Abstract
Background
Up to 60% of US visitors to Mexico develop travelers’ diarrhea (TD). In Mexico, rates of diarrhea have been associated with the rainy season and increase in ambient temperature. However, the seasonality of the various diarrheagenic Escherichia coli pathotypes in travelers has not been well described.
Objective
To determinate if ambient temperature and rainfall have an impact on the acquisition of TD due to different diarrheagenic E. coli pathotypes in Mexico.
Methods
We conducted a cohort study of US adult students traveling to Cuernavaca, Mexico that were followed during their stay and provided a stool sample with the onset of TD. The presence of E. coli was analyzed by a direct fecal multiplex PCR for common E. coli pathotypes including enterotoxigenic, enteropathogenic, enteroinvasive, shigatoxin producing and enteroaggregative E. coli (ETEC, EPEC, EIEC, STEC and EAEC respectively). The presence of pathotypes was correlated with daily rainfall, average, maximum and minimum temperatures.
Results
515 adults were enrolled from January 2006 to February 2007. The weekly attack rate of TD for newly arrived travelers was lower in the winter months (range 6.8 to 16.3%) than in summer months (range 11.5–25%; p=0.05). The rate of ETEC infection increased by 7% for each degree centigrade increase in weekly ambient temperature (P=0.003). In contrast, EPEC and EAEC were identified in similar proportions during the winter and summer seasons.
Conclusions
Temperature variations in Central Mexico influenced the rate of ETEC but not EAEC associated diarrhea in US visitors. This epidemiological finding could influence seasonal recommendations for the use of ETEC vaccines in Mexico.
Keywords: Travelers’ diarrhea, travel, seasonality, Enterotoxigenic E. coli, Enteroaggregative E. coli, Diarrheagenic E. coli
The frequency of Travelers’ diarrhea (TD) among international travelers to tropical and semitropical regions of the developing world ranges from 10% to 60%. The highest rates of TD are seen in Latin America, Africa and the Indian subcontinent [1].
Worldwide infectious diarrhea rates are influenced by seasonal changes. Striking examples include V. cholerae infection in Asia where the rates of infection double during the warm season [2]. In Mexican children, rates of diarrhea are also influenced by seasonal changes with rotavirus diarrhea predominating in winter months [3]. In the US, pediatric diarrhea rates also vary seasonally, with viral causes of diarrhea predominating during the winter months and enteroaggregative E. coli (EAEC) seen more commonly during spring time [4].
The microbiology of TD in US visitors to Mexico reflects the bacterial enteropathogens identified in Mexican children with diarrhea. Most TD acquired in Mexico is due to enterotoxigenic Escherichia coli (ETEC) and EAEC [5, 6]. Previous studies have shown that the TD overall and TD due to ETEC are more common during summer than during winter months [7–9]. In other regions of the world, investigators have also found seasonal variation in etiology of TD; for instance in a study conducted in Morocco, Campylobacter spp. was associated with TD during winter months and ETEC was seen more commonly identified during the fall months. This is felt to relate to an increase in the ambient temperature and rainfall favoring the growth and spread of bacteria that contaminate food and water. These changes may further evolve in response to current global climate changes. The aim of this study was to characterize seasonal differences in diarrheagenic Escherichia coli pathotypes as causes for TD over a thirteen month period in a popular tourist destination in Mexico.
SUBJECTS AND METHODS
The present study was conducted in two language schools in Cuernavaca, Mexico during the summer-fall months (May, June, July and August) of 2006 and winter months (January and February) of 2006 and 2007. Participants consisted of groups of newly arrived students from the U.S. that completed a diary that recorded the number and consistency of all stools passed and the presence of abdominal symptoms while in Mexico. Students were enrolled within 72 hrs of arrival and followed during their stay in Mexico with daily clinic visits. Acute diarrhea was defined as the passage of three or more unformed stools within a 24-hour period plus one or more gastrointestinal symptoms. A stool sample was collected at the time of the diagnosis. Appropriate treatment for TD was provided.
Stool samples from subjects with diarrhea were collected and transported at 4°C directly or in Cary-Blair transport medium to the laboratory for culture and examination for enteric protozoan parasites, including Giardia lamblia, Entamoeba histolytica, and Cryptosporidium species by an ELISA (Alexon). Stool samples were evaluated for the presence of mucus, fecal leukocytes, and occult blood by conventional methods.
An aliquot of stool was frozen and transported to Houston for PCR studies with probes specific for diarrheagenic E. coli virulence factors as previously described [10, 11]. We then correlated the frequencies of the enteropathogens identified in stools with the daily temperature (maximum, minimum, and average), and rain precipitation recorded at the MMCB 767260 weather station located in Cuernavaca Mexico.
The study was approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston.
STATISTICAL ANALYSIS
The statistical analysis was performed by using the STATA v.10 software package. Demographic differences were compared using two sided Chi-square for categorical variables and student t-test was used for linear variables. Simple linear regression, pairwise correlation and multiple logistic regression analysis were applied. The variables included in the multiregression analysis included gender, age at arrival, ethnicity, prior travel experience to a developing country, length of stay, season of travel and the presence of the different diarrheagenic E. coli pathotypes in diarrheal stools. Correlation coefficients between temperature, rainfall and the rate of diarrhea due to the different E. coli pathotypes were calculated by regression analysis. A P value < 0.05 was considered significant.
RESULTS
Five hundred and fifteen adult students were enrolled, 365 (70.8%) were enrolled during the summer–fall months and 150 (29.2%) were enrolled during winter months (Table 1). One hundred twenty three (23.8%) male students and 392 (76.1%) female students participated in the study. The mean age of the participants was 34 years (SD ± 15, range 18–83) with a mean length of stay in Mexico of 19 days (95% CI 18–20). A total of one hundred and ninety eight participants developed TD (38%) during their stay in Mexico with a mean onset of 9.2 days after arrival (95% CI 8.2–10.1). Among those who developed TD, 152 (72%) provided a stool sample for microbiological analysis when ill.
Table 1.
Demographic Characteristics of U.S. Students Participating in a Prospective Study on the Occurrence of Diarrhea during Short-term Stays in Mexico
| Demographic Characteristics |
Winter Travel (n 150) |
Summer Travel (n 365) |
P |
|---|---|---|---|
| Females | 125 (83%) | 267 (73%) | 0.01 |
| Males | 25 (17%) | 98 (27%) | 0.01 |
| Age ± SD (years) | 30.8 ± 16.1 | 35.5 ± 14.3 | 0.001 |
|
Length of Stay ±SD in Days (range) |
17.2 ± 2.9 16.7–17.7 |
19.8 ± 9.1 18.8–20.7 |
0.0009 |
|
Rate of Diarrhea per season (No. subjects with TD / No. Subjects enrolled) |
44/150 (29.3%) | 154/365 (42.6%) | 0.005 |
|
Provided stool sample for Microbiology |
35/44 (79%) | 117/154 (75%) | 0.8 |
|
Any E. coli pathotype found (in tested samples) |
16/35 (45.7%) | 94/117 (80.3%) | 0.08 |
|
Onset of diarrhea (Days of stay after arrival) |
8.48 (±5.2) | 9.38 (± 6.9) | 0.4 |
There were significant differences in the demographic characteristics of travelers in terms of age, rate of diarrhea and length of stay between summer and winter. Students taking classes during the winter were significantly younger (30 years, 95% CI 28–33) than those coming into Mexico during the summer (35 years, 95% CI 34–37, P=0.001). However, students traveling during the summer stayed longer than students traveling during the winter months (17.2, 95% CI 16.7–17.7 vs. 19.8, 95% CI 18.8–20.7 P=0.0009). During winter months the weekly attack rate of TD fluctuated between 6.8–16.3%, while during the summer months TD rates fluctuated between 11.5–25% (P=0.05).
One hundred and fifty two stool samples were tested for the presence of diarrheagenic E. coli virulence factors by PCR. ETEC and EAEC were the most commonly identified pathogens (Table 2). Genes characteristic for ETEC were found by PCR in 11.4% (4/35) of the stool samples provided during winter months and in 43.5% (51/117) of cases during summer months (OR 4.37, 95% CI 1.4–12.8, P=0.02), meanwhile EAEC genes were found in 22.8% (8/35) of the stool samples obtained during winter and 42.7% (50/117) during the summer months (OR 1.94, 95% CI 0.79–4.71 P=0.1). The proportions of infections due to EPEC and STEC were similar for both seasons (P = NS). Of interest, EIEC virulence factors were found in 11.1% (13/117) of stools collected during the summer and in none of the stools collected during the winter.
Table 2.
Detection rates for distinct E. coli pathotypes in the 152 fecal samples obtained from travelers with diarrhea according to season of travel
| No. positive samples/ No. tested samples (%) | ||||
|---|---|---|---|---|
| Pathotype | Winter | Summer | Odds Ratio (95% CI) |
P-value |
| ETEC | 4/35 (11.4) |
51/117 (43.5%) |
4.37 (1.4–12.8) |
0.02 |
| STEC | 4/35 (11.4) |
30/117 (25.6) |
NS | 0.1 |
| EAEC | 8/35 (22.8) |
50/117 (42.7) |
NS | 0.1 |
| EPEC | 8/35 (22.8) |
50/118 (42.7) |
NS | 0.1 |
| EIEC | 0/27 | 13/117 (11.1) 13/117 |
ND | 0.07 |
Abbreviations: ETEC, enterotoxigenic Escherichia coli; STEC, Shiga-toxin producing E. coli; EAEC, enteroaggregative E. coli; EPEC, enteropathogenic E. coli. EIEC, enteroinvasive E. coli, NS, Non- significant; ND= Not done.
A multiple logistic regression analysis done (Table 3) using the following variables: gender, ethnicity, race, and age in years on arrival, length of stay, prior travel history, and season of travel. In addition to the occurrence of TD, the different E. coli pathotypes (except for EIEC which was not identified in the winter months) were included as dependent variables in separate analyses. Based on logistic regression analysis and after adjusting for the other variables, length of stay (P=0.02) and travel during the summer season (P=0.05) were associated to the occurrence of TD by Pearson correlation. Diarrhea due to ETEC was also significantly increased during the summer months (OR 5.1, 95% CI 1.4–18.4, P= 0.01) after adjusting for all the other independents variables.
Table 3.
Multiple logistic regression analysis of risk factors for travelers’ according to E. coli pathotypes identified.
| Odds Ratios (95% CI) | |||||
|---|---|---|---|---|---|
| Diarrhea due to all causes |
ETEC | EAEC | EPEC | STEC | |
| Gender | 0.96 | 1.0 (0.4–2.5) |
1.1 (0.4–2.5) |
1.0 (0.9–1.0) |
0.51 |
| Age (years) | 0.98 | 0.98 | 0.96 | 0.9** (0.92–0.98) |
0.99 |
|
Length of Stay (days) |
1.0* (1.0–1.05) |
0.99 | 1.0 (0.9–1.0) |
1.0 (0.9–1.0) |
1.0 (0.9–1.0) |
| Race | 0.98 | 1.01 (0.6–1.4) |
0.87 | 0.98 | 0.96 |
| Ethnicity | 0.99 | 0.83 | 2.3 (0.7–7.6) |
2.3 (0.7–7.6) |
1.0 (0.2–4.2) |
| Prior travel | 0.92 | 1.4 (0.6–3.5) |
0.9 | 0.8 | 1.1 (0.4–3.1) |
|
Season (Winter or Summer) |
1.6* (0.98–2.6) |
5.1* (1.4–18.4) |
2.2 (0.7–6.8) |
2.4 (0.8–7.4) |
2.6 (0.7–9.6) |
p ≤ 0.05;
p ≤ 0.01
We examined the effect of weekly rainfall, and temperature (mean, maximum, minimum and average) on the travelers’ diarrhea attack rates due each E. coli pathotypes by pairwise correlation. The weekly attack rate of diarrhea due to ETEC showed a positive correlation with higher minimum (P=0.001) and average (P=0.002) temperatures, whereas STEC (Shiga-toxin Escherichia coli) showed correlation with the maximum (P=0.05) and average (P=0.01) temperatures (Table 4). No correlation was found between the weekly minimum, average or maximum temperatures and diarrhea due to EAEC or EPEC. Also, no correlation was found between rainfall and ETEC, EAEC, EPEC or STEC being identified in stools by PCR.
Table 4.
Correlation coefficients of ambient temperature (Min=Minimum, Max=Maximum and Average) and rainfall on the rate of diarrhea due E. coli pathotypes.
| Ambient Temperature^ | Rainfall^ | |||
|---|---|---|---|---|
| Pathotype | Average | Max | Min | - |
| ETEC rate | 0.63*** | 0.2 | 0.66*** | 0.1 |
| EAEC rate | 0.04 | −0.1 | 0.01 | −0.3 |
| EPEC rate | 0.1 | −0.27 | 0.2 | −0.02 |
| STEC rate | 0.4 * | 0.5** | 0.3 | 0.001 |
Temperature and rainfall coefficients as calculated by a regression model.
P ≤ 0.05;
P≤ 0.01;
P ≤ 0.001
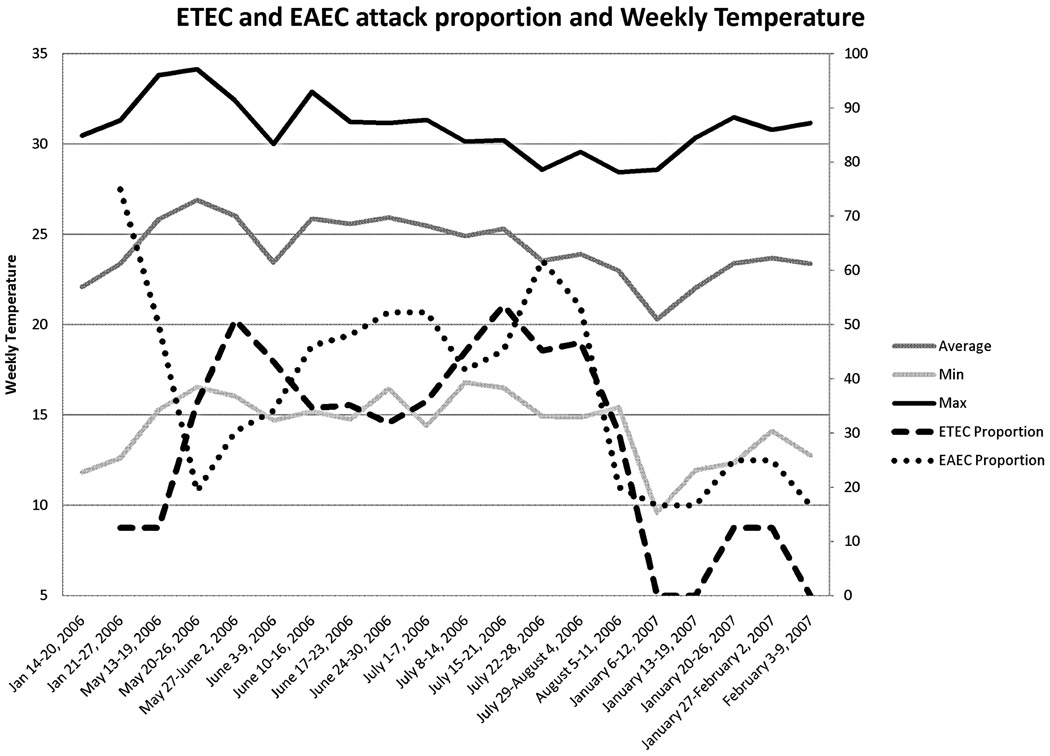
We observed a linear increase in the number of TD cases due to ETEC as the ambient temperature became warmer (Figure 1) in Cuernavaca. For each degree increase in the weekly average temperature, the attack rate of ETEC associated diarrhea increased by 7% as calculated by logistic regression (P=0.003; 95% CI 6-12%, r2= 0.40). An increase in the risk of developing ETEC associated diarrhea was also noted when we analyzed the minimum daily temperatures recorded. This analysis showed that for each centigrade increase in minimum daily ambient temperature, the odds of ETEC associated diarrhea increased by 7% (95% CI 3–11%; r2= 0.44, P=0.001). The increases in the maximum temperature had no significant effect on the attack rate. In contrast, to the rates for ETEC, the rates of EAEC associated diarrhea remained relatively constant despite seasonal temperature variations (P=0.1).
Figure 1.
Weekly proportion of ETEC and EAEC related travelers’ diarrhea and the weekly minimum, maximum and average temperature.
DISCUSSION
TD is caused by a variety of bacterial agents of which ETEC and EAEC are the most common identifiable pathogens [1]. In agreement with previously published studies on TD acquired in Guadalajara, Mexico in 1986–89 [9]. The present study found that the rates of TD were higher during summertime when compared to wintertime in central Mexico. This second study was conducted in Cuernavaca, Mexico, which is called “The city of eternal springtime” where temperature variations are milder.
The warmer and wetter summer months are associated with an increased occurrence of diarrhea [12]. Warmer climates may encourage propagation of enteric bacterial pathogens in food [13] and water [14] explaining the increase in bacterial diarrhea during the summertime. Furthermore, in the case of ETEC, seasonality also appears to influence the rates of toxin phenotypes identified. It has previously been suggested, that in Egypt ST producing ETEC strains are more commonly identified in the stools of children with diarrhea in the summer while LT (heat-labile toxin) producing ETEC strains are identified all year around [15]. In our study, the rates of LT and ST (heat-stable toxin) producing ETEC did not appear to vary according to seasonality.
In this report, we found that minimum and average temperatures are positively associated to higher rates of ETEC associated diarrhea. We hypothesize that since weekly maximum temperatures do not fluctuate as much as minimum temperatures, the analysis failed to show a statistical correlation with maximum temperatures.
When studied in the univariate analysis, the identification of STEC as defined by the presence of stx1 or stx2 in stools also showed a positive correlation with warmer temperature and summer time diarrhea, however only ETEC demonstrated a significant correlation when an adjusted multivariate analysis was performed.
An important observation in this study is that in contrast to ETEC, the rates for EAEC, the second most common bacterial cause of TD, remained similar in both seasons. This is consistent with a previous study carried out in Korea that failed to find a seasonal pattern for EAEC infection [16] and contrasts with a 12 month study in a US pediatric population, where Cohen et al. reported a seasonal peak of EAEC in children during March-April months; However a confounding variable in that study was that many of the EAEC cases were co-infected with Rotavirus [4]. Although EIEC was only identified in the summer additional studies are needed to determine if the occurrence of EIEC infection is also seasonal.
This study has several limitations that need to be considered. First, not every participant suffering from TD provided a stool sample, hence we evaluated the proportions for each diarrheagenic E. coli pathotype among collected stool samples rather than sick individuals to avoid assuming the proportions were the same. Second, during this cohort study we used direct stool PCR to differentiate between E. coli pathotypes rather to use different laboratory technique for each different pathotype, we did so in order to avoid having data obtained from different techniques with different sensibilities and specificities among them. Third, more participants were enrolled during the summer months.
This epidemiological finding could impact the recommended use of ETEC LT vaccines [17] during warmer and cooler months. However, additional studies using ETEC LT vaccines would need to be conducted in order to further evaluate the possible benefits during lower acquisition rate seasons. The difference between ETEC and EAEC rates in terms of seasonality suggests the two important causes of TD have different pathways of transmission and reservoirs in Mexico.
Acknowledgments
We are indebted to Judy Guillen, and the administration and staff of Universidad Internacional in Cuernavaca, Morelos, Mexico for their help in this project.
Financial support. This work was supported by the following sources: NIH R01 AI54948-01 and UL1 RR024148 to the Center for Clinical and Translational Sciences at the University of Texas Medical School at Houston, and NIH DK56338, which funds the Texas Gulf Coast Digestive Diseases Center
Footnotes
** Presented in part at the 48th Annual ICAAC / 47th IDSA Annual Meeting. Washington DC, USA. 2008. Abstract P-1613.
Declaration of Interests
The authors state they have no conflicts of interest to declare
References
- 1.DuPont HL. Systematic review: the epidemiology and clinical features of travellers' diarrhoea. Aliment Pharmacol Ther. 2009;30:187–196. doi: 10.1111/j.1365-2036.2009.04028.x. [DOI] [PubMed] [Google Scholar]
- 2.Islam MS, Sharker MA, Rheman S, et al. Effects of local climate variability on transmission dynamics of cholera in Matlab, Bangladesh. Trans R Soc Trop Med Hyg. 2009;103:1165–1170. doi: 10.1016/j.trstmh.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MB, Nataro JP, Bernstein DI, et al. Prevalence of diarrheagenic Escherichia coli in acute childhood enteritis: a prospective controlled study. J Pediatr. 2005;146:54–61. doi: 10.1016/j.jpeds.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 5.Flores J, DuPont HL, Jiang ZD, et al. Enterotoxigenic Escherichia coli heat-labile toxin seroconversion in US travelers to Mexico. J Trav Med. 2008;15:156–161. doi: 10.1111/j.1708-8305.2008.00187.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhi-Dong Jiang, Brett Lowe MP, Verenkar, et al. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay) J Infect Dis. 2002;185:497–502. doi: 10.1086/338834. [DOI] [PubMed] [Google Scholar]
- 7.Qadri F, Saha A, Ahmed T, et al. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun. 2007;75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattila L, Siitonen A, Kyronseppa H, et al. Seasonal variation in etiology of travelers' diarrhea. Finnish-Moroccan Study Group. J Infect Dis. 1992;165:385–388. doi: 10.1093/infdis/165.2.385. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson CD, DuPont HL, Mathewson IJ. Epidemiologic Observations on Diarrhea Developing in U.S. and Mexican Students Living in Guadalajara, Mexico. J Travel Med. 1995;2:6–10. doi: 10.1111/j.1708-8305.1995.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Saucedo C, Cerna JF, Villegas-Sepulveda N, et al. Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerg Infect Dis. 2003;9:127–131. doi: 10.3201/eid0901.01-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerna JF, Nataro JP, Estrada-Garcia T. Multiplex PCR for detection of three plasmid-borne genes of enteroaggregative Escherichia coli strains. J Clin Microbiol. 2003;41:2138–2140. doi: 10.1128/JCM.41.5.2138-2140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoge CW, Shlim DR, Echeverria P, et al. Epidemiology of diarrhea among expatriate residents living in a highly endemic environment. JAMA. 1996;275:533–538. [PubMed] [Google Scholar]
- 13.Lee MB, Middleton D. Enteric illness in Ontario, Canada, from 1997 to 2001. J Food Prot. 2003;66:953–961. doi: 10.4315/0362-028x-66.6.953. [DOI] [PubMed] [Google Scholar]
- 14.Wright RC. The seasonality of bacterial quality of water in a tropical developing country (Sierra Leone) J Hyg. 1986;96:75–82. doi: 10.1017/s0022172400062550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaheen HI, Abdel Messih IA, Klena JD, et al. Phenotypic and genotypic analysis of enterotoxigenic Escherichia coli in samples obtained from Egyptian children presenting to referral hospitals. J Clin Microbiol. 2009;47:189–197. doi: 10.1128/JCM.01282-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho SH, Shin HH, Choi YH, et al. Enteric bacteria isolated from acute diarrheal patients in the Republic of Korea between the year 2004 and 2006. J Microbiol. 2008;46:325–330. doi: 10.1007/s12275-008-0015-4. [DOI] [PubMed] [Google Scholar]
- 17.DuPont HL, Ericsson CD, Farthing MJ, et al. Expert review of the evidence base for prevention of travelers' diarrhea. J Travel Med. 2009;16:149–160. doi: 10.1111/j.1708-8305.2008.00299.x. [DOI] [PubMed] [Google Scholar]