounders as in Table 1 and Table 2, except that baseline hysterectomy status interacted with treatments and SNPs, thus allowing the treatment*SNP effect to be different in E-alone and E+P trial. Multiple comparison permutation adjusted p=0.014 for each SNP from three degree of freedom test of no interactions between treatment assignment (active vs. placebo), hysterectomy status and polymorphism that is linear in the number of minor alleles, adjusted for covariates as above.
Table 3.
Pairwise Linkage Disequilibrium Analysis for Estrogen Receptor Polymorphisms1
| D′ | |||||||
|---|---|---|---|---|---|---|---|
| ESR1 IVS1-401 | ESR1 Exon 1+30 | ESR1 IVS1-354 | ESR1 IVS1 1415 | ESR1 IVS1 1505 | ESR1 IVS1 1989 | ESR2 1730 | |
| ESR1 IVS1-401 | 0.7740 | 0.9980 | 0.9997 | 0.9997 | 0.6137 | 0.0022 | |
| ESR1 Exon 1+30 | 0.5724 | 0.8547 | 0.9232 | 0.7999 | 0.9737 | 0.0206 | |
| ESR1 IVS1-354 | 0.5838 | 0.4091 | 0.9998 | 0.9777 | 0.5283 | 0.0053 | |
| ESR1 IVS1-1415 | 0.4977 | 0.4055 | 0.8491 | 0.9930 | 0.8830 | 0.0259 | |
| ESR1 IVS1-1505 | 0.9617 | 0.5883 | 0.5823 | 0.5102 | 0.6215 | 0.0070 | |
| ESR1 IVS1-1989 | 0.0726 | 0.1746 | 0.0245 | 0.0582 | 0.0773 | 0.0930 | |
| ESR2 1730 | 0.0000 | 0.0003 | 0.0000 | 0.0005 | 0.0000 | 0.0009 | |
Shown in the upper triangle are Lewontin’s D′; shown in the lower triangle are squares of Pearson correlation
Discussion
We found no evidence that ER polymorphisms were associated with risk of CHD, stroke, or VTE, and the polymorphisms tested did not modify the effects of HT on CHD, stroke, and VTE overall. These data do not support the concept that variations in the estrogen receptor gene and consequent changes in biological effects estrogen to receptors may increase or decrease the adverse effects of HT. Our findings do not necessarily contradict the previously observed associations of estrogen receptor polymorphisms with atherosclerosis,17–19, 27 because the factors leading to precipitation of an acute event in response to HT may differ from those responsible for the buildup of atherosclerosis, but they are in agreement with some other studies in not finding associations of ER polymorphisms with the risks of CHD and stroke.24,26
There were some signals that the associations of ESR1 Exon 1+30 with CHD risk and fibrinogen varied by age and years since menopause, with higher per-minor allele ORs for CHD but with less favorable changes (decreases) in fibrinogen in younger women and women closer to the menopause. However, the clinical implications are unclear because the treatment effect of HT on CHD risk was not modified by ESR1 Exon 1+30 and there was no further interaction with age or years since menopause. Similarly, the clinical implications of the three-way interaction of ESR2 1730G with treatment and years since menopause on fibrinogen are unclear.
Change in PAP levels in response to HT were modified by 2 ESR-α polymorphisms (ESR1 IVS1-354 and ESR1 IVS1-1415) in high linkage disequilibrium. PAP was measured in an assay that detects only plasmin in complex with antiplasmin, and not free plasmin or antiplasmin.34 As such, it is a very good marker of active plasmin generation as part of the fibrinolytic response to clot formation. PAP has a low biological variability and an analytical CV of 1.7%, thus making it an excellent marker for epidemiological research.35
Whereas HT increased the levels of PAP overall,10,12 the presence of the minor alleles of these polymorphisms was associated with an attenuated response or a reduction in levels of PAP. However, the clinical importance of variation of PAP levels due to receptor polymorphisms in this cohort is unclear. As previously published, for CHD the baseline levels of PAP were not associated with risk, and did not interact with treatment assignment on CHD, and neither did treatment-induced increases in PAP levels.10 For ischemic stroke baseline PAP levels were also not associated with risk, and while higher baseline levels of PAP interacted with HT treatment to increase the risk of stroke, the HT treatment-induced increases did not increase the risk of stroke.12 For VTE baseline levels of PAP were associated with VTE risk, and both baseline PAP and change in PAP levels interacted with HT to further increase the risk of VTE (Cushman M, submitted for publication). The evidence from the WHI trials supports a role for activation of coagulation and fibrinolysis in explaining increased risks of stroke and VTE on hormone therapy, but not for the initially increased risk of CHD. However, as noted above, the risks of CHD, stroke, and VTE did not depend on ER polymorphisms and therefore it is unlikely that variation in PAP levels due to polymorphisms play an important role in baseline risk or in mediating HT-related risk for these conditions. The current findings, though biologically plausible, need to be replicated in other studies, and in general the relationships of ER polymorphisms to coagulation, fibrinolysis, and HT need further research.
Previous publications based on this dataset indicated that higher levels of baseline LDL-cholesterol levels, and possibly lower levels of HDL-C, were associated with greater risk of CHD due to HT.9–11 The British Women’s Heart and Health Study did not find a relationship between ESR1 haplotypes and HDL-C or any other cardiovascular risk factors; however, in the Rochester Family Heart Study ESR1 polymorphisms were related to increasing levels of Apo A-1, ApoA-2, and HDL-C.23,25 In the current study we did not find any significant relationships between ER polymorphisms and baseline levels of LDL-C or triglycerides. We also did not replicate a previous finding of increased HDL-C response to E+P in women with the minor allele of ESR1 IVS-401 and several related genotypes13 We did not confirm an association between ESR1 IVS-401 alleles and the response of E-selectin to HT, but our findings are in agreement with previous studies in finding no interactions of ER polymorphisms with the response of CRP to HT treatment.14,15
Strengths of the current study include its setting in a randomized controlled clinical trial of HT which allowed assessment of the interactions of the polymorphisms with HT on vascular disease and biomarkers in addition to the main effects of ER polymorphisms. The standardized and complete ascertainment and classification of clinical outcomes is also a strength. However, the study also has several weaknesses: the number of cases of CHD, stroke, and VTE were relatively small and it is possible that we may have missed some associations of ER polymorphisms with disease outcomes. Other biomarkers such as acquired activated protein C resistance and tissue factor pathway inhibitor were not included. Adherence to HT treatment regimens diminished over time and this too would have impaired the ability to demonstrate interactions with hormone therapy; however, during the first 4 years after randomization included in this analysis the great majority of participants reported full adherence to study treatment.1,5 The trials tested only one formulation of estrogen (conjugated equine estrogens) and it is possible that other formulations might have had different effects.
We conclude that ER polymorphisms may influence the pro-coagulant and subsequent fibrinolytic response to HT as measured by PAP, but we could not demonstrate that these polymorphisms modified the risk of CHD, stroke, or VTE associated with the first 4 years of HT. Therefore, screening for ER polymorphisms to identify women at less risk of adverse cardiovascular outcomes is not likely to be useful for making HT treatment decisions.
Supplementary Material
Acknowledgments
Sources of Funding: The Women’s Health Initiative is funded by contracts N01WH 2211, 2452, 32100-32102, 32105-32106, 32108-32109, 32111-32113, 32115, 32118-32119, 32122, 42107-42126, 42129-42132 and 44221 from the National Heart, Lung, and Blood Institute, National Institutes of Health. Study drugs were donated by Wyeth Research, St. Davids, Pennsylvania.
Footnotes
Trial Registration: http://www.clinicaltrials.gov identifier: NCT 00000611
Disclosure: Dr. Hsia is currently an employee of Aztra-Zeneca.
Contributor Information
Jacques Rossouw, Email: rossouwj@nih.gov, National Heart, Lung, and Blood Institute, Bethesda, MD.
Paul Bray, Email: paul.bray@jefferson.edu, Jefferson University, Philadelphia, PA.
Jingmin Liu, Email: jliu@whi.org, Fred Hutchinson Cancer Research Center, Seattle, WA.
Charles Kooperberg, Email: clk@fhcrc.org, Fred Hutchinson Cancer Research Center, Seattle, WA.
Judith Hsia, Email: Judith.Hsia@astrazeneca.com, Astra-Zeneca, Wilmington, DE.
Cora Lewis, Email: clewis@mail.dopm.uab.edu, University of Alabama, Birmingham, AL.
Mary Cushman, Email: Mary.Cushman@uvm.edu, University of Vermont, Burlington, VT.
Denise Bonds, Email: bondsde@nhlbi.nih.gov, National Heart, Lung, and Blood Institute, Bethesda, MD.
Susan Hendrix, Email: SHENDRIX@dmc.org, Detroit Medical Center, Detroit, MI.
George Papanicolaou, Email: gjp@nhlbi.nih.gov, National Heart, Lung, and Blood Institute, Bethesda, MD.
Tim Howard, Email: tdhoward@wfubmc.edu, Wake Forest University School of Medicine, Winston-Salem, NC.
David Herrington, Email: dherring@wfubmc.edu, Wake Forest University School of Medicine, Winston-Salem, NC.
References
- 1.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen J, Curb JD, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ Women’s Health Initiative Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a Randomized Trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 4.Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, Rosendaal FR Women’s Health Initiative Investigators. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292:1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J WHI Investigators. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 7.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R Women’s Health Initiative Investigators. Conjugated equine estrogens and coronary heart disease: The Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 8.Curb JD, Prentice RL, Bray PF, Langer RD, Van Horn L, Barnabei VM, Bloch MJ, Cyr MG, Gass M, Lepine L, Rodabough RJ, Sidney S, Uwaifo GI, Rosendaal FR. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med. 2006;166:772–780. doi: 10.1001/archinte.166.7.772. [DOI] [PubMed] [Google Scholar]
- 9.Bray PF, Larson JC, LaCroix AZ, Manson J, Limacher MC, Rossouw JE, Lasser NL, Lawson WE, Stefanick ML, Langer RD, Margolis KL Women’s Health Initiative Investigators. Usefulness of baseline lipids and C-reactive protein in women receiving menopausal hormone therapy as predictors of treatment-related coronary events. Am J Cardiol. 2008;101:1599–1605. doi: 10.1016/j.amjcard.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossouw JE, Cushman M, Greenland P, Lloyd-Jones DM, Bray P, Kooperberg C, Pettinger MS, Robinson J, Hendrix S, Hsia J. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the Women’s Health Initiative trials of hormone therapy. Arch Intern Med. 2008;168:2245–2253. doi: 10.1001/archinte.168.20.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil-Smoller S, Hendrix SL, Robinson JG, Lund B, Kuller LH for the Women’s Health Initiative Research Group. Lipoprotein particle concentrations may explain the absence of coronary protection in the Women’s Health Initiative Hormone Trials. Arterioscler Thromb Vasc Biol. 2008;28:1666–1671. doi: 10.1161/ATVBAHA.108.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kooperberg C, Cushman M, Hsia J, Robinson JG, Aragaki AK, Lynch JK, Baird AE, Johnson KC, Kuller LH, Beresford SAA, Rodriguez B. Can biomarkers identify women at increased stroke risk? The Women’s Health Initiative Hormone Trials. PLoS Clin Trials. 2007;2:e28. doi: 10.1371/journal.pctr.0020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrington DM, Howard TD, Hawkins GA, et al. Estrogen-receptor polymophisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. NEJM. 2002;346:967–974. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- 14.Herrington DM, Howard TD, Brosnihan B, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002;105:1879–1882. doi: 10.1161/01.cir.0000016173.98826.88. [DOI] [PubMed] [Google Scholar]
- 15.De Maat MPM, Madsen JS, Langdahl B, Bladberg EM, Tofteng CL, Abramsen B, Rejnmark L, Brixen K, Chrisenen K, Jespersen J, Kristensen SR. Genetic variation in estrogen receptor, C-reactive protein and fibrinogen does not predict the plasma levels of inflammation markers after longterm hormone replacement therapy. Thromb Haemostasis. 2007;97:234–239. [PubMed] [Google Scholar]
- 16.Lehtimaki T, Kunnas TA, Mattila KM, Perola M, Penttila A, Koivula T. Coronary wall atherosclerosis in relation to the estrogen receptor 1 gene polymorphism: an autopsy study. J Mol Med. 2002;80:176–180. doi: 10.1007/s00109-001-0311-5. [DOI] [PubMed] [Google Scholar]
- 17.Rokach A, Pollak A, Rosen L, Friedlander Y, Blumenfeld A, Reznik L, Dresner-Pollak R. Estrogen receptor alpha gene polymorphisms are associated with the angiographic extent of coronary artery disease. J Clin Endocrinol Metab. 2005;90:6556–6560. doi: 10.1210/jc.2005-0236. [DOI] [PubMed] [Google Scholar]
- 18.Alevizaki M, Saltiki K, Cimponeriu A, Kanakakis I, Xita N, Alevizaki CC, Georgiou I, Sarika H-L. Severity of cardiovascular disease in postmenopausal women: associations with common estrogen receptor α polymorphic variants. Eur J Endocrinol. 2007;156:489–496. doi: 10.1530/EJE-06-0685. [DOI] [PubMed] [Google Scholar]
- 19.Shearman AM, Cooper JA, Kotwinski PJ, et al. Estrogen receptorα gene variation and the risk of stroke. Stroke. 2005;36:2281–2282. doi: 10.1161/01.STR.0000181088.76518.ec. [DOI] [PubMed] [Google Scholar]
- 20.Shearman AM, Demissie S, Cupples AM, et al. Tobacco smoking, estrogen receptor α gene variation and low density lipoprotein cholesterol. Human Mol Genet. 2005;14:2405–2413. doi: 10.1093/hmg/ddi242. [DOI] [PubMed] [Google Scholar]
- 21.Schuit SCE, Hok-Hay SO, Witteman JCM, et al. Estrogen receptor α gene polymorphisms and risk of myocardial infarction. JAMA. 2004;291:2969–2977. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 22.Klos KLE, Boerwinkle E, Ferrell RE, Turner ST, Morrison AC. ESR1 polymorphism is associated with plasma lipid and apoprotein levels in Caucasians of the Rochester Family Heart Study. J Lipid Res. 2008;49:1701–1706. doi: 10.1194/jlr.M700490-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch W, Hoppmann P, Pfeufer A, Meuller JC, Schomig A, Kastrati A. No replication of association between estrogen receptor α gene polymorhisms and susceptibility to myocardial infarction in a large sample of patients of European descent. Circulation. 2005;112:2138–2142. doi: 10.1161/CIRCULATIONAHA.105.545913. [DOI] [PubMed] [Google Scholar]
- 24.Lawlor DA, Timpson N, Ebrahim S, Day INM, Smith GD. The association of oestrogen receptor α-haplotypes with cardiovascular risk factors in the British Women’s Heart and Health Study. Eur Heart J. 2006;27:1597–1604. doi: 10.1093/eurheartj/ehi833. [DOI] [PubMed] [Google Scholar]
- 25.Kjaergard AD, Ellervik C, Tybjaerg-Hansen A, et al. Estrogen receptor α gene polymorphism and risk of cardiovascular disease, cancer, and hip fracture. Cross-sectional, cohort, and case-control studies and a meta- analysis. Circulation. 2007;115:861–871. doi: 10.1161/CIRCULATIONAHA.106.615567. [DOI] [PubMed] [Google Scholar]
- 26.Bos MJ, Schuit SCE, Koudstaal PJ, Hofman A, Uitterlinden AG, Breteler MMB. Variation in estrogen receptor α gene and risk of stroke: the Rotterdam Study. Stroke. 2008;39:1324–1326. doi: 10.1161/STROKEAHA.107.494476. [DOI] [PubMed] [Google Scholar]
- 27.Christian RC, Liu PY, Harrington S, Ruan M, Miller VM, Fitzpatrick LA. Intimal estrogen receptor (ER) β, but not ERα expression, is correlated with coronary calcification and atherosclerosis in pre-and postmenopausal women. J Clin Endocrinol Metab. 2006;91:2713–2720. doi: 10.1210/jc.2005-2672. [DOI] [PubMed] [Google Scholar]
- 28.Rexrode KM, Ridker PM, Hegener HH, Buring JE, Manson JE, Zee RYL. Polymorphisms and haplotypes of the estrogen receptor β gene (ESR-2) and cardiovascular disease in men and women. Clinical Chemistry. 2007;53:1749–1756. doi: 10.1373/clinchem.2007.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straczek C, Alhenc-Gelas M, Aubry ML Scarabin P-Y on behalf of the Estrogen and Thromboembolism Risk (ESTHER) Study Group. Genetic variation at the estrogen receptor α locus in relation to venous thromboembolism risk among postmenopausal women. J Thromb Haemost. 2005;3:1535–1537. doi: 10.1111/j.1538-7836.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- 30.Oger E, Lacut K, Mercier B, Le Gal G, Leroyer C, Pasquier E, Ferec C, Mottier D. Estrogen receptor alpha polymorphism and venous thromboembolism in male and female: data from the EDITH study. Thromb Res. 2007;119:433–439. doi: 10.1016/j.thromres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Devlin P, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 32.Efron B, Tibshirani RJ. Monograph on Statistics and Applied Probability. New York: Chapman and Hall/CRC; 1993. An introduction to the bootstrap; p. 57. [Google Scholar]
- 33.Westfall PH, Young SS. Resampling-based multiple testing. New York: John Wiley&Sons; 1993. [Google Scholar]
- 34.Holvoet P, de Boer A, Verstreken M, Collen D. An enzyme-linked immunosorbent assay (ELISA) for the measurement of plasmin-a-2-antiplasmin complex in human plasma: application to the detection of in vivo activation of the fibrolytic system. Thromb Haemost. 1986;56:124–127. [PubMed] [Google Scholar]
- 35.Sakkinen PA, Macy EM, Callas PW, Cornell ES, Hayes TE, Kuller LH, Tracy RP. Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: assessment and implications for epidemiology. Am J Epidemiol. 1999;149:261–267. doi: 10.1093/oxfordjournals.aje.a009801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.