Abstract
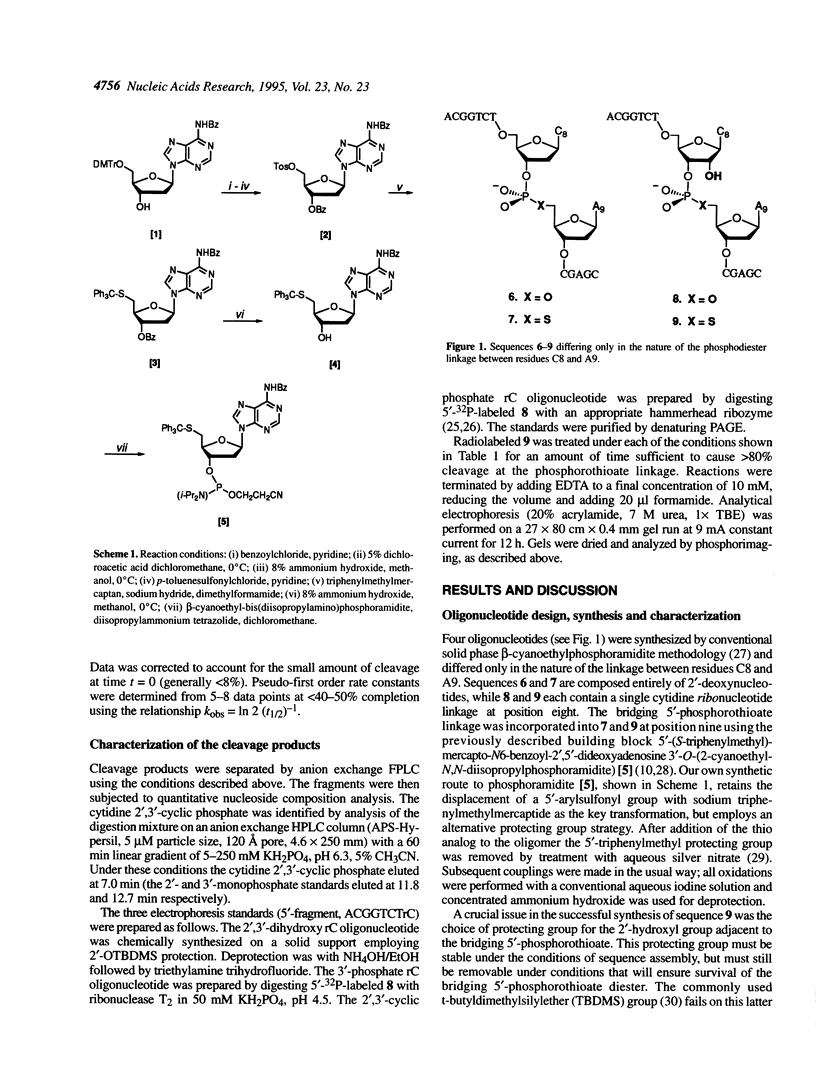
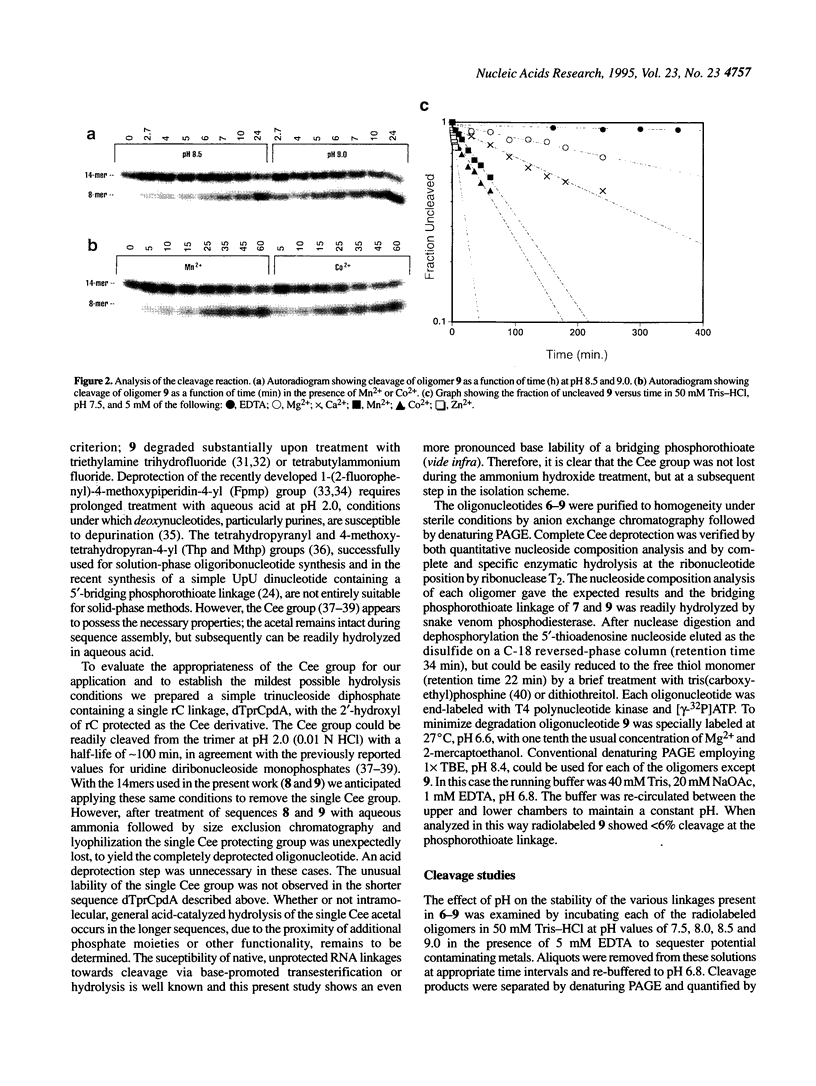
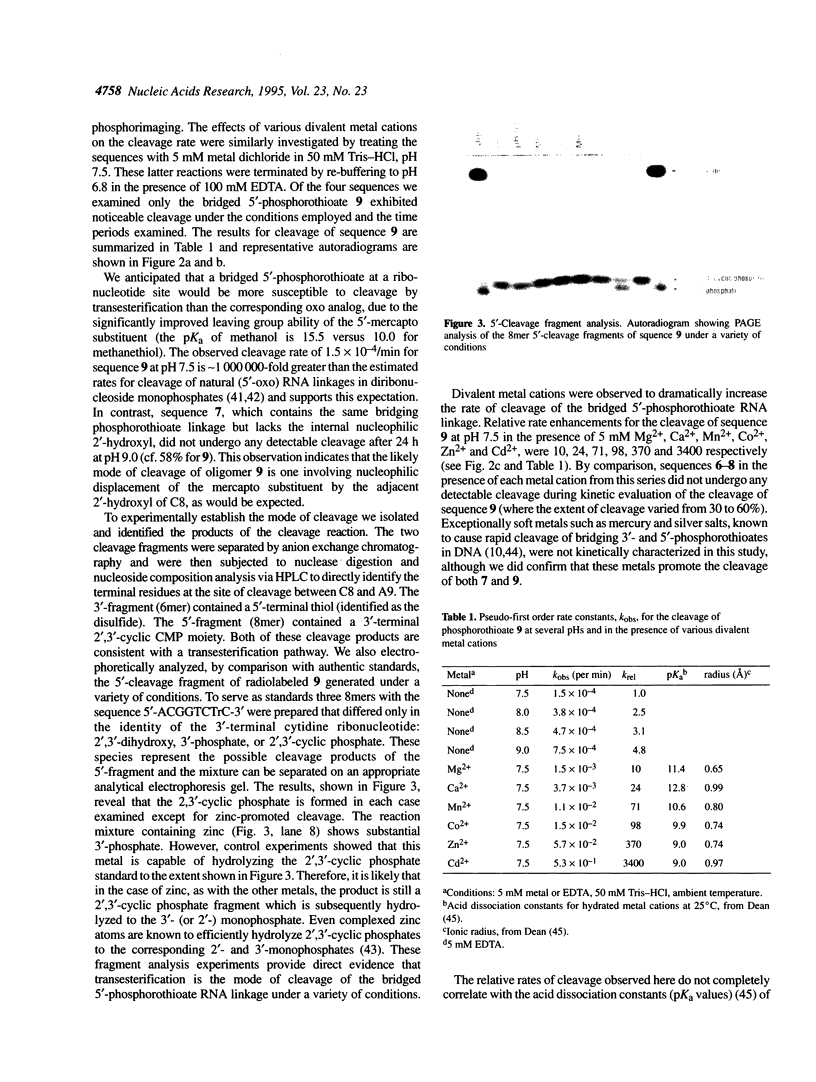
An oligonucleotide has been synthesized that contains a single bridging 5'-phosphorothioate at an RNA linkage (5'-ApCpGpGpTpCpTprCpsApCpGpApGpC-3'). This new phosphodiester linkage is found to be particularly susceptible to cleavage when compared with the corresponding oxo, deoxy and thiodeoxy derivatives. Divalent metal cations were observed to dramatically increase the cleavage rate. The products of the cleavage under a variety of conditions are a 5'-thiol-containing fragment (6mer) and a 2',3'-cyclic phosphate-containing fragment (8mer). The pseudo-first order rate constant, kobs, for cleavage at pH 7.5 (50 mM Tris-HCI) in the presence of 5 mM EDTA is 1.5 x 10(-4)/min. In the presence of 5 mM metal dichloride and 50 mM Tris-HCI, pH 7.5, the relative cleavage rate enhancements are 10, 24, 71, 98, 370 and 3400 for Mg2+, Ca2+, Mn2+, Co2+, Zn2+ and Cd2+ respectively. The rate enhancements correlate well with Pearson's HSAB principle, suggesting that cleavage is mediated in part by coordination of the metal to the 5'-mercapto leaving group. RNA linkages containing bridging 5'-phosphorothioates should prove valuable for studying the mechanistic details of a variety of RNA cleaving agents, such as ribozymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beese L. S., Steitz T. A. Structural basis for the 3'-5' exonuclease activity of Escherichia coli DNA polymerase I: a two metal ion mechanism. EMBO J. 1991 Jan;10(1):25–33. doi: 10.1002/j.1460-2075.1991.tb07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlen L. S., Sampson J. R., DiRenzo A. B., Uhlenbeck O. C. Lead-catalyzed cleavage of yeast tRNAPhe mutants. Biochemistry. 1990 Mar 13;29(10):2515–2523. doi: 10.1021/bi00462a013. [DOI] [PubMed] [Google Scholar]
- Breslow R., Huang D. L. Effects of metal ions, including Mg2+ and lanthanides, on the cleavage of ribonucleotides and RNA model compounds. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4080–4083. doi: 10.1073/pnas.88.10.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. S., Dewan J. C., Klug A. Crystallographic and biochemical investigation of the lead(II)-catalyzed hydrolysis of yeast phenylalanine tRNA. Biochemistry. 1985 Aug 27;24(18):4785–4801. doi: 10.1021/bi00339a012. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Hingerty B. E., Dewan J. C., Klug A. Pb(II)-catalysed cleavage of the sugar-phosphate backbone of yeast tRNAPhe--implications for lead toxicity and self-splicing RNA. Nature. 1983 Jun 9;303(5917):543–546. doi: 10.1038/303543a0. [DOI] [PubMed] [Google Scholar]
- Butzow J. J., Eichhorn G. L. Different susceptibility of DNA and RNA to cleavage by metal ions. Nature. 1975 Mar 27;254(5498):358–359. doi: 10.1038/254358a0. [DOI] [PubMed] [Google Scholar]
- Capaldi D. C., Reese C. B. Use of the 1-(2-fluorophenyl)-4-methoxypiperidin-4-yl (Fpmp) and related protecting groups in oligoribonucleotide synthesis: stability of internucleotide linkages to aqueous acid. Nucleic Acids Res. 1994 Jun 25;22(12):2209–2216. doi: 10.1093/nar/22.12.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Rider P. Chemical synthesis of oligonucleotides containing a free sulphydryl group and subsequent attachment of thiol specific probes. Nucleic Acids Res. 1985 Jun 25;13(12):4485–4502. doi: 10.1093/nar/13.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosstick R., Vyle J. S. Synthesis and properties of dithymidine phosphate analogues containing 3'-thiothymidine. Nucleic Acids Res. 1990 Feb 25;18(4):829–835. doi: 10.1093/nar/18.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm S. C., Derrick W. B., Uhlenbeck O. C. Evidence for the role of solvated metal hydroxide in the hammerhead cleavage mechanism. Biochemistry. 1993 Dec 7;32(48):13040–13045. doi: 10.1021/bi00211a013. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Gish G. Phosphorothioates in molecular biology. Trends Biochem Sci. 1989 Mar;14(3):97–100. doi: 10.1016/0968-0004(89)90130-8. [DOI] [PubMed] [Google Scholar]
- Gasparutto D., Livache T., Bazin H., Duplaa A. M., Guy A., Khorlin A., Molko D., Roget A., Téoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases. Nucleic Acids Res. 1992 Oct 11;20(19):5159–5166. doi: 10.1093/nar/20.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish G., Eckstein F. DNA and RNA sequence determination based on phosphorothioate chemistry. Science. 1988 Jun 10;240(4858):1520–1522. doi: 10.1126/science.2453926. [DOI] [PubMed] [Google Scholar]
- Grasby J. A., Connolly B. A. Stereochemical outcome of the hydrolysis reaction catalyzed by the EcoRV restriction endonuclease. Biochemistry. 1992 Sep 1;31(34):7855–7861. doi: 10.1021/bi00149a016. [DOI] [PubMed] [Google Scholar]
- Ikenaga H., Inoue Y. Metal(II) ion catalyzed transphosphorylation of four homodinucleotides and five pairs of dinucleotide sequence isomers. Biochemistry. 1974 Jan 29;13(3):577–582. doi: 10.1021/bi00700a027. [DOI] [PubMed] [Google Scholar]
- Jeltsch A., Alves J., Maass G., Pingoud A. On the catalytic mechanism of EcoRI and EcoRV. A detailed proposal based on biochemical results, structural data and molecular modelling. FEBS Lett. 1992 Jun 8;304(1):4–8. doi: 10.1016/0014-5793(92)80576-3. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Ohtsuka E. Effects of phosphorothioate and 2-amino groups in hammerhead ribozymes on cleavage rates and Mg2+ binding. Biochemistry. 1991 May 28;30(21):5145–5150. doi: 10.1021/bi00235a005. [DOI] [PubMed] [Google Scholar]
- Mag M., Lüking S., Engels J. W. Synthesis and selective cleavage of an oligodeoxynucleotide containing a bridged internucleotide 5'-phosphorothioate linkage. Nucleic Acids Res. 1991 Apr 11;19(7):1437–1441. doi: 10.1093/nar/19.7.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. S., Caruthers M. H. Phosphorodithioate DNA as a potential therapeutic drug. Science. 1993 Mar 12;259(5101):1564–1570. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- Piccirilli J. A., Vyle J. S., Caruthers M. H., Cech T. R. Metal ion catalysis in the Tetrahymena ribozyme reaction. Nature. 1993 Jan 7;361(6407):85–88. doi: 10.1038/361085a0. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Uhlenbeck O. C. Thiophosphate interference experiments locate phosphates important for the hammerhead RNA self-cleavage reaction. Nucleic Acids Res. 1990 Oct 25;18(20):6025–6029. doi: 10.1093/nar/18.20.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakov V. N., Rivkin M. I., Kumarev V. P. Some substrate properties of analogues of oligothymidylates with p-s-C5' bonds. Nucleic Acids Res. 1981 Jan 10;9(1):189–201. doi: 10.1093/nar/9.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Olsen D. B., Eckstein F. Inhibition of restriction endonuclease hydrolysis by phosphorothioate-containing DNA. Nucleic Acids Res. 1989 Nov 25;17(22):9495–9495. doi: 10.1093/nar/17.22.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry. 1989 Sep 5;28(18):7401–7408. doi: 10.1021/bi00444a038. [DOI] [PubMed] [Google Scholar]
- Slim G., Gait M. J. Configurationally defined phosphorothioate-containing oligoribonucleotides in the study of the mechanism of cleavage of hammerhead ribozymes. Nucleic Acids Res. 1991 Mar 25;19(6):1183–1188. doi: 10.1093/nar/19.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Beijer B., Rider P., Neuner P. The synthesis of protected 5'-mercapto-2',5'-dideoxyribonucleoside-3'-O-phosphoramidites; uses of 5'-mercapto-oligodeoxyribonucleotides. Nucleic Acids Res. 1987 Jun 25;15(12):4837–4848. doi: 10.1093/nar/15.12.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Eckstein F. Incorporation of phosphorothioate groups into fd and phi X174 DNA. Biochemistry. 1977 Aug 9;16(16):3633–3640. doi: 10.1021/bi00635a020. [DOI] [PubMed] [Google Scholar]
- Vyle J. S., Connolly B. A., Kemp D., Cosstick R. Sequence- and strand-specific cleavage in oligodeoxyribonucleotides and DNA containing 3'-thiothymidine. Biochemistry. 1992 Mar 24;31(11):3012–3018. doi: 10.1021/bi00126a024. [DOI] [PubMed] [Google Scholar]
- Westheimer F. H. Why nature chose phosphates. Science. 1987 Mar 6;235(4793):1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- Westman E., Strömberg R. Removal of t-butyldimethylsilyl protection in RNA-synthesis. Triethylamine trihydrofluoride (TEA, 3HF) is a more reliable alternative to tetrabutylammonium fluoride (TBAF). Nucleic Acids Res. 1994 Jun 25;22(12):2430–2431. doi: 10.1093/nar/22.12.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. H., Perreault J. P., Labuda D., Usman N., Cedergren R. Mixed DNA/RNA polymers are cleaved by the hammerhead ribozyme. Biochemistry. 1990 Dec 25;29(51):11156–11160. doi: 10.1021/bi00503a002. [DOI] [PubMed] [Google Scholar]
- Yarus M. How many catalytic RNAs? Ions and the Cheshire cat conjecture. FASEB J. 1993 Jan;7(1):31–39. doi: 10.1096/fasebj.7.1.8422972. [DOI] [PubMed] [Google Scholar]
- van Tol H., Buzayan J. M., Feldstein P. A., Eckstein F., Bruening G. Two autolytic processing reactions of a satellite RNA proceed with inversion of configuration. Nucleic Acids Res. 1990 Apr 25;18(8):1971–1975. doi: 10.1093/nar/18.8.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]