Abstract
Prostate cancer is the most common malignancy in older men. With the aging of the population, the number of older men with prostate cancer will grow rapidly. Androgen deprivation therapy (ADT) is the mainstay of treatment for men with systemic disease and is increasingly utilized as primary therapy or in combination with other therapies for localized disease. Side effects of therapy are multifold and include hot flashes, osteoporosis, and adverse psychological and metabolic effects. Recent research has illustrated that ADT can negatively impact the functional, cognitive, and physical performance of older men. Patients with prostate cancer, despite recurrence of the disease, have a long life expectancy and may be subjected to the side effects of ADT for many years. This review highlights the complications of ADT and approaches to management. We also provide recommendations for assessment and management of ADT complications among the most vulnerable and frail older male patients.
Keywords: Disability, Geriatric assessment, Prostate cancer, Vulnerable elders, Functional impairment, Androgen deprivation, Quality of life, Complications
1. Introduction
Prostate cancer is an age-associated disease. Over 70% of all cases of prostate cancer are diagnosed in men over 65 years of age, and the median age of men with prostate cancer is 79 years [1,2]. The risk of developing prostate cancer increases from 1 in 45 for those aged 40–59 years to 1 in 7 for those aged 60 or over [3]. Due largely to the use of more sensitive diagnostic techniques, particularly prostatic specific antigen (PSA) testing, prostate cancer is now diagnosed more frequently and at earlier stages [4]. Older men are more likely to be diagnosed with low- or intermediate-grade localized prostate cancer which may not impact their life expectancy, and thus a majority of older men with prostate cancer die from other diseases [5,6]. The potential lack of impact on life expectancy of untreated prostate cancer is illustrated by the high prevalence of the disease in autopsies. The prevalence of previously undetected prostate cancer in autopsies is from 10 to 20% in men between 50 and 60 years and is as high as 50% in men between 70 and 80 years [7,8]. It is estimated that due to the aging of the population, the prevalence of prostate cancer will quadruple by 2030 [9]. As a result, the use of prostate cancer therapies that adversely affect quality of life (QOL) is likely to increase. In addition, persons over the age of 80 are the fastest growing subgroup of the population [10]. These statistics portend an increasing number of older men, especially those in the “oldest-old” age group, with diagnosed prostate cancer who will likely experience complications from treatment for this disease.
Androgen deprivation therapy (ADT) is the most widely used therapeutic modality in prostate cancer [11]. Androgen suppression can be achieved by means of orchiectomy or the use of gonadotropin-releasing hormone agonists. ADT is the mainstay of initial therapy for systemic disease whether it is biochemical recurrence (i.e., PSA-rise only) after definitive localized therapy or overt metastatic disease. Although the timing of initiation of treatment for patients with asymptomatic disease recurrence is controversial [11,12], ADT is nonetheless increasingly employed earlier in the disease course [13–15]. While older men with prostate cancer have a higher incidence of low-risk disease characteristics, they are more likely to be treated with ADT than other modalities including watchful waiting [4,16]. ADT is increasingly being used as monotherapy or in combination with other modalities for clinically localized disease [17]. When initiated for systemic disease or as monotherapy for clinically localized disease, ADT is typically continued life-long, and many men live with the side effects of ADT for many years [18]. Commonly recognized adverse effects include hot flashes, decreased libido, and erectile dysfunction, and patients are often warned of these three common side effects [19]. However, there are numerous other potential complications. The impact of ADT on osteoporosis including risk of fracture has been well-described. More recently, ADT has been linked to worsening of comorbidities including diabetes and cardiovascular disease, especially in those patients who had these underlying conditions prior to initiation of treatment. The prevalence of functional, cognitive, and physical impairments in an at-risk population of elderly prostate cancer patients undergoing treatment with ADT is not as well-documented but has been illustrated in recent research [20–22].
Due to the potential interactions between age and ADT, it is imperative to recognize those side effects that can differentially affect the QOL and function of elderly men with prostate cancer and develop an approach to management that accounts for these issues. This review will provide an overview of common complications from ADT, provide recommendations for management, and highlight specific concerns for the geriatric population.
2. Forms of androgen deprivation therapy and efficacy in older prostate cancer patients
The American Society of Clinical Oncology (ASCO) Recommendations for Initial Hormonal Management of Androgen-Sensitive Disease defines ADT as “a treatment or procedure in which the androgen receptor of target cells is not activated via either reduction of testosterone production or androgen receptor blockade” and furthermore states that ADT “encompasses castration, antiandrogen therapies, and combinations thereof [23].”
Androgen deprivation can be achieved through several mechanisms with varying effects on peripheral testosterone levels. Dating back to the 1940s, bilateral orchiectomy was done to lower testosterone to castrate levels (<50 ng/ml). Prior to the advent of pharmaceutical mechanisms to achieve castrate-level hormones, orchiectomy had wide acceptance, and it is associated with the rapid onset of palliation, low cost, and lack of dependence on patient compliance. Although the procedure is well-tolerated with a low risk of infection, pain, or bleeding, the emotional burden associated with the permanence of physical castration instigated a search for other means of androgen deprivation.
The FDA approved the use of the first LHRH agonist in 1985. LHRH, released in pulses by the hypothalamus, triggers the release of FSH (follicle stimulating hormone) and LH (luteinizing hormone). A meta-analysis of the literature found no significant difference in outcomes between LHRH agonist use and orchiectomy [24]. LHRH agonist therapy is extremely expensive, with estimates of costs as high as $230 million dollars per year [25,26]. Unlike orchiectomy, testosterone levels can rise 4–6 months after cessation of LHRH agonist therapy, although not always to baseline levels [27]. However, the elderly are more likely to experience prolonged castrate levels of testosterone despite discontinuation of treatment and are less likely to have testosterone return to baseline levels [28].
Other methods to achieve androgen deprivation include DES, nonsteroidal antiandrogens, and steroidal antiandrogens. DES, a semi-synthetic estrogen compound, reduces testosterone to castrate levels within 2 weeks by inhibiting LHRH production from the hypothalamus. DES is not currently commercially available in North America. The nonsteroidal antiandrogens (bicalutamide, flutamide, and nilutamide) interfere competitively with androgen binding to the androgen receptor. As a result, testosterone levels are not reduced with these therapies and may actually increase. Data suggests that use of these agents as monotherapy may not be as effective as castration [29]. Nonsteriodal antiandrogens as single agents have different toxicity profiles than that of castration. Side effects, however, are generally reversible with discontinuation of therapy.
Options for androgen deprivation strategies generally include agents described above as monotherapy, a combined therapy approach (usually castration combined with antiandrogen), or intermittent androgen therapy. The rationale for combined therapy is that more complete androgen suppression can be achieved when an antiandrogen is added because castration, although it reduces testosterone levels, does not eliminate androgen production from the adrenal glands. Several meta-analyses and reviews have provided conflicting data [30–33]. The American Society of Clinical Oncology (ASCO) recommendations for the initial hormonal management of androgen-sensitive prostate cancer reviewed the available data. Although the panel felt that a small survival advantage may be present with combined androgen blockade versus castration alone, the use of this approach is associated with greater toxicity and is less cost effective [11].
Intermittent ADT describes a treatment approach in which LHRH agonist or combined therapy (LHRH agonist plus an antiandrogen) is initiated in a cyclical pattern depending on PSA levels. Treatment periods are cycled with off-treatment periods in which the PSA is allowed to rise to a predetermined level prior to ADT re-initiation. The benefits of this approach have not yet been proven in a prospective randomized trial, although some small studies suggest better sexual function and QOL during the off-period treatment [34]. Follow-up of two large randomized trials that are evaluating the efficacy and QOL differences between intermittent and continuous ADT is ongoing.
This review will further discuss complications of ADT and their management. Although side effects of all modes of therapy will be discussed, complications from castration will serve as the central focus.
3. Management of ADT effects on neuroendocrine and neuropsychological health
3.1. Hot flashes
Hot flashes, also known as hot flushes, significantly affect the quality of life (QOL) of patients who receive ADT. Hot flashes are sudden uncomfortable sensations of warmth in the face, neck, upper chest, and back, often accompanied by redness, profuse sweating, nausea, and anxiety. Although the exact etiology remains unclear, some have proposed that the decrease in testosterone secretion inappropriately stimulates the hypothalamic thermoregulatory center resulting in peripheral vasodilation [19,35]. Hot flashes, which for the most part occur spontaneously, may be triggered by stress, poor sleep, hot weather, changes in body position, or ingestion of hot liquids. Duration can last anywhere from seconds up to an hour in the most severe cases.
Hot flashes affect 58–80% of patients receiving ADT, with differing levels of severity [36,37]. A proportion of patients (15–27%) describe hot flashes as the most significant adverse QOL effect from ADT [38,39]. Debilitating hot flashes can result in ADT discontinuation. Hot flashes tend to become more frequent 3 months after starting treatment and persist long-term for most patients, contrary to the perception that they become less severe over time [19]. Although some scales to determine severity of hot flashes have been developed, none has been universally adopted. Nevertheless, practitioners should inform the patient about this potential side effect and, as a first step, have the patient keep a diary of the frequency and severity of events. A commonly utilized scale to keep track of hot flashes was developed by Moyad (Table 1) [40]. The frequency and severity of hot flashes should guide a discussion of interventions, but many men who suffer from hot flashes are not interested in taking medication [41,42].
Table 1.
The “Moyad” scale for determining frequency and severity of hot flashes (Moyad 10).
| Severity | Pointsa | Length | Quality |
|---|---|---|---|
| Mild | 1 | <1 min | Warm, involving limited body parts, no perspiration |
| Moderate | 2 | <5 min | Warm, uncomfortable, involving more of the body, perspiration, often requiring removal of clothing |
| Severe | 3 | >5 min | Burning quality, extremely uncomfortable, excessive perspiration, disruption of life activities |
Assign points for each hot flash and add points for each day for weekly discussions with a health professional.
Alternative or complementary therapies may alleviate mild-to-moderate hot flashes (and sometimes even severe hot flashes). Acupuncture is thought to work by raising serotonin levels which alter the temperature set point in the hypothalamus. Frisk et al. found that hot flashes were decreased in 29 men who received electrostimulation or traditional acupuncture weekly for 12 weeks [43]. In a study evaluating longer-term effects of acupuncture, 196 persons, predominantly with breast and prostate cancer, with a mean of 16 flashes per day, were taught to perform self-acupuncture for up to 6 years [44]. Following treatment, 114 (79%) achieved a 50% or greater reduction in hot flashes. Although acupuncture is an option for patients, larger and longer-term studies with defined placebo-control groups are required to determine the impact of acupuncture on hot flashes in older patients with prostate cancer receiving ADT.
Dietary supplements such as soy, black cohosh, and crushed flaxseed have also been investigated as therapies for reducing the severity of hot flashes in various populations [41]. In small randomized studies, these dietary supplements have been shown to help with hot flashes suffered by menopausal women [45–47]. These dietary interventions require more study in prostate cancer patients on ADT, but these supplements have not been shown to be harmful and may be helpful for some patients with mild-to-moderate hot flashes. On the other hand, one dietary supplement that had been previously recommended for palliation of hot flashes, Vitamin E at 400 IU twice daily, has now been shown to be associated with increased all-cause mortality and may have negative effects on cardiovascular health [48,49].
For those men who are motivated to reduce hot flash frequency and severity, hormonal and non-hormonal pharmacologic approaches are available. Hormonal approaches include progestational agents such as megestrol acetate, depot medroxyprogesterone, or estrogenic agents such as diethylstilbestrol or transdermal estrogen. Megestrol acetate is commonly utilized for the treatment of hot flashes in men receiving ADT and has been reported to reduce hot flashes by up to 85% [50]. A few case reports describe progression of prostate cancer with megestrol acetate; therefore, monitoring of disease is important [51,52]. Depot medroxyprogesterone acetate (MPA) has been studied as an intervention to reduce hot flashes in men on ADT. A multi-institutional retrospective study reviewed the efficacy of 150 mg or 400 mg IM of MPA in men on ADT [53]. Over 90% of patients had symptomatic improvement, and 48% achieved complete elimination of hot flashes. Side effects related to progestin use include sexual dysfunction, salt retention, and weight gain, although in general the medication is very well-tolerated. Estrogen therapies such as DES or transdermal estrogen can be effective in managing hot flashes, but benefits should be weighed carefully against toxicities and risks. Although the standard dose of DES is 1 mg/day, lower doses are effective and are associated with lower toxicity [54]. Cardiovascular complications including thromboembolism, myocardial infarction, and stroke are the main serious toxicities of DES at higher doses but may be less at lower dose levels. Although DES is not commercially available in the United States, low doses can often be prepared by a compounding pharmacist. Transdermal estrogen has also been used with success [55]. Gynecomastia and nipple tenderness can be significant consequences of estrogen therapy, and patients who choose to be treated should be offered prophylactic breast irradiation to prevent breast enlargement.
Non-hormonal treatments for hot flashes include antidepressants and gabapentin. Those antidepressants that are thought to have some efficacy generally fall in the class of the 5-hydroxytryptamine (5-HT) reuptake inhibitor family. The starting dose of sertraline is 25 mg/day, titrating by 25–75 mg/day or 100 mg/day as needed. Studies evaluating the efficacy of sertraline as a treatment for hot flashes caused by hormonal treatment for breast cancer have resulted in mixed outcomes [56,57]. One case report describes sertraline’s efficacy in relieving hot flashes in a man with advanced prostate cancer receiving ADT, but no other published studies are currently available [58]. Several small uncontrolled studies of venlafaxine HCl have demonstrated anywhere from a 50 to 68% reduction in hot flashes in breast or prostate cancer patients [59,60]. In a randomized study, the 75 mg dose has been associated with an approximately 60% reduction in hot flashes as compared to placebo in breast cancer patients [61]. Although currently no randomized placebo-controlled study of 5-HT uptake inhibitors in the treatment of hot flashes in men with prostate cancer has been published, the medications are generally well-tolerated and may have the added benefit of combating the psychological effects of ADT. Most recently, gabapentin has shown some promise in palliating hot flashes. Randomized controlled studies have shown that gabapentin at higher dose levels (900 mg/day) can cause a 45–50% reduction in hot flash frequency and severity scores in women with breast cancer or menopausal symptoms [62,63]. No randomized placebo-controlled study investigating gabapentin for men experiencing hot flashes from ADT has been published.
In summary, hot flashes can be a significant side effect for men on ADT and may result in discontinuation of therapy. There is some support for the use of medroxyprogesterone acetate, antidepressants, and gabapentin in palliating hot flashes, and these medications are generally well-tolerated. Larger randomized, placebo-controlled, double-blind studies are still needed to study the efficacy of these drugs and complementary approaches for men with prostate cancer on ADT. In addition, the risk of adding a medication for older men who may already be taking multiple medications and who may be more prone to side effects requires more study. Table 2 provides a summary of approaches for treating hot flashes.
Table 2.
Treatments for hot flashesa.
| Hot flash treatment | Summary of evidence |
|---|---|
| Complementary approaches | |
| Acupuncture | May be effective in mild-to-moderate hot flashes |
| Black Cohosh | In uncontrolled pilot studies, most complementary approaches are associated with a 30–50% reduction in hot flash frequency and severity |
| Flaxseed | |
| Soy | |
| Estrogenic therapies | |
| DES | Effective for moderate-to-severe hot flashes |
| Transdermal estrogen | Associated with >50% reduction in hot flash frequency and severity with many complete responders Increases risk for cardiovascular toxicity and is associated with gynecomastia and nipple tenderness |
| Progestin therapies | |
| Megestrol acetate | Effective for moderate-to-severe hot flashes |
| Medroxyprogesterone acetate | Associated with >50% reduction in hot flash frequency and severity with many complete responders Causes salt retention and weight gain, some reports of worsening prostate cancer progression with megestrol acetate |
| Antidepressants | |
| Sertraline | Effective for mild-moderate-severe hot flashes |
| Venlafaxine | 50–60% reduction in hot flash frequency and severity with venlafaxine Well-tolerated but may cause gastrointestinal side effects May also help with adverse psychological effects as related to ADT |
| Gabapentin | Effective for mild-moderate-severe hot flashes at 900 mg/day Generally well-tolerated |
Adapted from Moyad 10 steps; More research is required for all therapies with well-designed randomized, placebo-controlled, double-blinded studies for men receiving ADT for prostate cancer. As a result, data has been extrapolated from uncontrolled studies or randomized studies involving other populations.
3.2. Anemia
Normocytic normochromic anemia is a commonly encountered problem in men receiving ADT and may be associated with adverse QOL effects, especially fatigue which may contribute to frailty. Testosterone and other androgens stimulate erythroid stem cells and erythropoiesis, and a decrease in testosterone can cause anemia [38]. One study noted a greater than 10% decline in hemoglobin in 90% of patients with 13% experiencing a drop of 25% or more [64]. Other studies indicate that the majority of subjects experience a decline to 25–28 g/L within 4–6 months of ADT initiation [65,66]. Anemia tended to be even worse in those patients with combined androgen blockade than with LHRH agonist alone. Improvement in hemoglobin occurred slowly after cessation of therapy. Patients should be assessed for other risk factors for anemia prior to starting ADT (such as B-12, folate, or iron-deficiencies), and the underlying causes should be addressed in men with anemia prior to ADT initiation. Although subcutaneous recombinant human erythropoietin may correct anemia, the risk of this therapy is likely higher than the benefit [67,68].
3.3. Sexual health side effects
One of the most significant consequences to both men on ADT and their partners is effects on sexual function. ADT impacts both erectile function and libido. These effects are directly linked to testosterone decline and as a result, sexual side effects occur usually within the first year of ADT initiation [19]. Androgens are critical for penile-tissue development, growth, and maintenance of erectile function. In hypogonadal men, androgen supplementation improves overall sexual function in hypogonadal patients, nocturnal penile tumescence, erectile function (in patients who did not respond to phosphodiesterase type 5 inhibitor therapy initially) [69], and the well-being, mood, energy, and sexual function in aging men [70].
Erectile dysfunction (ED) is defined as a persistent inability to initiate or maintain an erection. Severity of ED is definitely worsened with continuous or combined androgen deprivation modalities. Monotherapy with single agent antiandrogen and intermittent androgen deprivation have been associated with better sexual outcomes. Long-term ADT causes a decrease in the frequency, rigidity, duration, and volume of erections during nocturnal penile tumescence measurement [71].
There are a number of potential therapies for ADT-associated sexual dysfunction. Standard treatments for erectile dysfunction may work in men with impotence from ADT. Oral phosphodiesterase inhibitors such as sildenafil, tadalafil, and vardenafil are options, although ADT may negatively influence the response to these medications [72]. These medications are also not recommended for men with significant coronary artery disease or those on nitrate therapy, both of which are more common in older men. Despite the current lack of long-term evidence of benefit in improvement of erectile dysfunction or libido, medical management is recognized as first-line therapy in men on ADT without contraindications to therapy [38]. Non-pharmaceutical modalities for treatment include mechanical erectile dysfunction therapies such as vacuum constriction devices, intracavernosal injections, and penile prostheses. In a Cochrane review of interventions to improve sexual function after treatment of cancer, phosphodiesterase-inhibitors after localized treatment for prostate cancer was found to be effective in treating ED (odds ratio, 10.09; 95% confidence interval, 6.20–16.43), although there was no significant consistent positive benefits with mechanical interventions [73]. In a survey of prostate cancer survivors with ED, although 85% complained about ED, only about one-third noted improvement with interventions [74]. Little is still known about the efficacy of these interventions in men on ADT.
Loss of sexual desire or libido is a common side effect of ADT and can negatively impact a man’s sexuality and self-identity [75]. Not all men have loss of libido, suggesting other underlying factors may impact severity such as age, fitness, and baseline testosterone levels. Couples may have sexual and communication issues prior to starting ADT [19]. Prior to starting therapy, men and their partners have different worries about sexual side effects; while men tend to worry about sexual functioning, women often are anxious about other aspects of the relationship being affected such as intimacy [76]. Counseling and education interventions have been shown to improve sexual function in survivors of prostate cancer and their partners, but there is limited information on the benefits that could be achieved with sexual rehabilitation of men on ADT and their partners [77].
Many older men have sexual dysfunction at baseline prior to ADT initiation, a baseline assessment of libido and ED is important. In addition, previous radiation and surgery for localized disease could exacerbate underlying sexual issues. It is important to note that severity of impotence does not correlate with “bother,” which may be related to the level of sexual function pre-therapy [78,79].
3.4. Neuropsychological side effects
3.4.1. Mood
Low testosterone levels have been shown to impact mood and self-esteem. ADT thus may be associated with an increased risk of depression. In a pilot cross-sectional study of 45 men, 13% of men receiving ADT had major depressive disorder, eight times the national rate of depression [80]. Men with a previous history of depression were more likely to develop a major depressive disorder. In contrast to these pilot results, an analysis of over 50,000 men from the Medicare-Surveillance, Epidemiology, and End Results (SEER) database revealed no statistical difference in the prevalence of depression between men on ADT and controls [81]. At the univariate level, 31.3% of those receiving ADT developed at least one depressive or cognitive diagnosis as compared with 23.7% in those who did not (p < .001). After controlling for variables such as comorbidity, tumor characteristics, and age, the risks associated with ADT were largely eliminated. A limitation for this study is that diagnosis variables are related to claims and mild to moderate depression in older men with multiple problems are likely under-reported. Although more research is necessary to better define the association between ADT and psychological problems, it is recommended by experts in the field that men on ADT be screened for depression and other mental health problems [19,40]. Partners are often the first to report a change in behavior or mood, and often comment on a perceived increase in emotional lability.
There has been little study on the effects of interventions on mood in prostate cancer patients on ADT. An antidepressant may be helpful for men who screen positively for depression. In addition, cognitive behavioral treatments have been shown to improve mood, quality of life, and stress in men with localized prostate cancer, and may be useful for men on ADT [82,83].
3.4.2. Cognition
Several published studies have described the cognitive changes associated with ADT. One of the first studies, published by Green et al., randomized 82 men with systemic prostate cancer to continuous leuproelin, continuous goserelin, cyproterone acetate, or close clinical monitoring [84]. The investigators found that men randomized to the LHRH analogue arms (leuproelin and goserelin) showed differential decrements in cognitive functioning. Twenty-four of 50 men randomized to active treatment had a clinically significant decline in one or more of 12 cognitive tests of attention or memory over a 6-month period. No-one randomized to the observation arm experienced any decline in performance on the tests. A follow-up study which compared the population at 12 months to 20 healthy controls noted that patients on ADT did worse on verbal and 1 of 3 executive tasks at 12 months as compared to controls and observation group [85]. Jenkins et al. reported the effects of neoadjuvant LHRH therapy on cognition in 32 men with localized prostate cancer as compared to 25 healthy men with no prostate cancer [86]. Subjects received 3–6 months of therapy and testing was performed at 3 months (while on therapy) and 9 months later when off-therapy. Although no difference in overall group effect of treatment was noted, differences in cognitive functioning were noted for some men using the reliable-change analysis. Spatial ability and verbal memory were most affected. Four studies have described the effects of combined androgen therapy (i.e., LHRH agonist plus antiandrogen) on cognition [87–90]. Taken as a whole, these small, uncontrolled studies, may support an ADT effect on cognition, especially verbal, spatial, and executive functioning. The clinical significance of these findings, however, remains unclear.
Currently, due to a lack of convincing evidence of an ADT effect on cognition, there are no definite recommendations for treatments that may prevent or treat cognitive impairment in this population. Two studies have evaluated the effects of estradiol administration in the modification of memory in men receiving ADT for prostate cancer. Taxel et al. conducted a double-blind, randomized controlled trial of 17-beta estradiol versus placebo in 27 older men receiving LHRH agonists for treatment of prostate cancer [91]. At 12 weeks, no differences were noted in neuropsychological outcomes despite significant difference in estradiol levels between the groups. Beer et al. evaluated the effects of transdermal estradiol over 4 weeks in 18 patients with prostate cancer receiving ADT as compared to a group of healthy age-matched controls and a group of prostate cancer patients receiving ADT without estradiol [92]. They found that men receiving ADT demonstrated declines in scores of immediate and delayed verbal memory and processing speed when compared to controls. Men receiving estradiol therapy plus ADT demonstrated improvement in verbal memory performance over time. These two studies are the only available published data evaluating interventional approaches to modify cognitive deficits in men receiving ADT.
Results of studies of ADT’s effects on cognition have been variable likely due to different patient populations studied, small samples, variable time points in testing, short exposure times prior to testing, and the variety of neuropsychological tests utilized. It is difficult to draw any specific conclusions on the magnitude or type of cognitive effect by ADT. In addition, the clinical significance of these subtle cognitive changes is yet to be made clear. One large population-based study using the SEER-Medicare database evaluated the risks of developing cognitive disorders (defined as senile dementia, memory disturbances, and cerebral degeneration) 6–60 months after receipt of ADT for prostate cancer [81]. Cognitive disorders were most commonly recognized in the prostate cancer group who received ADT (13.9) as compared to a prostate cancer group without ADT (10.2%) and noncancer control group (7.9%). These results were statistically significant in an unadjusted analysis, but the differences disappeared after controlling for other factors such as age, tumor grade, and comorbidity.
While it remains unclear whether ADT causes cognitive impairment, it is important to recognize underlying cognitive issues in patients presenting with prostate cancer. These disorders impact cancer care patterns and outcomes in older patients and can affect patient decision-making capacity. In addition, treatment decisions should be based on an evaluation of cognitive disability as these may impact life expectancy and ability to tolerate treatment [93]. Screening for these impairments both prior to and along the course of treatment may provide valuable information on underlying deficits. In a cross-sectional analysis, Joly et al. compared the cognitive function of 57 patients receiving ADT for nonmetastatic cancer to 51 healthy age-matched controls [20]. Although they found that there were no significant differences in prevalence of cognitive deficits between the two groups, there was a high prevalence of cognitive disorders overall with 23% of patients exhibiting moderate to severe impairment on the High Sensitivity Cognitive Screen. Our group also found a high prevalence of cognitive deficits in a group of older men (age 70 years or older) on ADT [22]. Eighty percent of our sample of 50 men were receiving ADT for biochemical recurrence versus overt metastatic disease with a median time on ADT of 36 months (range 3–96 months). The Short Portable Mental Status Questionnaire was utilized to screen for gross cognitive impairment. This tool has been linked to an increased risk for mortality in populations of community-dwelling older adults and is a brief measure of orientation, recall, and working memory [94]. Twenty-four percent of men had three or more errors (out of 10) on this measure potentially signifying cognitive impairment. Forty percent had one or more error/s. Although these cross-sectional data do not determine the specific effects of ADT on cognition, it is important to recognize that a high proportion of men with prostate cancer on ADT have significant impairment in cognitive abilities.
4. Management of ADT effects on metabolic and musculoskeletal health
4.1. Metabolic syndrome and cardiovascular health
Weight gain and increased body fat mass are common complications of ADT. This weight gain coupled with low activity levels secondary to fatigue and changes in lipid levels may increase the risk of cardiovascular toxicities. Several studies have noted increase in total cholesterol levels, as well as triglyceride levels. In one cross-sectional study, 58 men were investigated for the prevalence of metabolic syndrome [95]. The sample included 20 patients undergoing ADT for at least 12 months (ADT group), 18 age-matched patients with biochemical recurrence not on ADT, and 20 age-matched controls (control group). The prevalence of metabolic syndrome was higher in the ADT group compared with the non-ADT (p < .01) and control (p = .03) groups. Among the components of metabolic syndrome, men on ADT had a higher prevalence of abdominal obesity and hyperglycemia. Androgen-deprived men also had elevated triglycerides compared with controls (p = .02). The authors concluded that metabolic syndrome was present in more than 50% of the men undergoing long-term ADT, predisposing them to higher cardiovascular risk. In another study of 26 men treated with leuprolide for one year, subcutaneous fat mass in the abdominal area and HDL cholesterol were increased while waist-to-hip ratio, blood pressure, and C-reactive protein did not change significantly. These changes are in contrast to the more recognized “metabolic syndrome” which is characterized by an increased waist-to-hip ratio and blood pressure [96]. In a larger study, Yannucci et al. measured the fasting serum lipid, glucose and hemoglobin A1C levels in 1102 men at baseline and on ADT treatment at days 85 and 169 [97]. Significant increases in total cholesterol, triglyceride and high density lipoprotein-cholesterol were observed in patients while on LHRH agonist therapy.
Several recently published studies have demonstrated an increased risk of diabetes and cardiovascular toxicity in men on ADT in population-based analyses. Using Medicare claims, Keating et al. found that GnRH agonist therapy was associated with an increased risk of incident diabetes (adjusted HR, 1.44; p < .001), coronary artery disease (adjusted HR, 1.16; p < .001), myocardial infarction (adjusted HR, 1.11; p = .03), and sudden cardiac death (adjusted HR, 1.16; p = .004) [98]. Orchiectomy was significantly associated with diabetes (adjusted HR, 1.34; p < .001) but not the cardiovascular outcomes. Tsai et al. evaluated the risk of cardiovascular death in 3262 patients treated with radical prostatectomy and 1630 patients treated with external beam radiation therapy, brachytherapy, or cryotherapy for localized prostate cancer [99]. Competing risks regression analyses were performed to assess whether use of ADT was associated with a shorter time to cardiovascular death after controlling for age (as a continuous variable) and the presence of baseline cardiovascular disease risk factors. Among the 1015 patients who received ADT, the median duration of ADT use was 4.1 months (range = 1.0–32.9 months). Both ADT use (adjusted HR= 2.6; p = .002) and age (adjusted HR= 1.07; p = .003) remained associated with statistically significant increased risks of cardiovascular death in patients treated with radical prostatectomy. Among patients 65 years or older treated with radical prostatectomy, the 5-year cumulative incidence of cardiovascular death was 5.5% in those who received ADT and 2.0% in those who did not. Among patients 65 years or older treated with external beam radiation therapy, brachytherapy, or cryotherapy, ADT use was associated with a higher cumulative incidence of death from cardiovascular causes, but the difference did not reach statistical significance. In a third study, Saigal et al. retrospectively examined the cardiovascular risk of 22,816 newly diagnosed men in a population-based registry who were diagnosed between 1992 and 1996 [100]. The investigators found that newly diagnosed prostate cancer patients who received ADT for at least 1 year were found to have a 20% higher risk of serious cardiovascular morbidity than similar men who did not receive ADT. All the studies conducted so far have been retrospective analyses, and cardiovascular toxicity should be an endpoint studied in any future prospective randomized studies of ADT.
Older patients with prostate cancer have a high prevalence of comorbidities and a higher likelihood of dying from conditions other than prostate cancer. Pre-existing presence of metabolic syndrome and risk factors for cardiovascular toxicity should be assessed prior to initiation of ADT. Focused efforts to reduce cardiac risk factors through diet, exercise, lipid-lowering agents, and other cardioprotective agents (e.g., aspirin, beta-blockers) may help mitigate some of the risks of ADT. In addition, the risks of ADT in potentially causing metabolic syndrome or increasing the risk of cardiovascular toxicity should be explained to the patient and communicated to the patient’s other physicians, including his primary care doctor. If a patient who has underlying cardiac disease must be started on ADT, enlisting the expertise of a cardiologist should be strongly considered along with routine tests to evaluate for worsening of underlying disease.
4.2. Osteoporosis and fractures
Osteoporotic-related fractures are a significant health concern for the elderly with over 1.5 million fractures occurring yearly in the United States. Fractures are associated with back pain, decrease in functional capacity, increased risk for further fractures, higher health care costs, and a higher rate of institutionalization and hospitalizations [101,102]. In osteoporosis studies, fractures in men were associated with profound quality of life deficits especially in physical functioning [103]. In addition, mortality within a year after osteoporotic fracture is markedly increased; in one study, increased odds of death ranged from 1.45 to 3.17 depending on where the fracture occurred [103]. Lastly, costs for osteoporosis-related fractures are high with persons with fracture having over three times the overall health care costs as persons without osteoporosis [102]. Overall costs have been estimated at close to $17 billion per year.
An increased risk for loss of bone density, and a subsequent fracture, is a significant concern for older men on ADT. Bone mineral density is most often screened with dual-energy X-ray absorptiometry (DEXA) and the hip, spine, wrist, and femur are the typical locations screened. Screening tests provide a “T-score” which compares the measured density with that of a younger adult. T-scores of −1 to −2.5 demonstrate a risk for the disease or osteopenia and osteoporosis is generally demonstrated with T-scores <−2.5. While loss of bone density does not necessarily lead to osteoporosis or fractures, these are also common additional complications of ADT. In older men, age-related decreases in bioavailable testosterone and estradiol results in bone loss at a rate of approximately 1% per year at baseline [104]. Annual bone loss with ADT is more rapid; multiple studies have revealed bone loss rates from 1 to 4.6% yearly in men on ADT for nonmetastatic disease [101]. Although studies are generally small without adequate controls, they typically show statistically significant, clinically important declines in bone density in the lumbar spine, hip, femoral neck, and distal radius with 6–24 months of castration [34]. On the other hand, in a study which randomized 52 men to bicalutamide or LHRH agonist therapy, bicalutamide was associated with increases in bone mineral density [105]. Although the rate of bone loss is highest in the first year of therapy, the duration of ADT has been linked to greater magnitude of loss [106,107]. In one study of 390 men followed for over 10 years, osteoporosis was prevalent in hormone naive men with prostate cancer (35.4%) and increased to 80% in men on ADT for 10 years [106]. Other factors that are associated with bone loss in men on ADT include body mass index, alcohol use, and use of preventative modalities such as calcium/vitamin D and exercise. Bone loss can also be linked to other conditions such as hyperthyroidism, liver disease, and vitamin D malabsorption.
Despite increases in prevalence of bone loss and osteoporosis on ADT, there have been no prospective studies evaluating fractures due to ADT, mainly due to the large sample sizes required to study fracture-related endpoints [34]. However, retrospective studies utilizing large population-based databases have demonstrated that men on ADT are 13–30% more likely to develop a fracture as compared to prostate cancer patients not on ADT [108–112]. Age over 65 and comorbidity have been shown to be independent risk factors for fractures in this patient population [110].
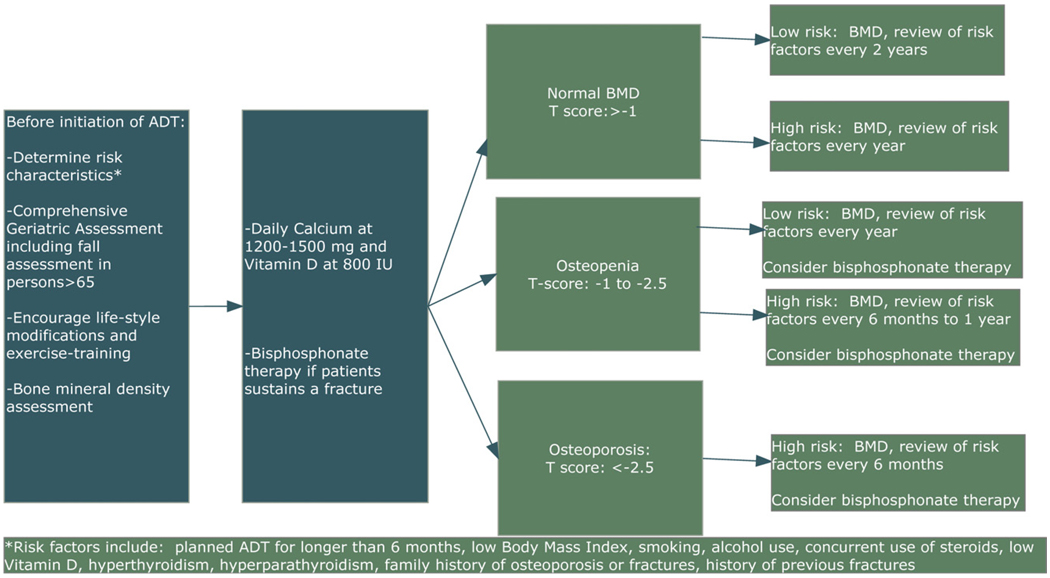
Given the above, monitoring for bone loss and risk of fractures is imperative in older men on ADT. Patients should undergo complete physical examination and medical history in order to assess for other risk factors for osteoporosis, falls, and fractures. In the elderly, conditions such as falls and reduced muscle strength or sarcopenia contribute to fractures and thus we recommend a baseline comprehensive geriatric assessment (CGA) to assess for these conditions prior to initiation of ADT (see Section 5), which can help with decision-making for treatment initiation and provide targets for multidisciplinary intervention in vulnerable and frail older men [21,22]. Because osteoporosis is prevalent in men prior to starting ADT, baseline bone mineral density assessment should be obtained with DEXA scan. Follow-up tests should be performed frequently (every 6–24 months) depending on baseline assessment of BMD and risk factors for development of osteoporosis and fractures (see Fig. 1) [101].
Fig. 1.
Prevention and treatment of osteoporosis in the older man on ADT.
The National Osteoporosis Foundation (NOF) recommends treatment for people with a T-score of −2 with no risk factors, and for T-scores below −1.5 with risk factors [113]. Risk factors for osteoporosis or fractures for prostate cancer patients include planned long-term ADT use, low body mass index, cigarette smoking, alcohol use, family history of osteoporosis or fractures, and previous history of fractures. All patients who are to undergo ADT should be counseled on smoking cessation, alcohol in moderation, and the benefits of exercise. Exercise, specifically weight-bearing and muscle-strengthening exercises, have been shown to increase BMD and reduce fracture risk in osteoporotic persons, although most of the studies were conducted in postmenopausal women [113,114]. Resistance exercise training for men on ADT can improve muscular strength [115]. Although no study has demonstrated reduced fracture risk in men on ADT as a result of exercise, experts in the field currently recommend that men on ADT be encouraged to actively participate in a regular exercise program that includes resistance training and weight-bearing exercise [40,101,113]. In older patients, it is imperative to assess fall risk, need for assistive devices, and conduct a home safety evaluation. Our research team found that close to 20% of men over the age of 70 report falls in the last 3 months, which exceeds the baseline risk for age-equivalent men [116]. A fall assessment includes a multidisciplinary objective evaluation of physical performance and a comprehensive fall history [117]. Prostate cancer specialists should enlist the expertise of health care professionals with specialized expertise in the care of older persons in order to provide the best care to the patient. When customizing an exercise program to prevent fracture risk in older persons, balance training as well as weight training should be recommended. Balance training and Tai Chi Chuan have been shown to reduce fall and fracture risk and improve BMD in frail older persons [118,119].
There are effective pharmaceutical interventions to help prevent and treat osteoporosis in men on ADT. Daily calcium (1500 mg in split doses) and vitamin D (800 IU) can both increase BMD and reduce the risk of fractures in older men with osteoporosis, and should be included in any osteoporosis prevention regimen [120]. These supplements may be useful in treating osteopenia and osteoporosis for men on ADT and they have minimal side effects. Medical options for treatment of osteoporosis include bisphosphonates, estrogen, and selective estrogen receptor modulators (SERMs). Bisphosphonates inhibit osteoclast activity thereby reducing bone resorption and are available in oral and intravenous formulations. Several randomized studies have shown that intravenous bisphosphonates (pamidronate or zolendronic acid) given every 3–4 months significantly prevented bone loss after 1 year in men with nonmetastatic prostate cancer on ADT [121,122]. Zoledronic acid (4 mg IV every 3 months) also has been shown to significantly improve bone mineral density at 1 year [123]. One study of 22 men on ADT who received zolendronic acid at 4 mg yearly also revealed statistically significant and clinically meaningful benefits in terms of prevention of bone loss [124]. Alendronate may be a possibility as an oral option, although few studies have been conducted with this agent in men on ADT [125,126]. It is important to note that studies thus far have not demonstrated prevention or improvement in risk or rate of fractures, although it has been shown that increasing BMD with bisphosphonate therapy reduces osteoporotic fractures in postmenopausal women [127]. Prior to initiation of bisphosphonate therapy, risk for side effects should be carefully considered. Bisphosphonate-induced nephrotoxicity can be a significant concern for cancer patients and health care professionals. Clinically significant deterioration in renal function is a risk with some bisphosphonates, requiring renal function monitoring and drug discontinuation [128]. In addition, renal impairment can progress to renal failure, prompting the need for renal dialysis and even death. Approximately 7% of the people with pre-existing chronic kidney disease would be at a greater risk for renal deterioration during long-term bisphosphonate therapy [129]. Other side effects can include a flu-like syndrome usually with the first infusion and a rare but significant potential complication of osteonecrosis of the jaw. Given this last concern, men starting ADT should have all dental work completed prior to initiation of bisphosphonates.
Besides bisphosphonate therapies, other options include estrogen therapy and SERMs. Estrogen therapy is associated with decreases in markers of bone resorption and prevention of bone loss in men on ADT; however, the studies are small and estrogen carries with it the risk of cardiotoxicity and thromboembolism. On the other hand, SERMs, such as raloxifene and toremifene, have beneficial effects on the bone, without significant cardiac toxicity. Smith et al. showed that raloxifene increases BMD in men on ADT [130]. One challenge, however, is the frequency of significant hot flashes associated with SERMs. Like with the bisphosphonates, more studies are needed to examine the impact of these drugs on fractures in men on ADT.
In summary, osteoporosis and fractures are important potential complications of ADT and may negatively impact QOL. Osteoporosis is a common scenario at baseline in frail older persons and is considered a geriatric syndrome. Geriatric syndromes in the elderly are associated with an increased risk of mortality [131]. As a result, the decision to start ADT in an older person should include an evaluation of bone mineral density and fall risk. A recent study of physician care practices revealed that only 9% of men on ADT had undergone a DEXA scan and only 15% received at least one intervention for prevention and treatment of ADT [132]. In another study of 174 men on ADT, only 34% received recommended osteoporosis management [133]. Intervention efforts to educate cancer specialists should also be undertaken in order to improve outcomes of this population.
4.3. Sarcopenia and physical function
4.3.1. Sarcopenia
Men with prostate cancer commonly gain weight on ADT. Median weight gain is close to 6 kg after 1 year on ADT, and some men gain as much as 10 kg [134] primarily related to an increase in fat mass [135]. In addition, while total body fat increases, lean muscle mass and muscular strength both decrease [115]. Loss of lean body mass (or sarcopenia), which is related directly to muscle strength and physical performance, is the most relevant component in the development of frailty [136]. Therefore, one could hypothesize that ADT could accelerate the onset of frailty in older men [21].
In the elderly, sarcopenia is a major contributing factor to falls, functional dependence, and frailty [137]. The prevalence of sarcopenia in community-dwelling older adults is estimated to be approximately 25% [138]. Age-related reductions in skeletal muscle mass are greater in men than in women, especially in the legs [139]. There is approximately a 5% loss of muscle mass per decade of life after the 4th decade, with more rapid loss after the age of 65 [139]. ADT likely hastens the development of sarcopenia. Several studies have reported statistically significant decreases in lean body mass within 3–6 months of LHRH agonist initiation [135,140,141].
In aging, loss of muscle mass can lead to physical weakness and disability. There are significant age-related decreases in strength, with a loss of approximately 20–40% when comparisons are made between younger adults in their twenties compared to older adults in their seventies and eighties, and even greater losses over 50% reported when comparisons were made with older adults in their nineties [142–144]. Men with prostate cancer on ADT also have muscle weakness. In a cross-sectional study, men on ADT had reduced upper limb strength and lower scores on a questionnaire-based physical function test as compared with age-matched controls [145]. Loss of lean body mass due to ADT could be a causal pathway to weakness leading to mobility disorders, functional dependence, and falls in older men, although these relationships are not yet established [21].
4.3.2. Physical function
Self-reported impairments in physical function are commonly reported in studies of QOL effects of ADT (Table 1). These QOL studies reveal that men on ADT report a decreased ability to accomplish physical tasks such as climbing stairs or walking distances. There have been few investigations of how ADT affects the physical abilities of older men and whether these physical deficits impair functional status (i.e., the ability of the person to care for himself). Joly et al. compared the physical function of 57 patients with nonmetastatic prostate cancer with 51 controls [20]. Physical and daily function were measured by the 6-min walk test, grip strength, the timed up and go test, and activities of daily living measures. In this study, performance on physical tests was similar in the two groups. However, patients tended to be the “young-old” elderly (i.e., <70 years old) and were excluded from the study if they had significant comorbidities. Thus, the patient population thus may not reflect the “true” prostate cancer patient population. In another study, physical function, walking speed, body composition, and Comorbidity Disease Index (CMDI) scores were assessed in a cohort of 100 participants that included men with prostate cancer who were not on ADT, men with prostate cancer who were on short-term ADT (<6 months), men with prostate cancer who were on long-term ADT (≥6 months), and control subjects who did not have prostate cancer [146]. Walking speed varied significantly across the four groups, even after adjusting for age, CMDI, and percentage of body fat. Age and CMDI were significantly associated with measurements of physical performance. Adjusted for covariates, men on long-term ADT walked 0.18 m/s slower than the control subjects. Physical function also varied significantly across the four groups. Our research team also evaluated physical functioning in older men (age ≥70) receiving ADT for asymptomatic prostate cancer and found that 56% of patients exhibited impaired scores on the Short Physical Performance Battery (see Table 3) [147]. Impairments were noted in all measures of the SPPB: balance, walking speed, and chair stands (a measure of quadriceps strength). In addition, 22% reported falls over the prior 6 months. This is double the 11% of patients who report falls in a general outpatient geriatrics population [148]. Since men on ADT have a high incidence of fractures, men should be screened for falls or fall risk and a multidisciplinary intervention to improve mobility outcomes should be considered. This intervention could include physical therapy, balance training, home safety evaluation, assist device evaluation, and fall education. These types of interventions have been shown to be beneficial in other vulnerable populations [117,149,150].
Table 3.
Summary of deficits within the physical performance measures in 50 older men on ADT.
| Test | Geriatric domain | Score range | Cut-off point associated with adverse outcomesa |
Median (range) | Impaired (%) |
|---|---|---|---|---|---|
| Short Physical Performance Battery (SPPB) | Objective evaluation of physical performance | 0–12 | ≤9 | 9 (0–12) | 56.0 |
| Number of falls in the last 6 months | Objective evaluation of physical performance | 0–∞ | >0 | 0 (0–4) | 22.0 |
| Physical disability assessment within the VES-13 | Self-perceived physical health | 0–2 | >0 | 0 (0–2) | 52.0 |
4.3.3. Management of musculoskeletal side effects
An exercise program tailored to the individual patient may help to combat muscle wasting and weakness that occurs with ADT. Few studies have evaluated the beneficial effects of exercise training on body composition of men on ADT. Galvao et al. examined the effect of progressive resistance training on muscle function, functional performance, balance, body composition, and muscle thickness in men receiving ADT for prostate cancer [151]. Ten men aged 59–82 years undertook progressive resistance training for 20 weeks at 6–12-repetition maximum for 12 upper- and lower-body exercises in a university exercise rehabilitation clinic. Outcome measures included muscle strength and muscle endurance for the upper and lower body, functional performance (repeated chair rise, usual and fast 6-m walk, 6-m backwards walk, stair climb, and 400-m walk time), and balance by sensory organization test. Body composition was measured by dual-energy X-ray absorptiometry and muscle thickness at four anatomical sites by B-mode ultrasound. Both muscle strength and muscle endurance increased significantly after training. Significant improvement occurred in the 6-m usual walk, chair rise, stair climbing, and balance. Muscle thickness increased by 15.7% (p < .05) at the quadriceps site. A larger study evaluated the effects of resistance training on muscle mass, fatigue, mood and QOL. In this study, 155 men were randomized to an intervention group that participated in a resistance exercise program three times per week for 12 weeks (82 men) or to a waiting list control group (73 men) [115]. The primary outcomes were fatigue and disease-specific QOL as assessed by self-reported questionnaires after 12 weeks. Secondary outcomes were muscular fitness and body composition. Men assigned to resistance exercise had less interference from fatigue on activities of daily living (p = .002) and higher QOL scores (p = .001) than men in the control group. Men in the intervention group also demonstrated higher levels of upper body (p = .009) and lower body (p < .001) muscular fitness than men in the control group. There was also good compliance in the exercise arm of the study.
Experts have recommended that all patients initiating ADT be counseled about a resistance and aerobic exercise program [40,42]. Despite lack of evidence of long-term benefits, it is likely that exercise can help alleviate many negative aspects of ADT including loss of lean muscle mass, muscular weakness, gain of fat mass, fatigue, functional and physical performance, and potentially reduce potential cardiovascular toxicity. Patients should be offered a referral to a licensed physical therapist or personal trainer to help develop a comprehensive program for exercise and interventions to reduce fall risk.
5. Overview of ADT effects on quality of life in older prostate cancer patients
Numerous studies have illustrated the negative effects of ADT on QOL. Cross-sectional studies have demonstrated worse global QOL scores, decreased energy, and lower social functioning and emotional well-being in men who receive ADT for any stage of prostate cancer as compared to those who receive other treatments including observation [78,152–154]. Potosky and his colleagues compared the QOL of 245 patients with localized prostate cancer who received ADT with the QOL of 416 men who did not receive ADT within the first year of diagnosis [78]. Patients who received ADT had a statistically greater incidence of sexual dysfunction, physical discomfort, and diminished vitality. In another study utilizing the Prostate Cancer Outcomes Survey, the QOL of 431 men who received primary ADT with either orchiectomy or LHRH agonist therapy was described [152]. In multivariate analyses, overall health outcomes were similar between patients receiving orchiectomy or LHRH agonist therapy, but LHRH patients reported more physical discomfort and were more likely to assess their overall health as fair or poor. Dacal et al. compared the QOL and comorbidity levels between prostate cancer patients with asymptomatic disease on ADT <6 months (n = 24), ADT ≥6 months (n = 29), on no therapy (n = 23), and healthy controls (n = 20) [155]. The authors found that men with prostate cancer who were receiving short- and long-term ADT had significantly lower scores on physical functioning and general health domains. Other domains including bodily pain, social functioning, and emotional and mental health components were not different between ADT and other groups. In addition, scores between men on short- and long-term ADT were not statistically different. In this study, only low testosterone and comorbidity contributed to QOL outcomes in regression analysis. Joly et al. also examined QOL in a cohort of men on ADT as compared to controls and found that men on ADT had significantly worse fatigue and symptoms from prostate cancer [20]. Conclusions from these cross-sectional studies are generally limited due to examination at few time points and omission of baseline information.
Longitudinal studies of men on continuous ADT demonstrate a negative impact of therapy on QOL, especially on energy, sense of well-being, and physical functioning [85,156–162]. These studies are summarized in Table 4. Patients who receive ADT for metastatic disease experience improvement in QOL, likely due to palliation of cancer-related side effects. Men who receive ADT for locally advanced disease or biochemical recurrence generally have worse global QOL scores over time, especially in regard to fatigue, physical function, and sexual function. This trend over time is more evident in longitudinal studies that included baseline assessments (i.e., before ADT initiation). Because older men without prostate cancer can demonstrate poor QOL on validated tools utilized for prostate cancer QOL assessments, it is important to include baseline information, and if this is not available, to compare outcomes with a carefully selected control group [163]. Existing longitudinal studies have important limitations in that they include small sample sizes over a short duration, and do not, for the most part, account for age and comorbidity [34]. One larger study evaluated the patient-reported outcomes of 1201 patients and 625 spouses at multiple centers before and after radical prostatectomy, brachytherapy, or external-beam radiotherapy [164]. In this study, neoadjuvant hormonal therapy was significantly associated with quality of life declines with sexual health (with radiotherapy), urinary incontinence (with brachytherapy), urinary irritation (with radiotherapy and brachytherapy), and vitality (with radiotherapy and brachytherapy). Neoadjuvant hormonal therapy was given with radiotherapy or brachytherapywas associated with long-lasting symptoms involving sexuality and vitality. Despite this recent addition to the literature, no studies have yet examined the age-related differences in QOL and interactions with geriatric domains with an emphasis on persons in the “oldest-old” subgroups.
Table 4.
Longitudinal studies that evaluate QOL in subjects receiving ADT.
| References | Patient population | QOL domains | Assessment time points | Main results |
|---|---|---|---|---|
| Lubecket al. [157] | Men during first year of therapy from CaPSURE database | SF-36, UCLA Prostate Cancer Index | No pre-treatment assessment; men were studied at study entry and quarterly thereafter | ADT group had poorer urinary and sexual function and a higher rate of urinary and sexual bother than surveillance group |
| 106 men who opted for surveillance and 167 men receiving ADT | Scores remained low during first year of treatment | |||
| Lubeck et al. [156] | Men during first year of therapy from CaPSURE database | SF-36, UCLA Prostate Cancer Index | No pre-treatment assessment; men were studied at study entry and quarterly thereafter | Low QOL scores immediately after treatment |
| 179 men who received primary ADT compared to men who received radical prostatectomy, radiotherapy, or observation | Scores improved in hormone therapy arm over a 1 year period | |||
| Herr and O’Sullivan [158] | 79 men who were started on ADT for locally advanced disease or biochemical relapse after local therapy as compared to 29 men who received no therapy and 36 who received local therapy alone | EORTC Prostate Cancer QOL Scale | Assessments at baseline, at 6 months, and 1 year | ADT patients had worsening fatigue, physical function, and sexual function along with a trend towards psychological distress at 1 year |
| Green et al. [85] | 62 men with non-localized prostate cancer were randomized to three different ADT regiments or observation | General QOL measures of depression, satisfaction, role functioning, sexual function; also, a comprehensive neuropsychological assessment | Assessments at baseline, 6 months, and 1 year | ADT patients had worse scores in sexual function than control group at 1 year |
| 15 controls | ||||
| Van Andel and Kurth [159] | Men who received ADT for asymptomatic, lymph node positive prostate cancer | EORTC Prostate Cancer QOL Scale | Assessment performed at study entry (6 months after diagnosis) and 12 months later | ADT patients had worse emotional function and overall QOL scores at baseline |
| 76 (31 on ADT and 45 on no ADT) completed baseline assessment and 61 completed follow-up (27 on ADT and 34 on no ADT) | ADT patients had worse sexual function and more hot flashes at baseline and 1 year | |||
| At 1 year, physical function and fatigue were worse in ADT patients but emotional well-being and overall QOL scores were similar to no ADT patients | ||||
| Arredondo et al. [160] | Men who received second treatment after radical prostatectomy, either radiation or hormonal therapy (n = 897) as compared to RT alone (n = 3231) | SF-36, UCLA Prostate Cancer Index | Assessment performed before and after RP and then biannually | Before and during second treatment, role-functioning and sexual functioning scales revealed clinically relevant decreases |
| Study does not distinguish between types of second treatment (i.e., ADT vs radiation) but no overt differences noted in QOL outcomes as related to treatment type | ||||
| Sanda et al. [164] | 1201 men who received localized therapy for prostate cancer | Expanded Prostate Cancer Index-26 | Assessment before treatment and at 2, 6, 12 and 24 months after treatment | Declines noted in sexual score and vitality score lasting over 2 years when neoadjuvant therapy was combined with radiotherapy |
| Distinction made between QOL scores of men who received neoadjuvant hormonal therapy in combination with radiotherapy or brachytherapy | ||||
| Litwin et al. [161] | 63 men with metastatic disease who received treatment with ADT | SF-36, UCLA Prostate Cancer Index | Assessment at baseline, 6, and 12 months after starting therapy | Significant improvements in 10 of the 14 domains of health-related quality of life |
| Moinpour et al. [162] | 739 with metastatic prostate cancer who underwent surgical castration and were randomized to flutamide or placebo | QOL assessments of diarrhea, gas pain, body image, physical functioning, and emotional functioning | Assessment at baseline, 1 month, 3, and 6 months | Improvements in QOL domains over time noted in both groups but more pronounced in placebo versus flutamide |
6. Approach to the assessment and management of ADT complications in vulnerable and frail older men
As discussed in the previous sections, ADT can be associated with serious side effects and complications in older men. Prior to initiation, an older man’s health status should be carefully assessed. This assessment should include examination of domains that could exacerbate pre-existing conditions and accelerate mortality. Recognition of these conditions would aid the physician in making the best treatment decision for an individual patient. For example, a comprehensive medical and geriatric assessment could provide information that can help estimate active remaining life expectancy (RLE). In an older person with prostate cancer, an estimation of RLE is imperative as many men die from conditions other than prostate cancer and may experience significant QOL effects from treatment [6]. Side effects from ADT may ultimately increase an older man’s overall mortality risk from resulting osteoporosis and falls leading to fractures and higher risk of significant cardiovascular disease. Table 5 illustrates life expectancy data for men at varying health statuses.
Table 5.
Upper, middle and lower quartiles of life expectancy: U.S. men.
| Quartilea | Age |
|||||
|---|---|---|---|---|---|---|
| 70 | 75 | 80 | 85 | 90 | 95 | |
| Top 25% | 18.0 | 14.2 | 10.8 | 7.9 | 5.8 | 4.3 |
| 50th percentile | 12.4 | 9.3 | 6.7 | 4.7 | 3.2 | 2.3 |
| Lowest 25% | 6.7 | 4.9 | 3.3 | 2.2 | 1.5 | 1.0 |
NCHS. Life tables of the United States, 1997. Adapted fromWalter LC and Covinsky KE. JAMA 2001;285(21):2750–6.
Top 25% represents very healthy men, 50th percentile represents those in average health, and the lowest 25% represents those in fair or poor health.
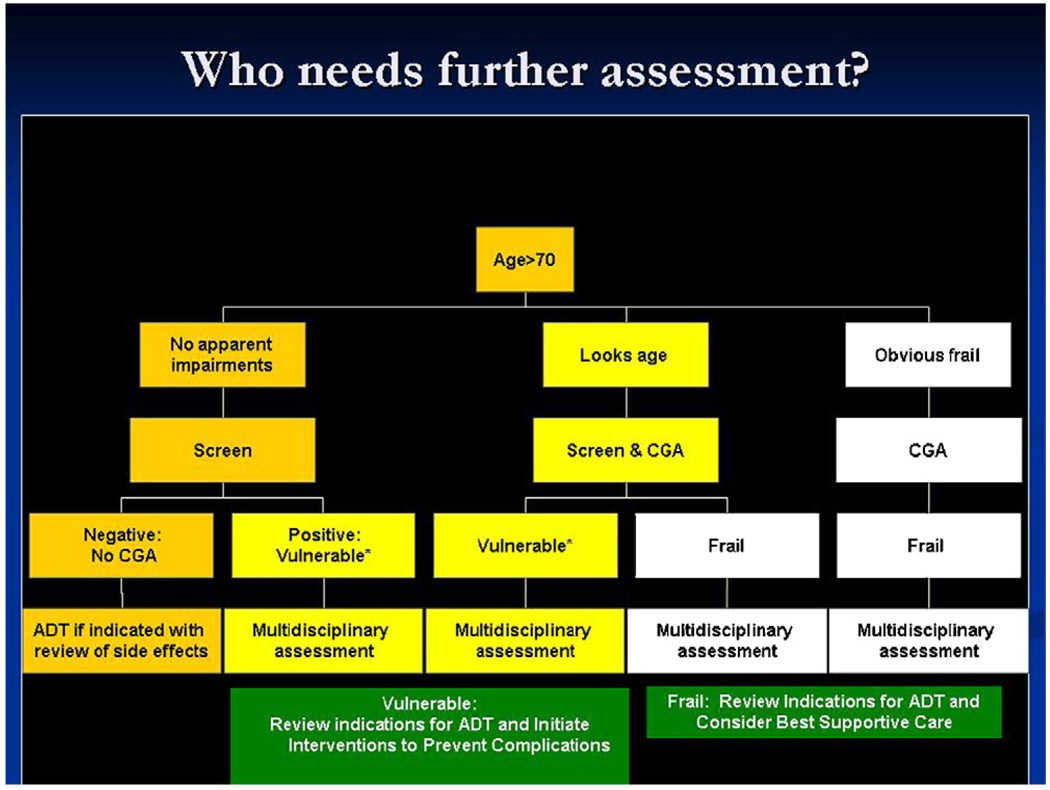
The spectrum of functional ability of an older person can range from those who are fully independent to those who are at moderate risk (i.e., vulnerable) of health deterioration to those at a high risk of functional decline (i.e., frail). Several clinical characteristics have distinct and possibly synergistic influences on underlying vulnerability and frailty in the elderly: disability, comorbidity, and geriatric syndromes. Functional and physical disability, comorbidities, and geriatric syndromes are linked with increased risk of hospitalization, higher health care costs, need for long-term care, and overall mortality [165–171]. Evaluation of comorbidity, disability and other geriatric impairments are essential to help identify vulnerable and frail older men with prostate cancer who are at risk for adverse outcomes from ADT. Several studies have noted a high prevalence of comorbidity, disability, and geriatric syndromes in elderly cancer patients [172,173]. A study of persons recently diagnosed with prostate cancer utilizing home health services in Ohio noted that only 12% had no comorbidity, disability, or geriatric syndromes. 20.7% had comorbidity, 2.5% had disability, and 5.9% had geriatric syndromes and 24.7% had all three. A multidimensional comprehensive geriatric assessment (CGA) should be a key part of the treatment approach for older cancer patients (especially aged 70 and over) and can help detect these underlying factors [174–176]. The CGA generally includes a compilation of validated tools to assess comorbidity, functional status, physical performance, cognitive status, psychological status, nutritional status, medication review, and social support [94,177–185]. Table 6 is an example of a CGA that has been utilized in the care of older persons with prostate cancer [22]. The Vulnerable Elder’s Survey-13 (VES-13) is a brief, functionally based screening tool that is predictive of functional decline or death over 2 years in a large cohort of Medicare beneficiaries [186]. This screening test is comparable to a full geriatric assessment in screening for those who may be at high risk of decline or death [22]. A multidisciplinary approach to assessment and care of older patients on ADT would help identify targets for interventions (see Fig. 2).
Table 6.
Components of the comprehensive geriatric assessment (CGA).
| Test | Geriatric domain | Number of questions | Administration | Score, range |
Cut-off point associated with adverse outcomes |
|---|---|---|---|---|---|
| Vulnerable elders survey-13 (VES-13) | Functionally based screening measure | 13 | Self-administered (5 min) | 0–10 | ≥3 |
| Activities of daily living (ADLs) | Function | 8 | Self-administered (5–10 min) | 0–16 | ≤14 |
| Instrumental activities of daily living (IADLs) | Function | 7 | Self-administered (5–10 min) | 0–14 | ≤12 |
| Short Physical Performance Battery (SPPB) | Objective evaluation of function/physical performance | Three separate physical performance tests | Administered by member of research team (10–15 min) | 0–12 | <9 |
| CALGBa adaptation Charlson comorbidity score | Comorbidity | 18 | Self-administered (15 min) | 0–54 | >10 |
| Number of medications | Comorbidity/toxicity potential from drug interactions | 1 | Self-administered (1–5 min) | 0–∞ | ≥5 |
| RAND medical social support scale | Social Support/access to medical care and support | 5 | Self-administered (1–5 min) | 0–5 | <4 |
| Short Portable Mental Status Questionnaire | Cognition/risk for dementia | 10 | Administered by member of research team (10–15 min) | 0–10 | >3 |
Cancer and Leukemia Group B.
Fig. 2.
Schema of CGA use to manage side effects of ADT.
Those persons with no apparent impairments are “fit” and should have no functional dependence, no comorbidities, and no geriatric syndromes. Vulnerable persons may have some dependence in instrumental activities of daily living, non-life threatening comorbidities, and no significant geriatric syndromes other than mild depression or a mild memory disorder. Frail persons can be characterized as those persons aged 85 years and over, those older persons with a dependence in an activity of daily living, three or more significant comorbidities or one comorbidity that is associated with limitation of daily life responsibilities. These definitions are adapted from Balducci and Extermann [188] and Basso et al. [189].
7. Conclusion
Prostate cancer is a major health issue for many older men. ADT has numerous side effects and toxicities, and many can be serious in the elderly. Despite these potentially serious consequences, ADT use is increasing in the elderly. The elderly have a higher likelihood of having conditions such as comorbidity, geriatric syndromes, and disability that are linked with vulnerability and frailty. These conditions must be identified prior to initiation of ADT as they may worsen as a result of ADT. The domains impacted by ADT include neuropsychological, sexual, body composition and physical performance, bone health, and comorbidities. Recognizing the complications that could affect the quality of life, function, and mortality of older persons with prostate cancer can help with treatment decision-making. It is imperative to assess the older person’s underlying health status before initiating therapy to better estimate whether the risks of ADT initiation and continued use outweigh the benefits for cancer-specific mortality. Physicians who treat older patients with prostate cancer should be aware of side effects and methods for prevention and treatment. Table 7 summarizes these approaches. Treating physicians should also inform patients and their primary care physicians about the many complications of ADT. A comprehensive multidisciplinary assessment of the older man on ADT can help target interventions to mitigate side effects and toxicities from treatment.
Table 7.
Summary of recommendations to prevent side effects in older men on ADT.
| Side effect | Screening | Prevention | Treatment |
|---|---|---|---|
| Hot flashes | Moyad screening tool | a | See Table 2 for various complementary or pharmacologic approaches for treatment |
| Anemia | Hemoglobin and hematocrit assessment prior to starting ADT and every 3–6 months | Assess and treat other known causes of anemia such as folate, vitamin B-12 or iron deficiency | Consider transfusion if severe anemia or symptomatic |
| Sexual side effects | Ask patient and partner about baseline sexual functioning | a | Sexual rehabilitation and counseling |
| Oral phosphodiesterase inhibitors Mechanical therapies | |||
| Cognitive side effects | Ask patient about baseline memory issues | a | Refer to specialist if concern for cognitive decline |
| Screen with validated cognitive scale such as Short Portable Mental Status Questionnaire or Mini-Mental Exam If memory problems or abnormality on screening tool, refer to specialist and for baseline neuropsychological testing Repeat screening and/or neuropsychological tests at yearly if memory symptoms worsen or if patient has baseline deficits prior to starting ADT |
|||
| Psychological side effects | Ask patient about symptoms of depression prior to starting ADT and at each visit | a | Antidepressant |
| Can use validated geriatric depression scales | Counseling | ||
| Metabolic syndrome and cardiovascular health | Assess history of coronary artery disease and diabetes | Inform other physicians including primary care about potential risk of worsening coronary artery disease and/or diabetes with ADT initiation | Close monitoring for progression of disease |
| Assess risk factors for coronary artery disease and diabetes | Consider referral to cardiac specialist for those with underlying coronary artery disease | Treatment of underlying and worsening coronary artery disease and diabetes per guidelines and in concert with primary care and specialist | |
| May need to confer with primary care physician | |||
| Osteoporosis and fractures | Assess risk factors for osteoporosis | Calcium 1500 mg daily | Bisphosphonates |
| Baseline and yearly bone mineral density scans | Vitamin D 600–800 IU daily | Resistance training | |
| Physical function and falls | Ask about fall history at baseline and every visit | Resistance training | If history of falls, evaluate for assist device |
| Assess performance with validated tool such as timed up and Go or Short Physical Performance Battery | Home safety evaluation | Refer to specialty falls clinic | |
| Refer to physical and occupational therapy |
No evidence-based data.
Biographies
Supriya Gupta Mohile, M.D., M.S. is a geriatric oncologist whose research focuses on the quality of life and health outcomes of vulnerable and frail older adults with cancer. She is an Assistant Professor at the University of Rochester’s James Wilmost Cancer Center and is the recipient of a Hartford Geriatrics Health Outcomes Research Award. Her clinical expertise focuses on the care of older patients with genitourinary and gastrointestinal malignancies.
Karen Mustian, Ph.D. is an exercise psychologist within the University of Rochester’s Cancer Control and Prevention Program. She is a NIH-funded academic researcher who focuses on the benefits of exercise on cancer-related fatigue and quality of life of cancer patients.
Kathyrn Bylow, M.D. is an Assistant Professor at the Medical College of Wisconsin. She is a geriatric oncologist with clinical and research expertise in improving the quality of life of older cancer patients.
William Hall, M.D. is director of the Center for Healthy Aging at the University of Rochester and previously served as chief of general medicine/geriatrics at the University of Rochester School of Medicine and chief of medicine at Rochester General Hospital. As an academic geriatrician, Dr. Hall’s career has been largely focused on aging.
William Dale, M.D., Ph.D.’s research focuses on medical decision-making, preventive care, and quality of life in the elderly. More specifically, his research is in the area of decision-making in prostate cancer, with a focus on anxiety related to screening, diagnosis, and treatment. Much of his research has focused on prostate cancer as a clinical model. He was awarded a Paul Beeson Career Development Award for his work.
Footnotes
Supported in part by: Hartford Geriatrics Health Outcomes Research Award (SGM) and Paul Beeson Career Development Award (WD).
Reviewers
Droz J.-P., Professor, Centre Léon Bérard, Department of Medical Oncology, 28 rue Laennec, F-29008 Lyon, France.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11(6):437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 Suppl. 1):3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu-Yao G, Stukel TA, Yao SL. Changing patterns in competing causes of death in men with prostate cancer: a population based study. J Urol. 2004;171(6 Pt 1):2285–2290. doi: 10.1097/01.ju.0000127740.96006.1a. [DOI] [PubMed] [Google Scholar]
- 6.Newschaffer CJ, Otani K, McDonald MK, Penberthy LT. Causes of death in elderly prostate cancer patients and in a comparison non-prostate cancer cohort. J Natl Cancer Inst. 2000;92(8):613–621. doi: 10.1093/jnci/92.8.613. [DOI] [PubMed] [Google Scholar]
- 7.Gatling RR. Prostate carcinoma: an autopsy evaluation of the influence of age, tumor grade, and therapy on tumor biology. South Med J. 1990;83(7):782–784. doi: 10.1097/00007611-199007000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Konety BR, Bird VY, Deorah S, Dahmoush L. Comparison of the incidence of latent prostate cancer detected at autopsy before and after the prostate specific antigen era. J Urol. 2005;174(5):1785–1788. doi: 10.1097/01.ju.0000177470.84735.55. discussion 8. [DOI] [PubMed] [Google Scholar]
- 9.Brawer MK, Crawford ED, Fowler J, Lucia MS, Schroeder FH. Prostate cancer: epidemiology and screening. Rev Urol. 2000;2 Suppl 4:S5–S9. [PMC free article] [PubMed] [Google Scholar]
- 10.Balducci L, Aapro M. Epidemiology of cancer and aging. Cancer Treat Res. 2005;124:1–15. doi: 10.1007/0-387-23962-6_1. [DOI] [PubMed] [Google Scholar]
- 11.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25(12):1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 12.Messing E. The timing of hormone therapy for men with asymptomatic advanced prostate cancer. Urol Oncol. 2003;21(4):245–254. doi: 10.1016/s1078-1439(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 13.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103(8):1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 14.Demers RY, Tiwari A, Wei J, Weiss LK, Severson RK, Montie J. Trends in the utilization of androgen-deprivation therapy for patients with prostate carcinoma suggest an effect on mortality. Cancer. 2001;92(9):2309–2317. doi: 10.1002/1097-0142(20011101)92:9<2309::aid-cncr1577>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Mohile SG, Petrylak DP. Management of asymptomatic rise in prostatic-specific antigen in patients with prostate cancer. Curr Oncol Rep. 2006;8(3):213–220. doi: 10.1007/s11912-006-0022-8. [DOI] [PubMed] [Google Scholar]
- 16.Harlan SR, Cooperberg MR, Elkin EP, et al. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. J Urol. 2003;170(5):1804–1807. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 17.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95(13):981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 19.Higano C. Androgen deprivation therapy: monitoring and managing the complications. Hematol Oncol Clin North Am. 2006;20(4):909–923. doi: 10.1016/j.hoc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Joly F, Alibhai SM, Galica J, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176(6 Pt 1):2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 21.Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: A conceptual review. Cancer. 2007;110(12):2604–2613. doi: 10.1002/cncr.23084. [DOI] [PubMed] [Google Scholar]
- 22.Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109(4):802–810. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 23.Loblaw DA, Mendelson DS, Talcott JA, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004;22(14):2927–2941. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- 24.Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132(7):566–577. doi: 10.7326/0003-4819-132-7-200004040-00009. [DOI] [PubMed] [Google Scholar]
- 25.Bonzani RA, Stricker HJ, Peabody JO, Menon M. Cost comparison of orchiectomy and leuprolide in metastatic prostate cancer. J Urol. 1998;160(6 Pt 2):2446–2449. doi: 10.1097/00005392-199812020-00015. [DOI] [PubMed] [Google Scholar]
- 26.Seidenfeld J, Samson DJ, Aronson N, et al. Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid Rep Technol Assess (Summ) 1999;(4) I–x, 1–246, I1–36, passim. [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy V, Norman AR, Shahidi M, et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU Int. 2006;97(3):476–479. doi: 10.1111/j.1464-410X.2006.06013.x. [DOI] [PubMed] [Google Scholar]
- 28.Pickles T, Agranovich A, Berthelet E, et al. Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer. 2002;94(2):362–367. doi: 10.1002/cncr.10219. [DOI] [PubMed] [Google Scholar]
- 29.Bales GT, Chodak GW. A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology. 1996;47(1A Suppl):38–43. doi: 10.1016/s0090-4295(96)80007-0. discussion 8–53. [DOI] [PubMed] [Google Scholar]
- 30.Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95(2):361–376. doi: 10.1002/cncr.10647. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt T. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD001526. CD001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt B, Wilt TJ, Schellhammer PF, et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology. 2001;57(4):727–732. doi: 10.1016/s0090-4295(00)01086-4. [DOI] [PubMed] [Google Scholar]
- 33.Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists’ Collaborative Group. Lancet. 2000;355(9214):1491–1498. [PubMed] [Google Scholar]
- 34.Alibhai SM, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol. 2006;60(3):201–215. doi: 10.1016/j.critrevonc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Spetz AC, Hammar M, Pettersson W, Varenhorst E. Hot flushes and prostate cancer: pathogenesis and treatment. BJU Int. 2002;90(4):476. doi: 10.1046/j.1464-410x.2002.t01-6-02967.x. author reply – 7. [DOI] [PubMed] [Google Scholar]
- 36.Schow DA, Renfer LG, Rozanski TA, Thompson IM. Prevalence of hot flushes during and after neoadjuvant hormonal therapy for localized prostate cancer. South Med J. 1998;91(9):855–857. doi: 10.1097/00007611-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Karling P, Hammar M, Varenhorst E. Prevalence and duration of hot flushes after surgical or medical castration in men with prostatic carcinoma. J Urol. 1994;152(4):1170–1173. doi: 10.1016/s0022-5347(17)32530-2. [DOI] [PubMed] [Google Scholar]
- 38.Holzbeierlein JM. Managing complications of androgen deprivation therapy for prostate cancer. Urol Clin North Am. 2006;33(2):181–190. doi: 10.1016/j.ucl.2005.12.008. vi. [DOI] [PubMed] [Google Scholar]
- 39.Spetz AC, Zetterlund EL, Varenhorst E, Hammar M. Incidence and management of hot flashes in prostate cancer. J Support Oncol. 2003;1(4):263–266. 9–70, 72–73. discussion 7–8, 71–2. [PubMed] [Google Scholar]
- 40.Moyad MA. Promoting general health during androgen deprivation therapy (ADT): a rapid 10-step review for your patients. Urol Oncol. 2005;23(1):56–64. doi: 10.1016/j.urolonc.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Moyad MA. Complementary/alternative therapies for reducing hot flashes in prostate cancer patients: reevaluating the existing indirect data from studies of breast cancer and postmenopausal women. Urology. 2002;59(4 Suppl. 1):20–33. doi: 10.1016/s0090-4295(02)01641-2. [DOI] [PubMed] [Google Scholar]
- 42.Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology. 2003;61(2 Suppl. 1):32–38. doi: 10.1016/s0090-4295(02)02397-x. [DOI] [PubMed] [Google Scholar]
- 43.Frisk J, Spetz AC, Hjertberg H, Petersson B, Hammar M. Two modes of acupuncture as a treatment for hot flushes in men with prostate cancer—a prospective multicenter study with long-term follow-up. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Filshie J, Bolton T, Browne D, Ashley S. Acupuncture and self acupuncture for long-term treatment of vasomotor symptoms in cancer patients—audit and treatment algorithm. Acupunct Med. 2005;23(4):171–180. doi: 10.1136/aim.23.4.171. [DOI] [PubMed] [Google Scholar]
- 45.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137(10):805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 46.Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol. 1998;91(1):6–11. doi: 10.1016/s0029-7844(97)00597-8. [DOI] [PubMed] [Google Scholar]
- 47.Pruthi S, Thompson SL, Novotny PJ, et al. Pilot evaluation of flaxseed for the management of hot flashes. J Soc Integr Oncol. 2007;5(3):106–112. doi: 10.2310/7200.2007.007. [DOI] [PubMed] [Google Scholar]
- 48.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 49.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 50.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331(6):347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 51.Sartor O, Eastham JA. Progressive prostate cancer associated with use of megestrol acetate administered for control of hot flashes. South Med J. 1999;92(4):415–416. doi: 10.1097/00007611-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Tassinari D, Fochessati F, Panzini I, Poggi B, Sartori S, Ravaioli A. Rapid progression of advanced “hormone-resistant” prostate cancer during palliative treatment with progestins for cancer cachexia. J Pain Symptom Manage. 2003;25(5):481–484. doi: 10.1016/s0885-3924(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 53.Langenstroer P, Kramer B, Cutting B, et al. Parenteral medroxyprogesterone for the management of luteinizing hormone releasing hormone induced hot flashes in men with advanced prostate cancer. J Urol. 2005;174(2):642–645. doi: 10.1097/01.ju.0000165570.28635.4b. [DOI] [PubMed] [Google Scholar]
- 54.Miller JI, Ahmann FR. Treatment of castration-induced menopausal symptoms with low dose diethylstilbestrol in men with advanced prostate cancer. Urology. 1992;40(6):499–502. doi: 10.1016/0090-4295(92)90401-h. [DOI] [PubMed] [Google Scholar]
- 55.Gerber GS, Zagaja GP, Ray PS, Rukstalis DB. Transdermal estrogen in the treatment of hot flushes in men with prostate cancer. Urology. 2000;55(1):97–101. doi: 10.1016/s0090-4295(99)00370-2. [DOI] [PubMed] [Google Scholar]
- 56.Kerwin JP, Gordon PR, Senf JH. The variable response of women with menopausal hot flashes when treated with sertraline. Menopause. 2007;14(5):841–845. doi: 10.1097/GME.0b013e31802e7f22. [DOI] [PubMed] [Google Scholar]
- 57.Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109(4):823–830. doi: 10.1097/01.AOG.0000258278.73505.fa. [DOI] [PubMed] [Google Scholar]
- 58.Roth AJ, Scher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology. 1998;7(2):129–132. doi: 10.1002/(SICI)1099-1611(199803/04)7:2<129::AID-PON294>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 59.Loprinzi CL, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol. 1998;16(7):2377–2381. doi: 10.1200/JCO.1998.16.7.2377. [DOI] [PubMed] [Google Scholar]
- 60.Quella SK, Loprinzi CL, Sloan J, et al. Pilot evaluation of venlafaxine for the treatment of hot flashes in men undergoing androgen ablation therapy for prostate cancer. J Urol. 1999;162(1):98–102. doi: 10.1097/00005392-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 61.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356(9247):2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 62.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005;366(9488):818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25(3):308–312. doi: 10.1200/JCO.2006.07.5390. [DOI] [PubMed] [Google Scholar]
- 64.Strum SB, McDermed JE, Scholz MC, Johnson H, Tisman G. Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br J Urol. 1997;79(6):933–941. doi: 10.1046/j.1464-410x.1997.00234.x. [DOI] [PubMed] [Google Scholar]
- 65.Chander S, Choo R, Danjoux C, et al. Effect of androgen suppression on hemoglobin in prostate cancer patients undergoing salvage radiotherapy plus 2-year buserelin acetate for rising PSA after surgery. Int J Radiat Oncol Biol Phys. 2005;62(3):719–724. doi: 10.1016/j.ijrobp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Asbell SO, Leon SA, Tester WJ, Brereton HD, Ago CT, Rotman M. Development of anemia and recovery in prostate cancer patients treated with combined androgen blockade and radiotherapy. Prostate. 1996;29(4):243–248. doi: 10.1002/(SICI)1097-0045(199610)29:4<243::AID-PROS5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 67.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299(8):914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 68.Guan X, Chen L. Role of erythropoietin in cancer-related anaemia: a double-edged sword? J Int Med Res. 2008;36(1):1–8. doi: 10.1177/147323000803600101. [DOI] [PubMed] [Google Scholar]
- 69.Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med. 2006;3(3):382–404. doi: 10.1111/j.1743-6109.2006.00245.x. discussion-7. [DOI] [PubMed] [Google Scholar]
- 70.Morley JE, Haren MT, Kim MJ, Kevorkian R, Perry HM., III Testosterone, aging and quality of life. J Endocrinol Invest. 2005;28(3 Suppl.):76–80. [PubMed] [Google Scholar]
- 71.Marumo K, Baba S, Murai M. Erectile function and nocturnal penile tumescence in patients with prostate cancer undergoing luteinizing hormone-releasing hormone agonist therapy. Int J Urol. 1999;6(1):19–23. doi: 10.1046/j.1442-2042.1999.06128.x. [DOI] [PubMed] [Google Scholar]
- 72.Teloken PE, Ohebshalom M, Mohideen N, Mulhall JP. Analysis of the impact of androgen deprivation therapy on sildenafil citrate response following radiation therapy for prostate cancer. J Urol. 2007;178(6):2521–2525. doi: 10.1016/j.juro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 73.Miles CL, Candy B, Jones L, Williams R, Tookman A, King M. Interventions for sexual dysfunction following treatments for cancer. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD005540.pub2. CD005540. [DOI] [PubMed] [Google Scholar]
- 74.Schover LR, Fouladi RT, Warneke CL, et al. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer. 2002;95(11):2397–2407. doi: 10.1002/cncr.10970. [DOI] [PubMed] [Google Scholar]
- 75.Aucoin MW, Wassersug RJ. The sexuality and social performance of androgen-deprived (castrated) men throughout history: implications for modern day cancer patients. Soc Sci Med. 2006;63(12):3162–3173. doi: 10.1016/j.socscimed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Boehmer U, Babayan RK. Facing erectile dysfunction due to prostate cancer treatment: perspectives of men and their partners. Cancer Invest. 2004;22(6):840–848. doi: 10.1081/cnv-200039641. [DOI] [PubMed] [Google Scholar]
- 77.Canada AL, Neese LE, Sui D, Schover LR. Pilot intervention to enhance sexual rehabilitation for couples after treatment for localized prostate carcinoma. Cancer. 2005;104(12):2689–2700. doi: 10.1002/cncr.21537. [DOI] [PubMed] [Google Scholar]
- 78.Potosky AL, Reeve BB, Clegg LX, et al. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002;94(6):430–437. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
- 79.Dale J, Jatsch W, Hughes N, Pearce A, Meystre C. Information needs and prostate cancer: the development of a systematic means of identification. BJU Int. 2004;94(1):63–69. doi: 10.1111/j.1464-410X.2004.04902.x. [DOI] [PubMed] [Google Scholar]
- 80.Pirl WF, Siegel GI, Goode MJ, Smith MR. Depression in men receiving androgen deprivation therapy for prostate cancer: a pilot study. Psychooncology. 2002;11(6):518–523. doi: 10.1002/pon.592. [DOI] [PubMed] [Google Scholar]
- 81.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166(4):465–471. doi: 10.1001/.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261–270. doi: 10.1207/s15324796abm3103_8. [DOI] [PubMed] [Google Scholar]
- 83.Penedo FJ, Dahn JR, Molton I, et al. Cognitive-behavioral stress management improves stress-management skills and quality of life in men recovering from treatment of prostate carcinoma. Cancer. 2004;100(1):192–200. doi: 10.1002/cncr.11894. [DOI] [PubMed] [Google Scholar]
- 84.Green HJ, Pakenham KI, Headley BC, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002;90(4):427–432. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 85.Green HJ, Pakenham KI, Headley BC, et al. Quality of life compared during pharmacological treatments and clinical monitoring for non-localized prostate cancer: a randomized controlled trial. BJU Int. 2004;93(7):975–979. doi: 10.1111/j.1464-410X.2004.04763.x. [DOI] [PubMed] [Google Scholar]
- 86.Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005;96(1):48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- 87.Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29(8):1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003;170(5):1808–1811. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- 89.Salminen E, Portin R, Korpela J, et al. Androgen deprivation and cognition in prostate cancer. Br J Cancer. 2003;89(6):971–976. doi: 10.1038/sj.bjc.6601235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin Cancer Res. 2004;10(22):7575–7582. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- 91.Taxel P, Stevens MC, Trahiotis M, Zimmerman J, Kaplan RF. The effect of short-term estradiol therapy on cognitive function in older men receiving hormonal suppression therapy for prostate cancer. J Am Geriatr Soc. 2004;52(2):269–273. doi: 10.1111/j.1532-5415.2004.52067.x. [DOI] [PubMed] [Google Scholar]
- 92.Beer TM, Bland LB, Bussiere JR, et al. Testosterone loss and estradiol administration modify memory in men. J Urol. 2006;175(1):130–135. doi: 10.1016/S0022-5347(05)00049-2. [DOI] [PubMed] [Google Scholar]
- 93.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25(14):1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 94.Pfeiffer E. A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 95.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24(24):3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 96.Smith MR, Lee H, McGovern F, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008 doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yannucci J, Manola J, Garnick MB, Bhat G, Bubley GJ. The effect of androgen deprivation therapy on fasting serum lipid and glucose parameters. J Urol. 2006;176(2):520–525. doi: 10.1016/j.juro.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 98.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 99.Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99(20):1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 100.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110(7):1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 101.Higano CS. Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: what do we really know? Nat Clin Pract Urol. 2008;5(1):24–34. doi: 10.1038/ncpuro0995. [DOI] [PubMed] [Google Scholar]
- 102.Orsini LS, Rousculp MD, Long SR, Wang S. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int. 2005;16(4):359–371. doi: 10.1007/s00198-004-1694-2. [DOI] [PubMed] [Google Scholar]
- 103.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 104.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 105.Smith MR, Goode M, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004;22(13):2546–2553. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 106.Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69(3):500–504. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 107.Ryan CW, Huo D, Stallings JW, Davis RL, Beer TM, McWhorter LT. Lifestyle factors and duration of androgen deprivation affect bone mineral density of patients with prostate cancer during first year of therapy. Urology. 2007;70(1):122–126. doi: 10.1016/j.urology.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 108.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23(31):7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 109.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 110.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175(1):136–139. doi: 10.1016/S0022-5347(05)00033-9. discussion 9. [DOI] [PubMed] [Google Scholar]
- 111.Daniell HW. Osteoporosis after orchiectomy for prostate cancer. J Urol. 1997;157(2):439–444. [PubMed] [Google Scholar]
- 112.Melton LJ, III, Alothman KI, Khosla S, Achenbach SJ, Oberg AL, Zincke H. Fracture risk following bilateral orchiectomy. J Urol. 2003;169(5):1747–1750. doi: 10.1097/01.ju.0000059281.67667.97. [DOI] [PubMed] [Google Scholar]
- 113.Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100(5):892–899. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 114.Gregg EW, Cauley JA, Seeley DG, Ensrud KE, Bauer DC. Physical activity and osteoporotic fracture risk in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1998;129(2):81–88. doi: 10.7326/0003-4819-129-2-199807150-00002. [DOI] [PubMed] [Google Scholar]
- 115.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 116.Bylow K, Dale W, Mustian K, et al. Falls and physical performance deficits in older prostate cancer patients on androgen deprivation therapy. Urology. 2008;72(2):422–427. doi: 10.1016/j.urology.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 118.Swanenburg J, de Bruin ED, Stauffacher M, Mulder T, Uebelhart D. Effects of exercise and nutrition on postural balance and risk of falling in elderly people with decreased bone mineral density: randomized controlled trial pilot study. Clin Rehabil. 2007;21(6):523–534. doi: 10.1177/0269215507075206. [DOI] [PubMed] [Google Scholar]
- 119.Lui PP, Qin L, Chan KM. Tai Chi Chuan exercises in enhancing bone mineral density in active seniors. Clin Sports Med. 2008;27(1):75–86. viii. doi: 10.1016/j.csm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 121.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345(13):948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 122.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169(6):2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 123.Ryan CW, Huo D, Demers LM, Beer TM, Lacerna LV. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006;176(3):972–978. doi: 10.1016/j.juro.2006.04.078. discussion 8. [DOI] [PubMed] [Google Scholar]
- 124.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25(9):1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 126.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146(6):416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 127.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 128.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 129.von Moos R. Bisphosphonate treatment recommendations for oncologists. Oncologist. 2005;10 Suppl. 1:19–24. doi: 10.1634/theoncologist.10-90001-19. [DOI] [PubMed] [Google Scholar]
- 130.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(8):3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 131.Naeim A, Reuben D. Geriatric syndromes and assessment in older cancer patients. Oncology (Williston Park) 2001;15(12):1567–1577. 80; discussion 81, 86, 91. [PubMed] [Google Scholar]
- 132.Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237–241. doi: 10.1002/cncr.20766. [DOI] [PubMed] [Google Scholar]
- 133.Yee EF, White RE, Murata GH, Handanos C, Hoffman RM. Osteoporosis management in prostate cancer patients treated with androgen deprivation therapy. J Gen Intern Med. 2007;22(9):1305–1310. doi: 10.1007/s11606-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith MR. Osteoporosis and obesity in men receiving hormone therapy for prostate cancer. J Urol. 2004;172(5 Pt 2):S52–S56. doi: 10.1097/01.ju.0000141820.17959.2f. [DOI] [PubMed] [Google Scholar]
- 135.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63(4):742–745. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 136.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J GerontolABiol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 137.Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37(4):477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 138.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 139.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279(2):E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 140.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy X-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167(6):2361–2367. discussion 7. [PubMed] [Google Scholar]
- 141.Boxer RS, Kenny AM, Dowsett R, Taxel P. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male. 2005;8(3–4):207–212. doi: 10.1080/13685530500361226. [DOI] [PubMed] [Google Scholar]
- 142.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 143.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263(22):3029–3034. [PubMed] [Google Scholar]
- 144.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. Across-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71(2):644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 145.Basaria S, Lieb J, II, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56(6):779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 146.Clay CA, Perera S, Wagner JM, Miller ME, Nelson JB, Greenspan SL. Physical function in men with prostate cancer on androgen deprivation therapy. Phys Ther. 2007;87(10):1325–1333. doi: 10.2522/ptj.20060302. [DOI] [PubMed] [Google Scholar]
- 147.Bylow K, Dale W, Mustian K, et al. Falls and physical performance deficits in older prostate cancer patients on androgen deprivation therapy. Urology. 2008;72(2):422–427. doi: 10.1016/j.urology.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fortinsky RH, Iannuzzi-Sucich M, Baker DI, et al. Fall-risk assessment and management in clinical practice: views from healthcare providers. J Am Geriatr Soc. 2004;52(9):1522–1526. doi: 10.1111/j.1532-5415.2004.52416.x. [DOI] [PubMed] [Google Scholar]
- 149.McClure R, Turner C, Peel N, Spinks A, Eakin E, Hughes K. Population-based interventions for the prevention of fall-related injuries in older people. Cochrane Database Syst Rev. 2005;(1) doi: 10.1002/14651858.CD004441.pub2. CD004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pynoos J, Rose D, Rubenstein L, Choi IH, Sabata D. Evidence-based interventions in fall prevention. Home Health Care Serv Q. 2006;25(1–2):55–73. doi: 10.1300/J027v25n01_04. [DOI] [PubMed] [Google Scholar]
- 151.Galvao DA, Nosaka K, Taaffe DR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–2052. doi: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 152.Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19(17):3750–3757. doi: 10.1200/JCO.2001.19.17.3750. [DOI] [PubMed] [Google Scholar]
- 153.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 154.Fowler FJ, Jr, McNaughton Collins M, Walker Corkery E, Elliott DB, Barry MJ. The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer. 2002;95(2):287–295. doi: 10.1002/cncr.10656. [DOI] [PubMed] [Google Scholar]
- 155.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54(1):85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 156.Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology. 1999;53(1):180–186. doi: 10.1016/s0090-4295(98)00408-7. [DOI] [PubMed] [Google Scholar]
- 157.Lubeck DP, Grossfeld GD, Carroll PR. The effect of androgen deprivation therapy on health-related quality of life in men with prostate cancer. Urology. 2001;58(2 Suppl. 1):94–100. doi: 10.1016/s0090-4295(01)01250-x. [DOI] [PubMed] [Google Scholar]
- 158.Herr HW, O’Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163(6):1743–1746. [PubMed] [Google Scholar]
- 159.van Andel G, Kurth KH. The impact of androgen deprivation therapy on health related quality of life in asymptomatic men with lymph node positive prostate cancer. Eur Urol. 2003;44(2):209–214. doi: 10.1016/s0302-2838(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 160.Arredondo SA, Latini DM, Sadetsky N, et al. Quality of life for men receiving a second treatment for prostate cancer. J Urol. 2007;177(1):273–278. doi: 10.1016/j.juro.2006.08.061. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 161.Litwin MS, Shpall AI, Dorey F, Nguyen TH. Quality-of-life outcomes in long-term survivors of advanced prostate cancer. Am J Clin Oncol. 1998;21(4):327–332. doi: 10.1097/00000421-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 162.Moinpour CM, Savage MJ, Troxel A, et al. Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst. 1998;90(20):1537–1544. doi: 10.1093/jnci/90.20.1537. [DOI] [PubMed] [Google Scholar]
- 163.Litwin MS. Health related quality of life in older men without prostate cancer. J Urol. 1999;161(4):1180–1184. [PubMed] [Google Scholar]
- 164.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 165.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 166.Fried TR, Mor V. Frailty and hospitalization of long-term stay nursing home residents. J Am Geriatr Soc. 1997;45(3):265–269. doi: 10.1111/j.1532-5415.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 167.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–1036. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- 168.Ettinger WH, Davis MA, Neuhaus JM, Mallon KP. Long-term physical functioning in persons with knee osteoarthritis from NHANES. I. Effects of comorbid medical conditions. J Clin Epidemiol. 1994;47(7):809–815. doi: 10.1016/0895-4356(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 169.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women’s Health and Aging Study. J Clin Epidemiol. 1999;52(1):27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 170.Luchi RJ, Gammack JK, Narcisse VJ, III, Storey CP., Jr Standards of care in geriatric practice. Annu Rev Med. 2003;54:185–196. doi: 10.1146/annurev.med.54.101601.152221. [DOI] [PubMed] [Google Scholar]
- 171.Naeim A, Hurria A, Leake B, Maly RC. Do age and ethnicity predict breast cancer treatment received? A cross-sectional urban population based study Breast cancer treatment: Age and ethnicity. Crit Rev Oncol Hematol. 2006 doi: 10.1016/j.critrevonc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 172.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006;24(15):2304–2310. doi: 10.1200/JCO.2005.03.1567. [DOI] [PubMed] [Google Scholar]
- 173.Flood KL, Carroll MB, Le CV, Ball L, Esker DA, Carr DB. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24(15):2298–2303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]
- 174.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55(3):241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 175.Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev. 2005;31(5):380–402. doi: 10.1016/j.ctrv.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 176.Lichtman SM. Guidelines for the treatment of elderly cancer patients. Cancer Control. 2003;10(6):445–453. doi: 10.1177/107327480301000602. [DOI] [PubMed] [Google Scholar]
- 177.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 178.Lawton MP. Scales to measure competence in everyday activities. Psychopharmacol Bull. 1988;24(4):609–614. [PubMed] [Google Scholar]
- 179.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 181.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 182.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289(13):1652–1658. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 183.Fillenbaum G, Heyman A, Williams K, Prosnitz B, Burchett B. Sensitivity and specificity of standardized screens of cognitive impairment and dementia among elderly black and white community residents. J Clin Epidemiol. 1990;43(7):651–660. doi: 10.1016/0895-4356(90)90035-n. [DOI] [PubMed] [Google Scholar]
- 184.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 185.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 186.Saliba D, Elliott M, Rubenstein LZ, et al. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 187.Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab. 2003;88(11):5240–5247. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- 188.Balducci L, Extermann M. Management of the frail person with advanced cancer. Crit Rev Oncol Hematol. 2000;33(2):143–148. doi: 10.1016/s1040-8428(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 189.Basso U, Tonti S, Bassi C, et al. Management of Frail and Not-Frail elderly cancer patients in a hospital-based geriatric oncology program. Crit Rev Oncol Hematol. 2008 doi: 10.1016/j.critrevonc.2007.12.006. [DOI] [PubMed] [Google Scholar]