Abstract
The development of implantable glucose sensors for use in diabetes treatment has been pursued for decades. However, enzyme-based glucose sensors often fail in vivo. In our previous work, we engineered a novel glucose indicator protein (GIP) that can sense glucose without relying on any enzymes and cofactors. Nevertheless, this GIP is unsuitable for blood glucose monitoring due to its low dissociation constant. Here, we report a novel approach to creating a new GIP that can be used to monitor blood glucose level. By disrupting pi-pi stacking around GIP’s glucose binding site through site-directed mutagenesis, we showed that GIP’s dissociation constant can be manipulated from 0.026 mM to 7.86 mM. This approach yielded four GIP mutants. We showed that one of the mutants can be used to detect glucose from 0 to 32 mM, while another mutant can be employed to visualize intracellular glucose (0–200 μM) within living cells through FRET imaging microscopy measurement.
Keywords: glucose sensor, glucose binding protein, glucose indicator protein, FRET imaging microscopy measurement, continuous glucose monitoring, site-directed mutagenesis of GBP
1. Introduction
Diabetes mellitus is a debilitating metabolic disease caused by lack of insulin production (type 1) or inappropriate cellular response to insulin (type 2). While diabetes is still incurable, it is manageable if the blood glucose concentration can be monitored appropriately. Ideally, the blood glucose concentration should be monitored continuously so that the insulin can be adequately administered to maintain glucose homeostasis in the body. Unfortunately, continuous glucose monitoring (CGM) is still challenging due in part to the scarcity of a methodology for developing reagentless glucose sensors that can be implanted in the body. Many existing electrochemical glucose sensors fit well for in vitro application, whereas in vivo application of these sensors has encountered many technical difficulties, including the instability of glucose oxidase at body temperature (House et al. 2007; Valdes and Moussy 2000), oxygen deficit in the body due to the relatively high or low glucose concentration (Pickup 1993; Rebrin et al. 1992), and allergic response to implanted glucose sensors (House et al. 2007; Moussy and Reichert 2000).
To circumvent these problems, many alternative technologies have been explored and developed in the past decades. Among these technologies, optical glucose sensors are promising due to their reagentless nature for glucose detection. These sensors utilize a signal transduction mechanism, i.e., fluorescence resonance energy transfer (FRET), in which the efficiency of energy transferred from one fluorescent donor molecule to its acceptor molecule depends upon glucose binding (Campbell 2009). Thus, they are equilibrium-based sensors that do not consume any substrates and do not require any cofactors for glucose detection. They meet one of the critical requirements, i.e., reagentless, for the development of implantable CGM sensors. In this direction, glucose-binding concanavalin A or borate-based artificial glucose-specific receptors have been examined for developing CGM sensors (Ballerstadt et al. 2007; Schultz et al. 1982). The lectin-associated cytotoxicity, however, impaired the use of these sensors for long-term CGM in the body (Ballerstadt et al. 2006). Thus, there is an urgent need for generating new molecules for developing implantable CGM sensors.
In our previous work, we constructed a glucose indicator protein (GIP) by fusing two fluorescent proteins, i.e., a green fluorescent protein (GFP) and a yellow fluorescent protein (YFP) to the N- and C- termini of a glucose binding protein (GBP) in a manner that a FRET signal can be transduced from conformational changes of the GBP upon glucose binding (Ye and Schultz 2003).. The GBP used in this study is a 32 kDa globular periplasmic binding protein that serves as a glucose transporter in E. coli. It possesses two distinct helical structured domains, each organized in an α/β folding motif involving the glucose binding hinge region (Careaga and Falke 1992). The binding of glucose to GBP results in a rearrangement of the flap region located on one side of the hinge β-sheet, yielding a conformational change (Flocco and Mowbray 1994). The protein adopts a “closed” form in the absence of glucose and an “open” form in the presence of glucose. Its Rg, the radius of gyration, increases from 0.54Ǻ to 1.77Ǻ when the degree of opening increases from 10 to 60 (Shilton et al. 1996). This glucose binding-induced conformational change alters the distance between GFP and YFP fused at the N- and C-termini of GBP, giving rise to a FRET signal for glucose detection. A microsensor for CGM was developed based on this GIP in our previous work (Garrett et al. 2008; Ye and Schultz 2003). Similar types of sensors have also been constructed and applied for visualizing intracellular glucose concentration within live cells (Fehr et al. 2004; Fehr et al. 2003; Ge et al. 2008; John et al. 2007; Khan et al. 2008; Takanaga et al. 2007). However, these fluorescent sensory proteins have a very narrow detecting range. They can only detect glucose in the micromolar range; thus, they are unsuitable for monitoring blood glucose level where concentration is usually in the mM (millimole) range.
The glucose binding dissociation constant, Kd of wild type GBP is around 5 μM (Miller et al. 1983; Ye and Schultz 2003). Gene mutagenesis has been applied to elevate the Kd value of GBP from 5 μM to 589 μM (Fehr et al. 2003). Nevertheless, this increase is still far below the physiological glucose concentration. In this study, we intended to manipulate the molecular structure of the GBP in a manner that a panel of GIPs can be constructed for CGM at different ranges, including the use for monitoring blood glucose and visualization of intracellular glucose within living cells.
2. Materials and methods
2.1. Site-directed mutagenesis of GBP
The sequence of GBP was PCR amplified from the plasmid pTAGBP (Ye et al. 2004) and subcloned into pGEM-9Zf (Promega, WI) through the Spe I and Hind III sites, followed by site-directed mutagenesis of GBP at the 16th amino acid residue of GBP. Overlapping PCR was performed to replace the wild type GBP in GIP (Ye and Schultz 2003) with mutated GBPs. The primer pairs employed to amplify the 5′-half of the GIP gene were 5′-AGGAGGAATAAACCATGGTGAGCA-3′ and 5′-GATTGTTACACCAATGCGAGTATC -3′, and the primer pairs used to amplify the 3′-half of mutated GBP were 5′-GGTGTAACAATCTATAAGTACGACG -3′ and 5′-AACACCGGAATGCTGGACTTGTTG -3′. The mutated GBP was subcloned into the GIP expression cassette through the Nco I and Bsm I sites, and the resultant plasmids were transformed into E. coli DH5α.
2.2. GIP expression and purification
All GIPs were fused with a (His)6 tag and expressed in E. coli DH5α with IPTG (isopropyl β-D-1-thiogalactopyranoside) induction. In brief, the recombinant E. coli harboring GIP expression plasmids were cultured at 37°C with vigorous shaking at 225 rpm until it reached ~0.7OD600. IPTG (1 mM) was added to the culture broth to induce the GIP expression. Cell pellets were collected 4~5 hours post induction by centrifugation at 4,000 rpm at 4°C for 15 minutes, followed by resuspension in a homogenization buffer (20mM Tris-HCl, 500 mM NaCl, and 1 mM Phenylmethanesulfonyl fluoride (PMSF)). An ultrasonic homogenizer was used to prepare cell-free protein extraction. The homogenized samples were centrifuged at 13,000 rpm at 4°C for 10 min, and the supernatants were collected and stored at −20°C until use. A HiTrap IMAC column (GE Healthcare Lifescience, NJ) was employed to purify the proteins, followed by dialysis against a sugar-free buffer (20 mM Tris-HCl, 5 mM Dithioerythritol, 150 mM NaCl, and 1 mM CaCl2) at 4°C using a Float-A-Lyzer Dialysis Tubing (Fisher Scientific, PA) with a 25 kDa molecular weight cutoff.
2.3. In vitro GIP characterization
FRET signal was determined by measuring the fluorescent intensities of ECFP and EYFP at 476 nm and 526 nm simultaneously when excited at 433 nm. FRET signal was defined as a peak emission intensity ratio at 526 nm and 476 nm (Garrett et al. 2008). The Perkin-Elmer LS 55 luminescence spectrophotometer (Perkin-Elmer Instruments, Beaconsfield, UK) was used to measure the fluorescent intensities. The slit widths for both excitation and emission wavelengths were set at 10 nm. Glucose titration curves were established at room temperature using a quartz cuvette filled with 600 μL of GIP, as described elsewhere (Ye and Schultz 2003). Apparent Kd were determined by fitting glucose titration curves to one-site saturation binding isotherm using Prism 5: S=(Rmax−R)/(Rmax−Rmin)=n[s]/(Kd +[s]), where S is saturation of the FRET; R is fluorescent intensity ratio of EYFP to ECFP; Rmax stands for the maximum ratio at the saturation with ligand; Rmin stands for minimum ratio in the absence of ligand; n represents the number of binding sites; and [s] is glucose concentration. At least three independent titrations were performed with each GIP. All experiments were performed in triplicate.
2.4. Hollow fiber CGM sensor fabrication
To fabricate a CGM sensor, GIPi-Thr was enclosed inside a dialysis hollow fiber composed of biocompatible and regenerated cellulose with inner diameter of 200 μm, wall thickness of 16 μm, and cutoff molecular weight of 18 kDa. The hollow fiber was cut into a 1.0-cm segment, fixed in a 7-μL quartz flow cell unit, and installed in the aforementioned luminescence spectrometer. The sensor was stored in a sugar-free buffer between the measurements, and sugar-free or sugar-containing media were perfused into the flow cell unit for CGM through ratiometric FRET measurement. All measurements were conducted at room temperature.
2.5. Visualization of glucose within living cells through FRET imaging microscopy measurement
Mouse myoblast cells (C2C12) from ATCC (CRL-1772) were routinely maintained in Dulbecco’s modified Eagles’s medium supplemented with 10% fetal bovine serum (ATCC), 100 U/mL of penicillin, 0.1 mg/mL of streptomycin, and 2 mM L-glutamine at 37°C under 5% CO2 atmosphere. For visualization of intracellular glucose, cells were seeded on a 40 mm glass cover slip one day prior to experiment and transiently transfected with GIP expression plasmids at 50–60% confluence using the Polyfect transfection reagent (Qiagen, Valencia, CA). The FRET imaging microscopy measurement was performed 30–40 h post transfection using an inverted Olympus IX71 fluorescence microscope (Tokyo, Japan) equipped with a cooled CCD camera (Qimaging Retiga 4200, Surrey, Canada) and an automated multifilter wheel (Sutter Instrument Company, Novato, CA) controlled by Lambda 10-3 (Sutter Instrument Company, CA) through Slidebook (Intelligent Imaging Innovations, Inc., Denver, CO). The GIP was excited at 436 nm and the emissions of ECFP and EYFP were monitored using two different filters, i.e., 480/40 emission filter for ECFP and 535/30 emission filter for EYFP (Chroma Technology Corp, Rockingham, VT). Exposure time and neutral density of the filters were optimized to minimize photo-bleaching the sensory proteins. Glass cover slips were inserted into a gas-tight, temperature-controlled FCS2 perfusion chamber (Bioptechs Inc., Butler, PA) and perfused with a bath solution at a flow rate of 1 mL/min using a gravity fed six-way perfusion device (Warner Instruments, Hamden, CT) controlled by cheminert multi-position valve with microelectric actuator (VICI Valco Instruments, Houston, TX). The bath solution is composed of NaCl (140 mM), KCl (5 mM), MgCl2 (1.1 mM), CaCl2 (2.5 mM), and HEPES (10 mM). Either 10 mM glucose or 10 mM N-methyl-d-glucamine was added to the bath solution to determine the changes in cellular glucose uptake rates in response to changes in extracellular glucose concentrations.
3. Results and discussion
3.1. Construction of a Panel of Glucose Indicator Proteins
To develop a GIP suitable for monitoring blood glucose concentration, the Kd of the GBP needs to be increased from μM to mM. There are two approaches for introducing a long range of ligand binding affinity into the GBP: i.e., the manipulation of electrostatic interaction (Loladze et al. 1999) or the change in conformational equilibrium (Marvin and Hellinga 2001). A ligand-free open form of GBP can be stabilized or destabilized by mutating those residues located to link local conformational changes to the global- or ligand-mediated hinge-bending motion (de Lorimier et al. 2002), by shifting the equilibrium from open to close, and subsequently reducing the glucose binding affinity. Similarly, mutation of these locations to smaller residues could minimize the energetic difference between the open and closed forms, thereby increasing the glucose binding affinity. A GBP crystal structure shows that two aromatic residues, Phe16 and Trp183, engage in hydrophobic stacking with glucose as well as galactose (Borrok et al. 2007), and GBP Phe16 mutation can disrupt pi-pi stacking around glucose (Der and Dattelbaum 2008). Crystallographic analysis revealed that D-glucose or D-galactose is sandwiched in the GBP binding pocket by facing Phe16 and Trp183 of GBP through extensive van der Waals interactions (Vyas et al. 1994), indicating the superpositioning effect of the 16th and 183rd amino acids in the sugar binding site. Thus one can expect that replacing the amino acid residues with other amino acids at these sites may lead to a significant alternation of GBP’s glucose binding affinity. Fehr et al.’s work supports this hypothesis (Fehr et al. 2003). Following this lead, we performed the site-directed mutagenesis to replace the phenylalanine at the 16th amino acid residual site of GBP with a variety of amino acids such as valine, cysteine, threonine, and alanine. These mutants were then employed to construct a panel of GIPs by sandwiching them with ECFP and EYFP, as described elsewhere (Garrett et al. 2008; Ye and Schultz 2003). The resultant GIPs were designated as GIPi-Val, GIPi-Cys, GIPi-Thr, and GIPi-Ala. The molecular structure of these GIPs can be found in our previous work (Veetil et al. 2010; Ye and Schultz 2003). We also constructed a GIPi-Null in which the amino acid residue at the 16th site of GBP was deleted. This is to determine whether the 16th residue is essential to GBP’s glucose binding affinity.
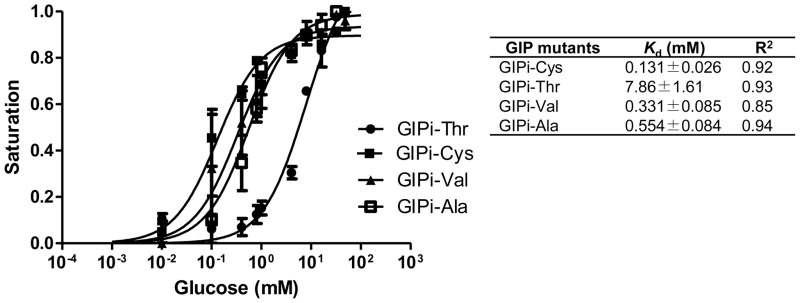
We determined the Kd values of the GIP mutants. The titration of the purified GIPs presented a glucose dose-dependent decrease in FRET (Fig. 1). Among the four mutants, the dissociation constants of GIPi-Cys, GIPi-Val, and GIPi-Ala are 0.13, 0.33, and 0.55 mM, respectively. The threonine substitution at the 16th amino acid position of GBP (GIPi-Thr) elevated the GIP’s Kd value to 7.9 mM, allowing its use for detecting glucose concentration up to 32 mM, suitable for monitoring the blood glucose level. In contrast, the deletion of the 16th amino acid of GBP resulted in no response to glucose concentration, suggesting the loss of its binding ability for glucose (data not shown). This result demonstrated that the removal of the 16th amino acid residue of GBP could disrupt the glucose binding site and thus lead to the loss of its binding ability for glucose.
Fig. 1.
Nonlinear regression of glucose binding isotherm curves of mutated GIPs. Data shown are the mean of three experiments and error bars stand for standard deviations of the mean. Symbol: Kd, dissociation constant of GIP; R2, the square of correlation coefficient. The calculation of Kd value was performed using steady-state intensity data fitting to one-site saturation binding isotherm with nonlinear regression, as described elsewhere (Garrett et al. 2008).
3.2. Fabrication of a millimole glucose sensor for CGM using mutated GIP
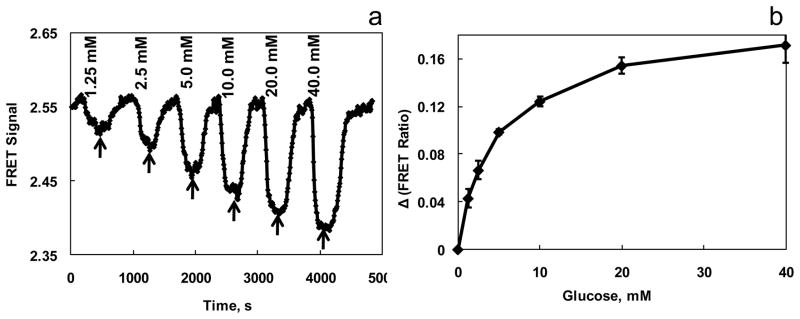
Using GIPi-Thr, we explored the feasibility of developing a glucose sensor for CGM within the physiological range of blood glucose concentration. The GIP was enclosed in a dialysis hollow fiber and installed in a 7 μL quartz cuvette in a continuous flow assembly to constitute a biosensor for CGM, as described previously (Ye and Schultz 2003). The FRET signals of the sensor were determined by measuring fluorescence intensity at 475 and 525 nm simultaneously while excited at 435 nm. Sugar-free solution was perfused through the sensor to yield a baseline of the sensor. For CGM, solutions containing glucose concentrations from 1.25 to 40 mM were flown continuously through the sensor assembly, and corresponding FRET signals were recorded. Between each glucose concentration, the flow was shifted to a sugar-free binding buffer (Fig. 2a). The experimental results presented as a response curve (signal, Δ FRET ratio against glucose concentration) clearly demonstrated the response of GIP to the glucose concentration in a physiological range (Fig. 2B).
Fig. 2. Continuous glucose monitoring through ratiometric FRET measurement with the GIPi-Thr sensor.
(a) Buffers containing various glucose concentrations from 1.25 mM to 40 mM were flown through the sensor assembly and the FRET signals were recorded continuously every 6 s. the sensor was excited at 435 nm. Its emission at 475 and 525 nm were monitored with a luminescence spectrophotometer. The slit widths were set at 10 nm for both excitation and emission. The arrows indicate the flow of sugar-free bath into the sensor assembly. (b) CGM experiments were repeated (n=3) and its glucose response curve was determined. The Δratio reflects the differences between the peak height at a given glucose concentration and the baseline in CGM curve. The measurements were performed at room temperature.
3.3. Construction of a molecular probe using mutated GIP for CGM within living cells through FRET imaging microscopy measurement
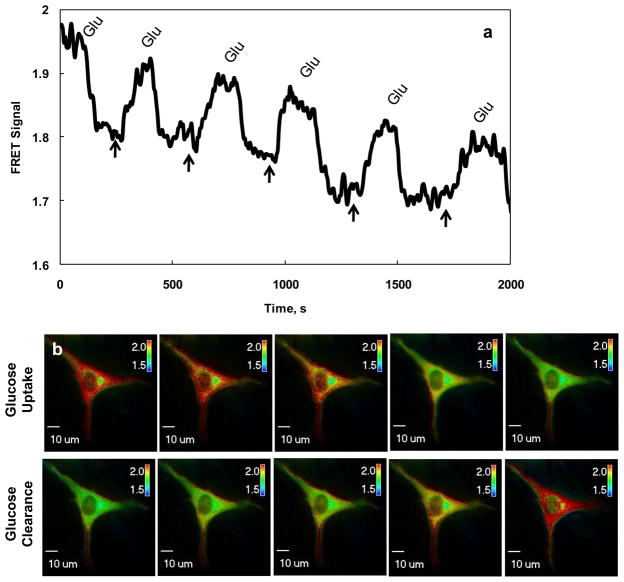
As GIPi-Cys′ Kd is about 0.131 μM close to the range of intracellular glucose concentration (Cline et al. 1999), we decided to explore the feasibility of developing a molecule probe for visualizing the intracellular glucose concentration within living cells through FRET imaging microscopy measurement. The GIPi-Cys encoding plasmids were transiently transfected into C2C12 cells for monitoring intracellular glucose concentration through FRET imaging microscopy measurement. FRET images were taken 36 h post transfection, and pseudo-color images of the cells expressing GIP were generated using Slidebook’s 2-channel corrected FRET module based on the pixel-by-pixel intensities of the images (Sorkin et al. 2000). The FRET signals were measured in six-second intervals during the perfusion of bath solutions containing 0 or 10 mM glucose. The images in Fig. 3 were captured with 200 ms exposure and 2×2 binning in a single C2C12 cell. We observed the uniform expression of the GIPi-Cys in cytosol of the cells (Fig. 3b). The top panel of Fig. 3b shows that the visualization of cellular glucose uptake occurred after 10 mM of glucose containing medium was flown into the culture chamber. The increase in intracellular glucose concentrations led to the color of the images turning from red to green, indicating a reduction of FRET signals due to the increase of intracellular glucose concentrations. In contrast, the color gradually shifted from green to red when sugar-free medium was flown into the cell culture chamber, revealing an increase in FRET signals owing to the decrease of intracellular glucose concentration caused by the removal of glucose from the medium. The changes in FRET signals in response to changes in intracellular glucose concentrations were quantified, as shown in Fig. 3a. Clearly, the FRET signals measured through FRET imaging microscopy measurement show superior response of the probe to the changes in intracellular glucose in live cells. The FRET signals started dropping when 10 mM glucose containing medium was flown into the culture chamber until it became stable. When glucose was removed from the medium, indicated by the arrows in Fig. 3a, the FRET signal started increasing until reaching a stable level, suggesting the reduction of intracellular glucose concentration due to the removal of glucose from the medium. The reversible changes in FRET signals during the repeated on-off of extracellular glucose supplement suggested that GIPi-Cys indicator worked well for visualizing glucose changes in live cells. The time constants for glucose uptake and clearance were calculated as 31 and 101 seconds, respectively by first order exponential curve fitting (Veetil et al. 2010). We also observed shifting of FRET signal baseline from 2.0 to approximately 1.67 with the repeated switch of 10 mM glucose bath to glucose-free medium. This observation is in consistence with others work. It has been found that the intracellular glucose concentration cannot reduce to its original level even after extracellular glucose was completely removed from culture medium through perfusion (John et al. 2007). This happens due in part to the synthesis of glucose from glycogen or other nonsugar substrates after removal of extracellular glucose from the medium.
Fig. 3. Visualization of intracellular glucose in single live cell with GIPi-Cys.
(a) The measurement of FRET signals in response to changes in intracellular glucose in single live cells and (b) pseudo-colored ratiometric FRET images of cells cultured in different glucose concentrations. The murine myoblast cells (C2C12) were cultured in a medium perfusion chamber and were transiently transfected with pGIPs-Cys encoding the GIPi-Cys indicator. The ratiometric FRET images were taken 36 h post transfection. To determine the response of GIPi-Cys to changes in intracellular glucose concentrations, 10mM glucose or N-methyl-d-glucamine containing media were perfused into the chamber. The FRET images showing changes in intracellular glucose concentrations, caused by changes in extracellular glucose concentrations, were taken using an Olympus inverted FRET microscope IX 71. The response curve shown in (a) was determined from the images taken in (b) using a FRET imaging microscopy measurement software (Slidebook). LUT in (b) represents the FRET signals.
4. Conclusion
In this work, we demonstrate that a panel of GIPs can be generated for CGM using site-directed mutagenesis of GBPs. Particularly, we showed at the first time that a GIP suitable for monitoring blood glucose level can be constructed using one of these mutants. With this GIP, it is possible to fabricate an implantable glucose sensor for CGM. Although only buffer systems, not human blood samples, were used to test these sensors, the experimental results shown in this study serve as proof-of-concept data for further investigation. Moreover, as shown in our previous works (Veetil et al. 2010), the GIPs are very stable at room temperature. It can be stored at room temperature for up to three weeks without losing functionality. We have also shown that a sensor developed with GIP can work continuously up to three weeks (Veetil et al. 2010). Using another GIP mutant we revealed the feasibility of developing a molecular probe for visualizing glucose uptake and clearance within living cells through FRET imaging microscopy measurement. Acquiring this key information could be critical to understanding the mechanism of glucose homeostasis in blood, providing better insights to explore unique diabetes therapy.
On the other hand, there is a wide range of glucose concentrations that have been observed in various types and various states of cells (Ishihara et al. 1993; Poitout et al. 1996). For instance, the mean intracellular glucose concentration in normal subjects is approximately 100 μM, but it is close to 200 μM in patients with diabetes (Cline et al. 1999). It has also been found that the intracellular glucose concentration can fall below 0.1 mM if the glucose uptake becomes slow; it can raise above 0.5 mM if the glucose uptake rate is high (John et al. 2007). Although a study showed that the sensitivity of the FRET sensor can be improved by adjusting the structure of the linker between CFP-GBP and GBP-YFP (Takanaga et al. 2007), the construction of a panel of GIPs which glucose binding affinities vary in line with required glucose working ranges can be beneficial. We demonstrated here that such a panel of GIPs can be developed by site-directed mutagenesis of GBP at its 16th amino acid residues.
Acknowledgments
This research was partially supported by NIH grant EB006378-01 and Arkansas Biosciences Institute grant 0402-27504-21-726.
References
- Ballerstadt R, Evans C, Gowda A, McNichols R. J Diabetes Sci Technol. 2007;1(3):384–393. doi: 10.1177/193229680700100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerstadt R, Evans C, McNichols R, Gowda A. Biosens Bioelectron. 2006;22(2):275–284. doi: 10.1016/j.bios.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Borrok MJ, Kiessling LL, Forest KT. Protein Sci. 2007;16(6):1032–1041. doi: 10.1110/ps.062707807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE. Anal Chem. 2009 doi: 10.1021/ac802613w. [DOI] [PubMed] [Google Scholar]
- Careaga CL, Falke JJ. J Mol Biol. 1992;226(4):1219–1235. doi: 10.1016/0022-2836(92)91063-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. N Engl J Med. 1999;341(4):240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- de Lorimier RM, Smith JJ, Dwyer MA, Looger LL, Sali KM, Paavola CD, Rizk SS, Sadigov S, Conrad DW, Loew L, Hellinga HW. Protein Sci. 2002;11(11):2655–2675. doi: 10.1110/ps.021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der BS, Dattelbaum JD. Anal Biochem. 2008;375(1):132–140. doi: 10.1016/j.ab.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Fehr M, Lalonde S, Ehrhardt DW, Frommer WB. J Fluoresc. 2004;14(5):603–609. doi: 10.1023/b:jofl.0000039347.94943.99. [DOI] [PubMed] [Google Scholar]
- Fehr M, Lalonde S, Lager I, Wolff MW, Frommer WB. J Biol Chem. 2003;278(21):19127–19133. doi: 10.1074/jbc.M301333200. [DOI] [PubMed] [Google Scholar]
- Flocco MM, Mowbray SL. J Biol Chem. 1994;269(12):8931–8936. [PubMed] [Google Scholar]
- Garrett JR, Wu X, Jin S, Ye K. Biotechnol Prog. 2008;24:1085–1089. doi: 10.1002/btpr.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Rao G, Tolosa L. Biotechnol Prog. 2008;24(3):691–697. doi: 10.1021/bp070411k. [DOI] [PubMed] [Google Scholar]
- House JL, Anderson EM, Ward WK. J Diabetes Sci Technol. 2007;1(1):18–27. doi: 10.1177/193229680700100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. Diabetologia. 1993;36(11):1139–1145. doi: 10.1007/BF00401058. [DOI] [PubMed] [Google Scholar]
- John SA, Ottolia M, Weiss JN, Ribalet B. Pflugers Arch. 2007 doi: 10.1007/s00424-007-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, Gnudi L, Pickup JC. Biochem Biophys Res Commun. 2008;365(1):102–106. doi: 10.1016/j.bbrc.2007.10.129. [DOI] [PubMed] [Google Scholar]
- Loladze VV, Ibarra-Molero B, Sanchez-Ruiz JM, Makhatadze GI. Biochemistry. 1999;38(50):16419–16423. doi: 10.1021/bi992271w. [DOI] [PubMed] [Google Scholar]
- Marvin JS, Hellinga HW. Nat Struct Biol. 2001;8(9):795–798. doi: 10.1038/nsb0901-795. [DOI] [PubMed] [Google Scholar]
- Miller DM, 3rd, Olson JS, Pflugrath JW, Quiocho FA. J Biol Chem. 1983;258(22):13665–13672. [PubMed] [Google Scholar]
- Moussy F, Reichert WM. Diabetes Technol Ther. 2000;2(3):473–477. doi: 10.1089/15209150050194341. [DOI] [PubMed] [Google Scholar]
- Pickup JC. Diabetes Care. 1993;16(2):535–539. doi: 10.2337/diacare.16.2.535. [DOI] [PubMed] [Google Scholar]
- Poitout V, Olson LK, Robertson RP. Diabetes Metab. 1996;22(1):7–14. [PubMed] [Google Scholar]
- Rebrin K, Fischer U, Hahn von Dorsche H, von Woetke T, Abel P, Brunstein E. J Biomed Eng. 1992;14(1):33–40. doi: 10.1016/0141-5425(92)90033-h. [DOI] [PubMed] [Google Scholar]
- Schultz JS, Mansouri S, Goldstein IJ. Diabetes Care. 1982;5(3):245–253. doi: 10.2337/diacare.5.3.245. [DOI] [PubMed] [Google Scholar]
- Shilton BH, Flocco MM, Nilsson M, Mowbray SL. J Mol Biol. 1996;264(2):350–363. doi: 10.1006/jmbi.1996.0645. [DOI] [PubMed] [Google Scholar]
- Sorkin A, McClure M, Huang F, Carter R. Curr Biol. 2000;10(21):1395–1398. doi: 10.1016/s0960-9822(00)00785-5. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Chaudhuri B, Frommer WB. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbamem.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes TI, Moussy F. Diabetes Technol Ther. 2000;2(3):367–376. doi: 10.1089/15209150050194233. [DOI] [PubMed] [Google Scholar]
- Veetil JV, Jin S, Ye K. Biosens Bioelectron. 2010 doi: 10.1016/j.bios.2010.08.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas MN, Vyas NK, Quiocho FA. Biochemistry. 1994;33(16):4762–4768. doi: 10.1021/bi00182a003. [DOI] [PubMed] [Google Scholar]
- Ye K, Jin S, Bratic K, Schultz JS. J Molecular Catalysis B: Enzymatic. 2004;28:201–206. [Google Scholar]
- Ye K, Schultz JS. Anal Chem. 2003;75(14):3451–3459. doi: 10.1021/ac034022q. [DOI] [PubMed] [Google Scholar]