Clinicians have few objective methods with which to assess the severity of acute asthma exacerbations in pediatric patients. Percent predicted forced expiratory volume in 1-second (%FEV1) by spirometry is the accepted criterion standard, but is frequently not available in acute care settings. Prior pediatric investigations have noted modest correlation of accessory muscle use with lower %FEV1 but have not reported severity-dependent associations of accessory muscle use by muscle groups in use with %FEV1 or hospitalization decisions.1,2 Because this is an easily identifiable physical finding, we sought to examine associations of individual accessory muscle groups with both %FEV1 and hospitalization in pediatric patients with acute asthma exacerbations.
We prospectively enrolled participants ages 5 to 17 years with doctor-diagnosed asthma and signs or symptoms of an acute exacerbation who presented to our urban, tertiary care pediatric emergency department (PED). We defined accessory muscle use as any visible use of the scalene, sternocleidomastoid-suprasternal, intercostal or subcostal muscles.3 Accessory muscle use was further quantified for analyses as none, one group, or two or more muscle groups in order to examine severity-response relationships. The clinical team determined need for hospitalization and was blinded to our assessment of accessory muscle use and %FEV1 values.
We assessed associations of pre-treatment accessory muscle use with simultaneous %FEV1 for participants meeting all American Thoracic Society (ATS) spirometry criteria, and with hospitalization for the entire cohort.4,5 We used the Kruskal-Wallis and Chi-square tests, respectively, for these analyses, and a multivariable model that adjusted for age, gender and race. The study protocol was approved by our institutional Human Research Protection Program.
Between April, 2008, and August, 2010, 672 eligible patients were enrolled and 604 unique participants were included for analysis. Median [IQR] age was 8.75 years [6.8, 11.3], 61% male, and 57% African-American. Global Initiative for Asthma (GINA) chronic control levels for the preceding 3-month period were [n(%)]: controlled 104(17) partially controlled 198(33), and uncontrolled 302(50). Disposition included 77.5% discharged to home, 16% admitted to the floor, and 6.5% to the PICU. Of the entire cohort (n=604), accessory muscle use was noted in 318 (53%).
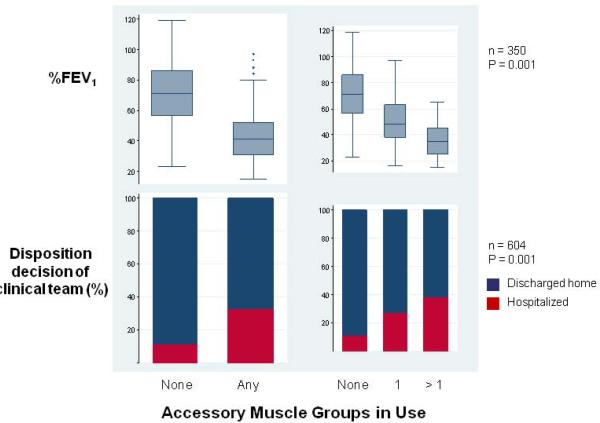
All participants attempted spirometry, and 350 (58%) met all ATS acceptability criteria. These participants were less likely to be hospitalized (19% vs. 28%, P=0.01) and, if hospitalized, were less likely to be admitted to the PICU (4% vs. 10%, P=0.007). There was no increase in the odds of obtaining spirometry meeting ATS criteria versus not meeting ATS criteria with use of any (OR=1.18, 95% CI: 0.84, 1.65) or of 2 or more groups of accessory muscles vs. none (OR=1.25, 95% CI: 0.83, 1.90). Median [IQR] %FEV1 for this group was 52 [38, 74]. %FEV1 was 71% [56, 86] in those without use of any, 48% [37, 63] in those with use of one and 35% [25, 44] in those with use of two or more accessory muscle groups (P=0.001, Kruskal-Wallis, Figure 1). The association of any accessory muscle use with decreased %FEV1 remained after controlling for age, gender and race in multivariable linear regression (β = −28, 95%CI: −32, −24, P=0.0001).
Figure 1.
% predicted FEV1, (%FEV1) and emergency department disposition according to use of none, one or more than one group of accessory muscles before treatment. Top: Boxes comprise interquartile ranges with median lines across boxes. Fences are 1.5 value of IQR beyond IQR bounds. Bottom: Disposition of entire study cohort according to accessory muscle use.
Hospitalization occurred in 33(12%) of those without, 44(27%) of those with use of one group, and 59(38%) of those with use of more than one accessory muscle group (P=0.001, Pearson test). Accessory muscle use (any vs. none) remained associated with increased odds of hospitalization when adjusted for age, gender and race in the multivariable model (OR=4.2, 95%CI: 2.6–6.6, P<0.001).
Accessory muscle use in pediatric patients with acute asthma exacerbations is associated with statistically significant and clinically important decreases of %FEV1 and with greater likelihood of hospitalization. These associations are strengthened by the severity-dependent nature of these associations. Accurate accessory muscle use assessment is of value to clinicians because it is simple, low cost, relatively objective, and physiologically plausible as it is indicative of increased work of breathing. Additionally, participants with accessory muscle use are as likely to perform ATS criteria spirometry as those without accessory muscle use, suggesting that efforts to obtain this test of lung function are warranted.
Limitations include that 42% of participants could not perform spirometry due to young age or severity of illness. This may have introduced bias, but had these studies been available, the values of %FEV1 with accessory muscle use would likely have been lower.
Concern has long been expressed that new imaging and laboratory technology may replace clinicians' use of and skill in the physical examination.6,7 Indeed, Sir William Osler's teaching maxim “The whole art of medicine is in observation” is relevant to the findings of our study.8 Observation for use of accessory muscles has clinical value and should be made during the care of patients with acute asthma exacerbations, and is associated with significant physiologic and clinical outcomes.
Acknowledgement
The authors acknowledge the work of Donald J. Resha, EMT-P, who assisted with participant enrollment for this study.
Donald J. Resha, EMT-P Department of Pediatrics Vanderbilt University School of Medicine
This research was funded by the National Institutes of Health NIH/NHLBI [K23 HL80005-01A2] (Dr. Arnold); NIH/NCRR [UL1 RR024975] (Vanderbilt CTSA/REDCap database); and NIAID [K24 AI77930] (Dr. Hartert)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statements Dr. Arnold holds two patents along with Precision Pulsus, Inc. (Apnea detection system and Method and apparatus for measuring pulsus paradoxus).
Ms. Gebretsadik has no conflict of interest to disclose.
Dr. Sheller has no conflict of interest to disclose.
Dr. Abramo has no conflict of interest to disclose.
Dr. Hartert has received lecture fees from Merck and has received industry-sponsored grants from MedImmune.
Reference List
- 1.Langhan ML, Spiro DM. Portable spirometry during acute exacerbations of asthma in children. J Asthma. 2009;46:122–125. doi: 10.1080/02770900802460522. [DOI] [PubMed] [Google Scholar]
- 2.Kerem E, Canny G, Tibshirani R, Reisman J, Bentur L, Schuh S, et al. Clinical-physiologic correlations in acute asthma of childhood. Pediatrics. 1991;87:481–486. [PubMed] [Google Scholar]
- 3.Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): a responsive index of acute asthma severity. J Pediatr. 2000;137:762–768. doi: 10.1067/mpd.2000.110121. [DOI] [PubMed] [Google Scholar]
- 4.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 5.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 6.Jauhar S. The demise of the physical exam. N Engl J Med. 2006;354:548–551. doi: 10.1056/NEJMp068013. [DOI] [PubMed] [Google Scholar]
- 7.Noehren TH, Kopp JB. Teaching physical diagnosis of the chest. Dis Chest. 1955;27:333–334. doi: 10.1378/chest.27.3.333. [DOI] [PubMed] [Google Scholar]
- 8.Osler WI. The natural method of teaching the subject of medicine. JAMA: The Journal of the American Medical Association. 1901;XXXVI:1673–1679. [Google Scholar]