Abstract
L-type voltage-gated calcium channels (LTCCs) have long been considered as crucial regulators of neuronal excitability. This role is thought to rely largely on coupling of LTCC-mediated Ca2+ influx to Ca2+-dependent conductances, namely Ca2+-dependent K+ (KCa) channels and nonspecific cation (CAN) channels, which mediate afterhyperpolarizations (AHPs) and afterdepolarizations (ADPs), respectively. However, in which manner LTCCs, KCa channels, and CAN channels co-operate remained scarcely known. In this study, we examined how activation of LTCCs affects neuronal depolarizations and analyzed the contribution of Ca2+-dependent potassium- and cation-conductances. With the use of hippocampal neurons in primary culture, pulsed current-injections were applied in the presence of tetrodotoxin (TTX) for stepwise depolarization and the availability of LTCCs was modulated by BAY K 8644 and isradipine. By varying pulse length and current strength, we found that weak depolarizing stimuli tend to be enhanced by LTCC activation, whereas in the course of stronger depolarizations LTCCs counteract excitation. Both effect modes appear to involve the same channels that mediate ADP and AHP, respectively. Indeed, ADPs were activated at lower stimulation levels than AHPs. In the absence of TTX, activation of LTCCs prolonged or shortened burst firing, depending on the initial burst duration, and invariably augmented brief unprovoked (such as excitatory postsynaptic potentials) and provoked electrical events. Hence, regulation of membrane excitability by LTCCs involves synchronous activity of both excitatory and inhibitory Ca2+-activated ion channels. The overall enhancing or dampening effect of LTCC stimulation on excitability does not only depend on the relative abundance of the respective coupling partner but also on the stimulus intensity.
Keywords: excitability, hippocampus, afterpotential
in central neurons, L-type voltage-gated calcium channels (LTCCs) localize primarily to the somatodendritic compartment (37). Influx of Ca2+ via these channels was shown to couple to gene transcription and was suggested to play a role in excitation-transcription coupling during differentiation and development (6, 13, 32, 45). Furthermore, forms of long-term synaptic plasticity (e.g., long-term potentiation) were shown to require LTCCs (28, 29). Additionally, LTCCs were suggested not only to provide Ca2+ for downstream effectors but also to act as crucial instant regulators of neuronal excitability (31). This notion was based on the observation that LTCCs activate a Ca2+-dependent potassium current that gives rise to postburst afterhyperpolarizations (AHPs) and spike frequency adaptation. Later, it was found that LTCCs can also induce afterdepolarizations (ADPs), e.g., by activation of a neuronal Ca2+-dependent nonspecific cation (CAN) current (30). This divergent coupling has since been confirmed in various neuronal populations, and its role is still the focus of current research (23, 25, 28). Hence, LTCCs may regulate neuronal excitability in opposing manners, depending on the prevalence of coupling to potassium or cation channels. So far these coupling modes have always been studied independently. Nevertheless, it is likely that they coexist in neurons, and a report on nigral dopamine neurons indeed pointed in that direction (35). However, there is still no information available on the relation between excitatory and inhibitory coupling modes of LTCCs. Thus the question if and how LTCCs affect neuronal excitability still needs to be elucidated.
To address this issue, the present study was initiated to test for and characterize the role of LTCCs in electrical activities of hippocampal neurons in primary culture, with particular emphasis on coupling to Ca2+-dependent conductances. We report on profound bimodal LTCC-mediated effects on membrane voltage occurring over a broad potential range and provide evidence for concomitant depolarizing and hyperpolarizing coupling modes, which operate in a stimulus-dependent manner: weak stimuli favor the excitatory LTCC coupling mode, which is suggested to involve CAN channels, whereas strong stimuli induce a LTCC-mediated deceleration of excitation via inhibitory coupling involving KCa2.x channels.
MATERIALS AND METHODS
Primary cell culture of hippocampal neurons.
Hippocampi were dissected from neonatal Sprague-Dawley rats, which had been killed by decapitation in full accordance with all rules of the Austrian animal protection law (see http://ris1.bka.gv.at/Appl/findbgbl.aspx?name=entwurf&format=pdf&docid=COO_2026_100_2_72288) and the Austrian animal experiment by-laws (see http://www.ris2.bka.gv.at/Dokumente/BgblPdf/2000_198_2/2000_198_2.pdf). Primary cultures of hippocampal neurons were prepared as described previously for mass cultures in microchambers created by glass rings (3), with the following minor modifications. The isolated brains were immediately transferred into ice-cold buffer containing (in mM) 137 NaCl, 4.5 KCl, 1.1 Na2HPO4 × 2 H2O, 1.1 KH2PO4, 6.1 glucose, and 1 kynurenate with pH 7.3 adjusted with NaOH.
The culture medium used was based on DMEM high glucose with l-glutamine (purchased from PAA, Pasching, Austria) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen, Lofer, Austria) and 12.5 mg/ml ITS (insulin:transferrin:Na-selenite = 25:25:0.025, Roche Diagnostics, Vienna, Austria), 10 nM progesterone, and 100 μM putrescine (both from Sigma, Vienna, Austria), with or without antibiotics (25,000 IU/l penicillin and 25 mg/l streptomycin, PAA). From the final single cell suspension we seeded about 35,000 cells per glass ring and exchanged the initial medium after 24 h for antibiotics-free medium. Cytosine (1 μM) arabinoside was added at day 4 to 5 after the preparation to reduce the proliferation of nonneuronal cells. Neurons were cultured for 20–30 days at 37°C and 5% CO2. To compensate for evaporation from the medium during that time, 150 μl autoclaved and sterile filtered purified water was added once a week.
Electrophysiology.
Recordings of membrane voltage were performed using a Multiclamp 700B amplifier (Axon Instruments) in the current clamp mode. Signals were low-pass filtered at 10 kHz and digitized with a Digidata 1440A digitizer (Axon Instruments) at a sampling rate of 20 kHz. Patch pipettes were made of borosilicate capillaries (Science Products GB150–8P) with a Sutter P97 horizontal puller. Tip resistances lay between 3, 5, and 5 MΩ. Pipette solutions contained (in mM) 120 potassium gluconate, 1.5 sodium gluconate, 3.5 NaCl, 1.5 CaCl2, 0.25 MgCl2, 10 HEPES, 10 glucose, and 5 EGTA. pH was adjusted to 7.3 by KOH. For perforated patch recordings, 500 μg/ml amphotericin B (from Streptomyces species, compound purchased from Sigma-Aldrich) was added to the pipette solution. Current clamp recordings were started after the series resistance had dropped below 30 MΩ, which usually occurred within 15 to 30 min after seal formation. Under these conditions, voltage-clamp experiments showed stable LTCC-mediated currents that amounted on average to 25% of total voltage-gated calcium channel currents (n = 32, unpublished results). To assure that only viable cells were used, the following inclusion criteria had to be met: a membrane voltage of at least −50 mV and the capability of generating overshooting action potentials, which was always tested before the recordings. Experiments were performed at room temperature, and cells were superfused continuously with standard external solution containing (in mM) 140 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 20 glucose (pH was adjusted to 7.4 by NaOH). In some experiments, the concentration of Na+ was reduced to 1.5 mM by an equimolar substitution of NaCl with CholinCl (equals low[Na+] solution). LTCC activity was modulated by application of the dihydropyridines (DHP) isradipine (LTCC antagonist) and BAY K 8644 (BayK, LTCC agonist), both at 3 μM. In a few experiments the concentration of BayK was varied as indicated. Superfusion was performed with a DAD-12 drug application system (Adams & List, Westbury, NY) with a micromanifold that held 12 channels converging into a 100 μM diameter quartz outlet. The tip of the outlet was positioned in close proximity (∼250 μm) to the patch-clamped cell (Adams & List). Activation of LTCCs was provoked by incremental current injections (typically 5 injections of equally increasing amplitude, e.g., injection 1 of 75 pA to injection 5 of 375 pA in a typical experiment, separated by 30-s intervals) to depolarize the neurons experimentally beyond the LTCC activation threshold. Duration of current injection was 8 s in most of the experiments, but pulse duration was varied in some experiments, as indicated. Unless stated otherwise, the recordings were made in the presence of 500 nM tetrodotoxin (TTX) in the external solution.
Drugs.
Atropine sulfate, d-(−)-2-amino-5-phosphonopentanoic acid (AP5), 4-aminopyridine, BayK, bicuculline methiodide (BMI), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), dimethyl sulfoxide (DMSO), flufenamic acid, isradipine, and bulk chemicals were purchased from Sigma-Aldrich; apamin, XE 991 dihydrochloride, and UCL 1684 were from Tocris Bioscience (Bristol, UK); and TTX was purchased from Latoxan (Valence, France). Since some of these drugs were dissolved in dimethyl sulfoxide (DMSO), the concentration of this solvent was kept constant at 0.3% in all solutions. Control solution contained 0.3% DMSO only, whereas DMSO-soluble compounds were diluted from concentrated stock solutions so as to obtain the same final concentration of DMSO, or more solvent was added as required.
Data analysis and statistics.
Levels of stimulations for all series of incremental current injections were quantified from recordings performed in the presence of isradipine by determination of the depolarization reached after 1 s (a duration after which current injections can be assumed to have caused steady state with respect to the purely passive depolarizations determined by the membrane time constant). Afterpotentials were quantified by measuring the area (mV·ms) between the baseline (equals membrane voltage before the current injection) and the recorded trace, from the end of the current injection to the repolarization back to the baseline level. The relative frequency of events in every voltage bin was determined as the number of registrations of a certain response mode or type of afterpotential divided by the total number of registrations in that voltage bin. A threshold search algorithm (“event detection”) performed by Clampfit 10.2 (Axon Instruments) was used for determining burst durations. The threshold was set to 15 mV above the baseline, which was adjusted to the resting membrane potential. The threshold for noise rejection was set to 1 ms.
GraphPad Prism version 5.03 was used for preparation of graphs and for statistical testing, for regression analysis (results are given as regression coefficient r2), and for nonparametric correlation analysis (results are given as Spearman rank correlation coefficient rs). Data are represented as means ± SE. Unpaired t-test, Mann-Whitney test, Kruskal-Wallis one-way ANOVA with Dunns post hoc test, and Wilcoxon signed-rank test were selected as required by the type of the data. Dose-response data were fitted using an equation describing a sigmoidal curve and allowing for variable slope (four parameter logistic equation, Graphpad software). For analyzing the frequency distributions of the LTCC onset, a single Gaussian distribution was compared with a sum of two Gaussian distributions using an F-test. In each data set, the unimodal fit corresponded to the null hypothesis, which was rejected, if the bimodal model fitted significantly better than the unimodal model. Where the sum of two Gaussian distributions was preferred, the resulting peaks (p1 and p2) were tested for significant difference by a two-tailed t-test. Variance homogeneities were tested by F-test and Gaussian distribution by Kolmogorov-Smirnov test.
RESULTS
Active responses in TTX-treated primary hippocampal neurons are largely due to Ca2+ influx via LTCCs.
To investigate the role of LTCCs in neuronal depolarizations together with the implication of coupling to Ca2+-dependent conductances, we applied TTX (0.5 μM) to avoid concomitant activation of voltage-dependent Na+ (Nav) channels in continuous superfusion, depolarized the neurons by incremental current injections, and modulated the activity of LTCCs by addition of BayK (LTCC agonist) and isradipine (antagonist). It is important to note at the beginning that, in the presence of both TTX and isradipine, responses to current injections had a rather monotonic appearance (see the isradipine traces in Fig. 1), even when they were long (e.g., 8 s) and large (e.g., reaching up to −25 mV). However, overlaying the traces evoked by a series of incremental current injections indicated the presence of outwardly rectifying conductances, the presence of which differed among the neurons. Hence, evoked depolarizations in the presence of TTX and isradipine consisted of the purely passive response, e.g., the voltage response determined by the membrane capacitance and membrane resistance, as well as voltage effects arising from noninactivating conductances. However, when isradipine was omitted, active responses were elicited (Fig. 1A), and these were potentiated in the presence of BayK (Fig. 1B). Such active responses were never evoked in isradipine, not even at the strongest levels of depolarization (Fig. 1, A–C). Hence, active responses evoked by depolarizing current injections were dominated by LTCC-mediated effects.
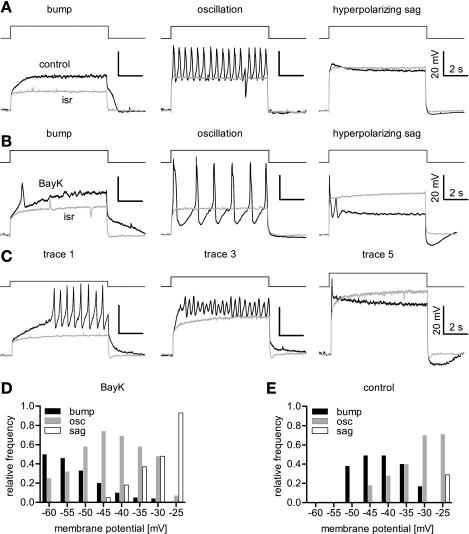
Fig. 1.
L-type voltage-gated calcium channels (LTCC)-mediated voltage responses evoked by depolarizing current injections. Rectangular current pulses were applied to depolarize the neurons that were bathed in tetrodotoxin (TTX) to avoid activation of voltage-dependent Na+ (Nav) channels. A–C: three major LTCC-mediated response modes were identified in comparisons of current injection-induced depolarizations recorded in the presence of isradipine (isr) and either DMSO or BAY K 8644 (BayK). Traces recorded in the presence of DMSO or 3 μM BayK are depicted in black, those recorded in 3 μM isradipine in grey. The effect modes were termed bumps, oscillations (osc), and hyperpolarizing sags, as indicated. Out of a series of 5 current injections of increasing amplitude, the recordings shown are either from different neurons (A, B) or from a single neuron (C), albeit at different depolarization levels: a bump occurred at the smallest depolarization (trace 1 out of 5 recordings), which then turned into oscillatory activity at intermediate voltages (trace 3, but also second half of trace 1), whereas the hyperpolarizing sag was seen with the strongest level of depolarization (trace 5). Traces 2 and 4 are not shown. D and E: relative-frequency distribution of effect modes plotted against the level of depolarization (evaluated in the presence of isradipine) for BayK-induced events (D) and events occurring under control conditions (E).
Three types of LTCC-mediated voltage responses.
Active responses obtained in the presence of BayK compared with the traces recorded in isradipine showed up as sustained elevated depolarizations, as oscillatory voltage changes, or as transient depolarizations and were denominated as “bumps,” “oscillations,” and “hyperpolarizing sags,” respectively, according to their coarse appearance. The term “bump” was used to describe responses where depolarizations rose above the response elicited in the presence of isradipine. The term “oscillation” was used for oscillatory membrane voltage changes, irrespective of the oscillation amplitude or frequency. “Hyperpolarizing sag” finally denominates responses that, after an initial rise above the trace recorded in isradipine, already during the pulse hyperpolarize toward or even below the response elicited in the presence of the LTCC inhibitor, so that the voltage response declines to or even traverses the isradipine trace in overlays. Examples illustrating these effect modes are shown in Fig. 1B. It was possible to observe all three effect modes in one cell, provided that depolarizations covered a sufficiently broad range: in that case, bumps occurred at lower depolarized voltages, oscillations at medium, and hyperpolarizing sags at the strongest levels of depolarization (Fig. 1C). Hence, cells could be further analyzed with respect to their typical LTCC-mediated response mode at discrete levels of depolarization (determined in the presence of isradipine, as indicated above). The analysis of 62 cells is illustrated in Fig. 1D, where the relative frequency of response modes is shown for the overall voltage-range represented in 5-mV bins. Bumps were most abundant at lower voltages but were rarely (≤5%) seen at depolarizations beyond −35 mV. Oscillations predominated in the voltage range of −50 mV to −35 mV, constituting up to 74% of responses at −45 mV, but they were less frequently observed at lower and higher voltages. Hyperpolarizing sags represent the typical response mode of strong depolarizations where they were seen in 93% of the cases (e.g., at −25 mV) but did not occur at potentials negative to −45 mV. Correlation analysis of response modes versus depolarizing voltage yielded rs values of −1.0 for bumps, 0.98 for sags, and −0.04 for oscillations, respectively. This indicates that there is a strong negative correlation between bumps and membrane voltage, a strong positive correlation between sags and membrane voltage, whereas no such correlation could be assigned to the oscillations. Each response mode could also be elicited under control conditions (Fig. 1E); overall, a similar trend was observed with respect to voltage dependency: bumps decreased in frequency as the voltage was raised, e.g., from a maximum of 49% at −45 to −40 mV, to 17% at −30 mV, whereas oscillatory activity increased over the voltage range of −45 mV to −25 mV from 18% to 71%. Hyperpolarizing sags were only seen at the highest depolarized level under control conditions, constituting 29% of responses at −25 mV. Correlation analysis yielded rs values of −0.64 for bumps and 1.0 for oscillations, indicating a moderate negative and strong positive correlation, respectively, between the response mode and the depolarizing voltage.
The experiments presented above were performed in the presence of TTX, which entirely blocked action potential generation in our preparation. Hence, excitatory inputs from other cells are unlikely to contribute to the responses elicited in the neurons tested. To confirm this, we performed similar experiments in the continuous presence of the glutamate receptor blockers AP-5 (25 μM) and CNQX (10 μM). Indeed, no change in the response modes became evident under this condition (data not shown). Thus LTCC-mediated responses arise endogenously from LTCC-mediated Ca2+ influx with a likely contribution of Ca2+-dependent conductances.
ADP and AHP are activated in a diametrical stimulus-dependent manner.
Afterpotentials are readily identifiable representations of Ca2+-dependent conductances. However, the respective channels are likely activated already during the pulse and may contribute to the voltage responses described above. In current clamp recordings, functional coupling of LTCCs to Ca2+-dependent cation or potassium channels appears as ADPs or AHPs following neuronal excitation. We set out in the next series of experiments to investigate, under which stimulatory conditions afterpotentials could be elicited. Therefore, current pulse duration was varied from 50 ms to 10 s. Recordings were made under control conditions (DMSO only) in the presence of BayK (3 μM) and isradipine (3 μM), respectively. Afterpotentials were identified in overlays of the trace recorded either under control conditions or in BayK, with the trace recorded in the presence of isradipine (Fig. 2A). As expected, the frequency (not shown) and the magnitude (Fig. 2B) of the afterpotentials increased by application of the LTCC agonist. Interestingly, the type of afterpotential that followed depolarizing pulses crucially depended on the length of the stimulus in that ADPs predominantly followed short pulses, whereas longer-lasting stimulations favored the occurrence of AHPs. As can be seen in Fig. 2B, where the afterpotential area (given as mV·ms) is plotted against the pulse length, the shortest pulses induced ADPs, which declined as the duration was prolonged (n = 7 to 9 for each duration). From >1 s durations onwards, stimulations were followed by AHPs, both under control conditions and in the presence of BayK. AHPs reached maximum values at 8 s long stimuli, further increasing the duration to 10 s did not cause any additional augmentation.
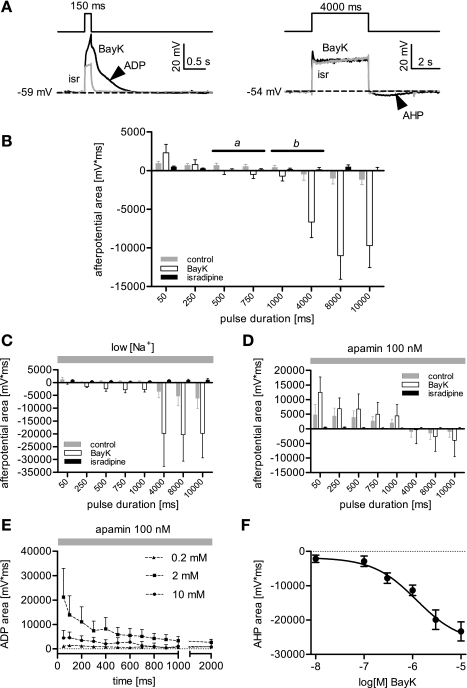
Fig. 2.
Dependence of afterpotentials on stimulus duration, external Ca2+, and BayK concentration. To evoke afterpotentials, hippocampal neurons were depolarized by pulsed current injections, the duration of which was increased from 50 ms up to 10 s (in 50 ms-steps from 50 ms up to 1 s, and in 1 s-steps from 1 s to 10 s). A: sample traces show overlays of voltage responses recorded in the presence of the LTCC antagonist isradipine (grey trace) and the LTCC agonist BayK (black trace) at a pulse duration of 150 ms and 4 s, respectively. An afterdepolarization (ADP, arrowhead, depicting the positive direction after event in the left traces) is evoked by short stimulation (150 ms) and an afterhyperpolarization (AHP, arrowhead, depicting the negative direction after event in the traces on the right) is induced by longer lasting stimulation (4s). B: afterpotential area is plotted against the pulse duration. For clarity, only 8 time points are depicted in the graph. Afterpotentials were quantified as the area between the recorded trace and the baseline (dashed line in A), from the end of the current injection to the repolarization back to the baseline level. Data are shown as means area ± SE from experiments performed in the presence or absence of agonist and antagonist, as indicated (n = 7–9 for this graph and n = 5 for C to F). Under control, pulses shorter than 1 s elicited ADPs (positive direction bars), whereas the long-lasting stimuli induced AHPs (negative direction bars). The range of an approximate turning point along the pulse duration axes is indicated by the solid line marked with b. In the presence of BayK the turning point from ADP to AHP is shifted to shorter stimuli and lies in a range indicated by the solid line marked with a. C: summary of experiments as in B but after reduction of external Na+ from 140 mM to 1.5 mM (low[Na+]). ADPs are reduced or, in the presence of BayK, no longer induced by the current injections, but DHP-sensitive AHPs are elicited and increase with longer pulse durations. Note that AHPs appear already at shorter pulse durations in the presence of BayK than under control conditions. D: summary of experiments as in C but performed in the presence of 100 nM apamin. ADPs are largest at 50-ms pulse duration and then continuously decrease with longer stimulation. Note that the longest pulses in the series, namely 8 and 10 s, induce a hyperpolarizing afterpotential (negative direction bars). E: dependence of BayK-induced afterpotentials on stimulus duration from experiments as shown in D but at different concentrations of external Ca2+. Data are only shown for pulse durations from 50 ms up to 2,000 ms. No distinct ADPs are induced when external Ca2+ was in the submillimolar range (e.g., 0.2 mM). Hence, ADPs require 2 mM external Ca2+ for induction and decrease with pulse duration. Raising external Ca2+ concentration ([Ca2+]o) to 10 mM gave smaller ADPs, and pulse prolongation caused a further reduction. F: dose-response curve for dihydropyridine (DHP)-sensitive AHPs evoked by 8 s-lasting current pulses with respect to the concentration of BayK (0.01–10 μM, n = 3 to 5 for each data point). The calculated Hill slope was 0.93 with an EC50 of 1.2 μM. TTX was present in all experiments at a concentration of 500 nM.
Reportedly, ADPs are mediated by nonspecific cation channels, and KCa2.x (SK) channels are one of the candidates for the molecular entities carrying the current underlying AHPs. Hence, we employed Na+-exchange experiments and the KCa2.x channel-blockers apamin or bicuculline methiodide (BMI), and in a few experiments UCL 1684, for further studies on afterpotentials. When external Na+ was reduced from 140 mM (standard external solution) to 1.5 mM (low [Na+] solution), under control conditions ADPs were reduced and AHPs increased. In the presence of BayK, AHPs were detected already at shorter pulses, but the reinforcing effect of increasing the pulse duration remained unaltered (Fig. 2C). However, when apamin was applied (in standard external solution), DHP-sensitive AHPs were not elicited with pulses ≤4,000 ms (these AHPs were also blocked by 30 μM BMI and 30 nM UCL 1684, data not shown). Instead, in the presence of BayK massive ADPs were induced by short pulses, the magnitude of which declined in the course of pulse prolongation (Fig. 2D). Longer pulses evoked negative afterpotentials that were considerably smaller than in the absence of apamin (e.g., reduced by 76% at 8-s long pulses). We repeated these experiments and varied the concentration of external Ca2+ ([Ca2+]o). As illustrated in Fig. 2E, ADPs were largely reduced when [Ca2+]o was lowered to micromolar levels and required millimolar [Ca2+]o for full induction. However, raising [Ca2+]o further to 10 mM reduced ADPs considerably, suggesting a bimodal dependence on Ca2+. To test whether solely changing the divalent cation concentration, e.g., because of surface charge screening effects, was responsible in any considerable manner for the effect shown in Fig. 2E, we applied 100-ms long current pulses and varied the current strength (as above). A comparison was made between ADPs obtained in the presence of 2 mM external Ca2+ with those evoked at 10 mM Ca2+ in the bath solution with the same current injection level and with higher currents, depolarizing the neurons by additional 10 mV. Increasing the depolarization in 10 mM external Ca2+ did not increase the ADPs to values obtained with 2 mM external Ca2+, indicating that a lowered depolarization directly at the membrane in case of high charge screening was not causing the smaller ADPs (data not shown). On the other hand, AHPs were affected by changes in Ca2+ availability in the opposite manner: at a given degree of depolarization, AHPs could be dose dependently augmented by BayK (Fig. 2F).
In the next step, we tested for a dependency of the afterpotentials on the stimulus intensity. To do this, we applied 8-s long stimuli and varied their magnitude in the course of five incremental current injections. Because afterpotentials could be more reliably induced in the presence of BayK, this analysis was done for experiments where the LTCC agonist was present. When ADPs were elicited by long stimuli, their induction was entirely dependent on membrane voltage. This is shown in the sample traces in Fig. 3A and in the graph shown in Fig. 3B, where the afterpotential area (mV·ms) is plotted against the voltage range reached during the pulse with the same stimulus but in the presence of isradipine (see materials and methods). The area of ADPs decreased with the extent of the preceding depolarization. However, a similar analysis on AHPs revealed that they had an opposite dependence and increased as the depolarizations grew (Fig. 3C). Correlation analysis yielded rs values of −0.90 for ADPs and 1.0 for AHPs, suggesting that the formation of afterpotentials is strongly correlated in a negative (ADPs) or positive manner (AHPs) with the membrane voltage. We established frequency distributions of afterpotential types using 5 mV binning of the preceding depolarized voltage. As illustrated in Fig. 3, D and E, ADPs were most abundant at smaller stimulations but decreased in frequency as the voltage responses increased. Hence, there was a negative correlation between depolarization during the pulse and the ADP. In contrast, AHPs were in positive correlation with the depolarization during the pulse and increased in frequency as the voltage responses rose. This held true both under control conditions (Fig. 3D) and after BayK administration (Fig. 3E), although the distributions were shifted to the left in the case of the LTCC agonist. The rs values for all indicated correlations were larger than 0.95 and −0.95, respectively.
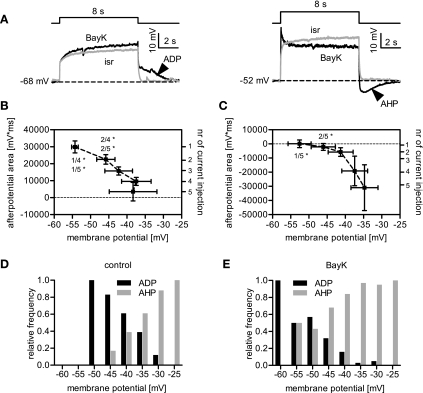
Fig. 3.
Dependency of afterpotentials on the stimulus amplitude. A: when neurons were depolarized for 8 s using five incremental current injections (in the presence of 500 nM TTX), ADPs followed the current pulses at milder depolarizations, whereas AHPs were elicited by strong stimulation. This is illustrated by sample traces recorded in the presence of either BayK (black traces) or isradipine (isr, grey traces), for a smaller (left) and a larger current-induced depolarization (right). Arrowheads highlight the afterpotentials. B and C: depolarizations induced by each incremental current step were grouped according to the pulse number (see y-axis on the right), and the x-axis values are depicted as means membrane voltage ± SE obtained in isradipine. The y-axis values (left axis) represent the afterpotential measured as area (mV·ms) ± SE in the respective depolarization group. B: data obtained from 7 cells displaying an ADP, showing that ADPs decrease in the course of growing depolarizations. C: data obtained from 7 cells developing an AHP with increasing depolarization. Statistical analysis in B and C was performed using Kruskal-Wallis one-way ANOVA, and pairs of data were compared using Dunns post hoc test (the numbers in the graph indicate the pairs that were tested against each other; *P < 0.05). D and E: relative-frequency distribution of afterpotentials plotted against the level of depolarization (evaluated in the presence of isradipine) for events occurring under control conditions (D) and BayK-induced events (E).
Interrelation between ADPs/bumps and AHPs-hyperpolarizing sags.
Our data suggest that LTCC activation typically leads to bumps and ADPs at lower levels of depolarizations and to sags and AHPs at stronger depolarization levels. To corroborate that response modes and afterpotentials are interrelated, we submitted their frequency distributions to a Spearman rank correlation analysis. For data obtained in the presence of BayK, an identical correlation coefficient rs of 0.95 was obtained for bump and ADP on the one hand, and for sag and AHP on the other hand, indicating positive correlations between these pairs. The correlation between bumps and ADPs could also be identified for control data (rs = 0.82), but due to their infrequent occurrence under control conditions, not for hyperpolarizing sags (data not shown).
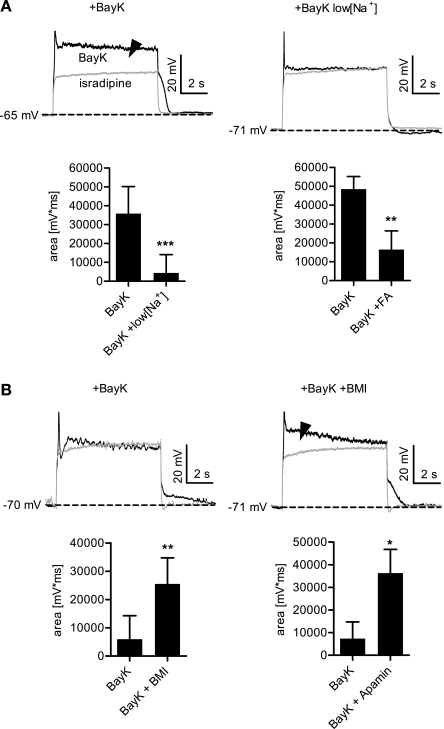
Since bumps correlated with ADPs we tested whether they were also mediated by Na+-conducting channels. Indeed, reduction of external sodium largely reduced bumps and gave the voltage responses a saglike appearance (see Fig. 4A). This finding is reflected in the decrease of the area (mV·ms) between the isradipine trace and the trace recorded in the presence of BayK (Fig. 4A). A similar reduction was seen when these experiments were repeated in the presence of 1 μM atropine, which indicates that this effect does not involve significant contribution of muscarinic receptor activation by the Na+ substitute choline (data not shown). Hence, bumps are mediated by Na+-conducting channels. A reduction in area was also obtained using the nonspecific CAN inhibitor flufenamic acid (Fig. 4A), which also reduced ADPs (data not shown). Since sags were found to correlate with AHPs, we tested whether sags were also mediated by KCa2.x channels by investigating the effects of BMI and apamin on this response mode. Indeed, both compounds reduced the hyperpolarizing sag obtained after BayK administration. As shown for BMI, this is reflected in the increase of the area (mV·ms) between the isradipine trace and the trace recorded in the presence of BayK (Fig. 4B). Similar results were obtained with apamin (Fig. 4B). Hence, hyperpolarizing sags are largely due to activation of KCa2.x channels.
Fig. 4.
Ion conductances responsible for de- and hyperpolarizing LTCC-mediated effects on membrane voltage. A: bumps are attenuated upon reduction of external Na+. The area between the response recorded in isradipine and the one recorded in BayK was evaluated in cells with a clearly discernible bump. An example is shown in the left traces, the arrowhead indicates the measured area. Thereafter, the recordings were repeated in low [Na+] solution as shown by right traces. The left bar graph summarizes the results of identical experiments on 12 cells. Application of low [Na+] solution largely reduced the bumps (by 88% on average). A qualitatively similar result was obtained with flufenamic acid (FA, bottom right), which reduced the bumps by 66% on average (n = 8). B: hyperpolarizing sags are attenuated by blockade of KCa2.x channels. Similar experimental approach as in A, except that cells with a clearly discernible hyperpolarizing sag were used and that a solution with 30 μM bicuculline methiodide (BMI) or 100 nM apamin was tested instead of low [Na+]. Bottom left summarizes the BMI results (n = 8). Application of BMI led to the appearance of an area or augmented the area between the response recorded in isradipine and the one recorded after application of BayK. Apamin had a similar effect, shown in bottom right (n = 7). ***P < 0.001, **P < 0.01, *P < 0.05, Wilcoxon signed-rank test.
Physiological implications.
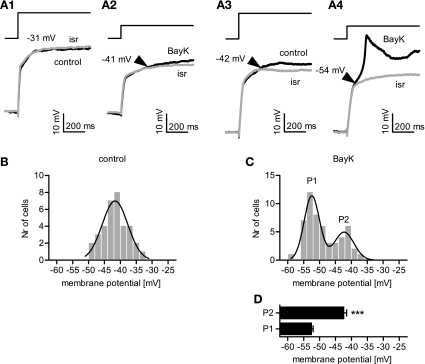
Overlaying and comparing the traces recorded in the presence of isradipine and those recorded under control conditions (35 cells) or in the presence of BayK (53 cells) allowed us to determine the activation threshold of LTCCs under current clamp conditions (Fig. 5A). It should be noted that under control conditions no LTCC-mediated effects were observed in seven cells, although the neurons were depolarized to at least −32 mV. However, LTCC activity was seen in the presence of BayK in these cells, hence their onsets were included in the analysis, but they were not available for the control data set. Onsets of DHP-sensitive depolarizations occurred within a voltage range of −49 to −34 mV under control and −59 to −37 mV when BayK was present. The distribution of onsets over the entire voltage range is shown in Fig. 5, B and C. The data obtained under control conditions could be satisfactorily fitted by a Gaussian function, which yielded a mean of onset of −41.7 mV. In contrast, the onset distribution in the presence of BayK could not be satisfactorily fitted by a single Gaussian function. Instead, a fit using the sum of two Gaussian functions was statistically preferred and yielded means of onset of −52.4 mV and of −42.3 mV, respectively. Hence, LTCCs operate over a broad voltage range and may be involved in a variety of physiological electrical signals, from subthreshold excitatory postsynaptic potentials to complex discharge patterns.
Fig. 5.
Current-pulse-induced depolarizations reveal differences in LTCC-activity onsets. A: recordings of the voltage response were performed under control conditions, in 3 μM BayK, and in 3 μM isr, respectively. Traces recorded under control or in BayK (black traces) were superimposed with the recording obtained in the presence of isr (grey traces) to identify the deflection where an active response starts to drift apart from the largely passive response (indicated by the arrowheads near the traces). A1–A4: sample traces showing the initial 800 ms of the voltage responses to current injections: it can be seen in A1 that a depolarization up to −31 mV did not reveal any active LTCC-mediated component under control, in that the control trace does not deviate from the recording made in the presence of isr. In the same neuron but in the presence of 3 μM BayK, a deflection occurs at a considerably lower level of depolarization, namely −41 mV (A2). In contrast, in another neuron, a depolarization to −42 mV (A3) already caused a deflection between the traces recorded under control conditions and after addition of isr; and in the presence of BayK the onset of LTCC activity was determined at −54 mV (A4). B and C: onsets of LTCC activity determined in this manner from a large number (Nr) of cells was subjected to 2 mV binning and is shown as frequency distribution for control data and data obtained in the presence of BayK. Under control (B), the distribution of LTCC onsets ranged from −49 mV to −34 mV (n = 35) and could be described by a single Gaussian curve with its peak at −41.7 ± 4.0 mV (r2 = 0.91). When 3 μM BayK was applied (C), the LTCC onset distribution was shifted to more negative membrane potentials ranging from −59 mV to −37 mV (n = 53) and could be described using a sum of two Gaussian functions with a peak P1 at −52.4 ± 2.4 mV and a peak P2 at −42.3 ± 3.1 mV (P2)(r2 = 0.92). D: onsets accounting for P1 and P2 were plotted against the membrane potential (n = 37 and 16, respectively). ***Significant difference between the two peaks P1 and P2 (P < 0.001, unpaired t-test). Results are given as means ± SE.
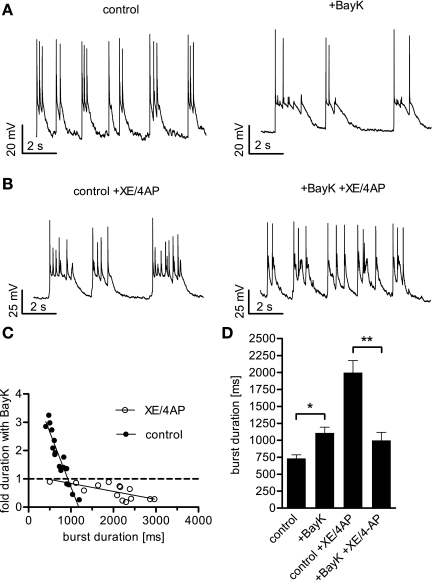
However, the data presented so far were obtained under conditions where discharge activity was reduced by blockade of TTX-sensitive Nav channels for a better resolution of LTCC-mediated effects. To see whether the findings obtained may be extended to normal neuronal electrical activities, we omitted TTX and studied the effect of LTCCs on hippocampal burst firing (Fig. 6A). In addition to intrinsic differences in burst duration, particularly long-lasting bursts were induced by coapplication of the potassium channel blockers XE 991 (XE) and 4-aminopyridine (4AP) (Fig. 6B). XE is a specific blocker of Kv7 channels, which give rise to excitability controlling M currents, whereas 4AP inhibits several repolarizing voltage-dependent K+ (Kv) channels (1, 22, 43). Indeed, on average, bursts lasted for 734 ± 52 ms under control conditions and were prolonged to 1,998 ± 179 ms by coapplication of XE and 4AP. Experiments on 17 cells revealed that in the case of unstimulated discharge activity application of BayK prolonged or shortened bursts, depending on the duration of these discharge events under control conditions (Fig. 6C). The turning point for the effect mode was at about 900 ms. In contrast, all long-lasting bursts obtained in the presence of XE/4AP (1–3 s durations) were shortened by BayK administration (n = 14). Taking together the results from all cells, BayK increased burst durations 1.5-fold (to 1,108 ± 88 ms) but shortened bursts by 50% (to 999 ± 118 ms) when XE/4AP was present (Fig. 6D). Hence, the effect of LTCC activation on neuronal electrical activity depended on the discharge pattern. Short events were prolonged, whereas longer lasting electrical activity was restrained.
Fig. 6.
Effect of LTCC activation on neuronal activity depends on the extent of depolarization. When BayK was added to neurons displaying a burst firing mode, the duration of the depolarization wave was increased or decreased, depending on the length of the burst before DHP application. A: recordings from a burst firing neuron under control conditions and in the presence of BayK reveal a prolonging effect of LTCC activation on the depolarization waves. B: recordings from a bursting neuron treated with the potassium channel blockers XE 991 (XE, 10 μM) and 4-aminopyridine (4AP, 100 μM) showing particularly long-lasting depolarization waves that were shortened after addition of BayK. C: plot of fold change induced by BayK against burst duration in otherwise untreated cells (solid circles) and in cells after coapplication of XE and 4AP (open circles). The solid lines were generated by linear regression analysis yielding r2 = 0.89 for data obtained under control conditions and r2 = 0.50 for data obtained in the presence of XE/4AP. The intersection of the regression line for control data with the dashed line representing unaltered burst duration is at 924 ms. D: summary of the overall enhancing effect of BayK on burst durations in otherwise untreated neurons, the prolonging effect of XE/4AP, and the shortening effect of BayK on burst discharge in the presence of these potassium channel blockers. Bars represent the means ± SE of the median burst duration. The n value was 17 cells for burst activity under control conditions and 14 cells for burst activity in the presence of XE/4AP. * and **Statistically significant difference of P < 0.05, and P < 0.01, respectively, as determined by a Mann-Whitney test.
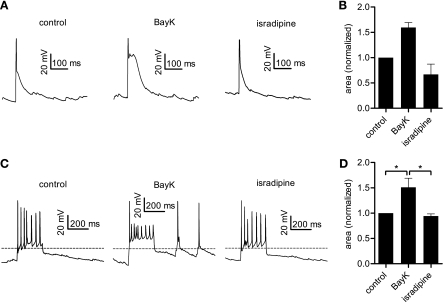
In the course of these TTX-free experiments, short unprovoked depolarizing events (probably spontaneous excitatory postsynaptic potentials) were routinely observed. When BayK was applied, such short depolarizations appeared prolonged when compared with signals recorded under control conditions. An example is shown in Fig. 7A. We evaluated this effect for three cells with comparable events (duration under control conditions ≤100 ms) and determined an average increase in area-below-the curve of 60% induced by BayK and a decrease of 33% in the presence of isradipine, both compared with control (Fig. 7B). We explored this effect further by stimulating neurons with a brief (250 ms long) current injection, again in the absence of TTX. Example traces are shown in Fig. 7C. Administration of BayK augmented these brief depolarizations, and the effect could be best evaluated as the rise in the area above a virtual baseline (set to −50 mV) (Fig. 7D), amounting to an 51% increase, on average. In the presence of isradipine, the area-above-baseline fell to 6% below the control value (n = 5). This again highlights the enhancing effect of LTCC activity in short excitatory events.
Fig. 7.
Brief electrical events are enhanced by LTCC activation. A: original traces showing solitary short depolarizations (probably spontaneous excitatory postsynaptic potentials) that where consecutively observed in one neuron under control conditions and in the presence of 3 μM BayK or 3 μM isradipine, as indicated. B: analysis of similar recordings from three different neurons. The bars depict the area under the initial 100 ms of the depolarizing event for the three conditions. Data obtained in the presence of the LTCC modulators were normalized to the corresponding control value and are depicted as means ± SE. C: original traces show the effect of BayK and isradipine application on brief depolarizing events elicited by 250-ms long current injections. D: area above −50 mV (indicated by the dashed line in C) is depicted normalized to the corresponding control value. Results are given as means ± SE (n = 5). *Statistically significant difference of P < 0.05.
DISCUSSION
LTCCs are considered as important elements in synaptic plasticity and neuronal excitability (5). In addition to long-term effects, LTCC-mediated Ca2+ influx can also affect membrane voltage and discharge properties in an instantaneous manner by providing depolarizing cation influx and via activation of Ca2+-dependent conductances (30, 31). So far, studies have either focused on LTCC-mediated after-depolarizing or after-hyperpolarizing conductances. However, the interplay of these conductances, in particular with respect to LTCC activation, has not been addressed. The present study demonstrates that these opposing mechanisms occur in the same neuron and depend on the intensity and duration of depolarizations.
Methodological considerations.
We performed current clamp recordings on hippocampal neurons in primary culture to record membrane voltage. The perforated patch method was employed to keep disturbances of the intracellular milieu at a minimum. DHPs were used to modulate the availability of LTCC channels. BayK acts as an LTCC agonist by induction of mode 2-gating (long single channel opening) (33). Isradipine was used as antagonist because of its relatively high potency and low potency difference between the two neuronal LTCC isoforms Cav1.2 and Cav1.3 (8.5-fold, as compared, for example, with nimodipine where the difference is 20-fold) (18, 41). Despite this difference, the concentration of 3 μM can be assumed to block both Cav1.2 and Cav1.3 channels, especially at potentials that would activate LTCCs. Although nonspecific effects were reported for various DHPs (7), these often require relatively high concentrations and should be negligible in the case of 3 μM isradipine. Moreover, we identified LTCC effects as being inhibited by isradipine and augmented by BayK. Hence, the responses that were elicited (or augmented) by BayK and inhibited by isradipine can be bona fide considered as LTCC-dependent events. In addition to warrant specificity, the use of BayK can be envisaged to model effects of Ca2+-dependent facilitation, which is also characterized by long-channel openings, e.g., mode-2 gating (10).
It should be noted that this study was performed on cells originally dissociated from whole hippocampus, which may thus represent various hippocampal neuronal types, e.g., pyramidal cells, granule cells, and interneurons originating from all hippocampal divisions (e.g., CA1, CA3, and dentate gyrus). Although data were acquired form various types of neurons (this heterogeneity may in fact explain the noted differences in other conductances, for example, outwardly rectifying potassium conductances), only three major LTCC-mediated response modes could be exemplified. This observation, together with the fact that these response modes are not strictly separated but can occur in a single neuron type (in dependence of the stimulation strength) indicates that general somatodendritic mechanisms are more relevant to the role of LTCC in excitability than for example morphological or functional peculiarities (e.g., type of neurotransmitter released at the terminals).
In several experiments, long-lasting stimulation protocols of up to 10-s durations were applied. This was done solely to let effects grow to optimal perceptibility; although typically evident early on after the beginning of the stimulus, bumps fully developed over a time scale of seconds, and hyperpolarizing sags and AHPs reached maximum values at 8 s long stimuli. Such stimuli may well exceed depolarizing events occurring under physiological conditions in a neuronal cell. However, it should be noted that while long-duration protocols were chosen to clearly demonstrate the various response modes, the turning point from excitatory to inhibitory coupling, as revealed by changing the current pulse duration, was not far from 1 s and was diminished well below 1 s after potentiation of LTCCs (see Fig. 2B). This timeframe can certainly be envisaged to lie within a physiologically relevant range. In line with this notion, hippocampal burst-firing discharge activity was modulated by LTCC activation in a manner predicted by the current pulse experiments (compare Fig. 2B and Fig. 6C). Hence, the dynamic interplay of excitatory and inhibitory coupling of LTCCs appears well suited to contribute to the shaping of physiological neuronal events.
Our experiments using ion exchange experiments and pharmacological tools indicate that LTCC-dependent response modes can largely be explained by an interplay of LTCC-, CAN channel- and KCa2.x channel-mediated currents. However, contributions of other voltage-dependent conductances are very likely and may in fact be responsible for some variability in the respective responses among the neurons investigated. Clear evidence of outwardly rectifying conductances that limited the experimentally induced depolarizations has already been noted. Nevertheless, we obtained no evidence that other conductances (e.g., those modulated solely by voltage changes) were present in an extent capable of generating profound secondary effects that could obscure the effects that were primarily induced by LTCC activation. For example, depolarizations appeared rather uniformly shaped, closely resembling purely passive responses to the current injections, when both TTX-sensitive sodium channels and LTCCs were blocked (see the traces recorded in the presence of isradipine in Figs. 1, 3, and 4). Importantly, this was true even for the strongest levels of stimulation, e.g., depolarizations toward −25 mV (see for example Fig. 1, right traces). This argues against complex contributions of other than LTCC or LTCC-coupled conductances.
Diametrical stimulus dependency of ADP and AHP.
Correlation analysis indicated that bumps and ADPs, as well as sags and AHPs, may be mechanistically linked. Evidence that this is correct comes from the observation that both sags and AHPs require apamin-sensitive channels and that both bumps and ADPs are dependent on (TTX insensitive) Na+-conducting channels. We suggest that the afterpotentials are decaying representations of the conductances that are responsible for the different response modes. This notion is supported by their identical dependence on stimulation strength.
Short and/or small depolarizations favored the appearance of ADPs, whereas long and/or large depolarizations elicited AHPs. Furthermore, the stimulus dependency could be altered by augmentation of Ca2+ influx with BayK, and ADP and AHP were affected by changing the Ca2+ gradient and the concentration of the DHP agonist, respectively. Collectively, the data suggest that ADPs can be induced by less LTCC activity than that required for generation of an AHP. What could be the mechanistic basis for an alternate Ca2+ dependence of ADPs and AHPs? On the one hand, Ca2+-responsive elements of channels underlying ADP and AHP may have differing Ca2+ affinities. An alternative possibility was that the spatial relations between LTCCs and these downstream channels are different. Whereas inhibition by apamin, BMI, and UCL 1684 indicates that AHPs are mediated by SK channels, the situation in the case of ADPs is less clear; in this study ADPs were abolished by reduction of external sodium ions. Hence, the channels mediating these afterpotentials are permeable to Na+ but not Ca2+ or other cations, and they were sensitive to changes of the availability of Ca2+, indicating that they are activated by cytoplasmic Ca2+ rises. Moreover, ADPs were sensitive to flufenamic acid. These attributes indicate an involvement of CAN channels, which have been characterized primarily in electrophysiological terms, but the molecular identity of these channels is still unknown (21, 23). Hence, no comparison can be made between Ca2+ affinities of KCa2.x channels and the channels underlying the CAN current, and data regarding the localization of these channels (in particular with respect to LTCCs) are also unavailable. Consequently, the reason for an alternate Ca2+ dependence of ADPs and AHPs remains elusive at present.
An apamin-sensitive slow AHP in hippocampal neurons.
In hippocampal neurons as well as many other neurons, AHPs come in three different kinetically defined types: fast (fAHP), medium (mAHP), and slow AHP (sAHP), their induction being largely dependent on the excitation pattern. Whereas fAHPs are thought to be due to the activity of big conductance KCa channels (KCa1.1), the mAHP was reported to be apamin sensitive and thus mediated by small conductance KCa channels (14, 34). Finally, the molecular identity of channels underlying the sAHP remained obscure (4). Hence, it may appear somewhat surprising that long-lasting AHPs were identified to be sensitive to apamin in this study. Probably a finding in motoneurons provides a clue to explain this discrepancy: in addition to an apamin-sensitive mAHP that is induced via Ca2+-influx through N and/or P/Q-type Ca2+ channels colocalized with SK channels, LTCC Ca2+-influx mediates a slow apamin-sensitive SK current, and it was suggested that this is because SK channels were not as spatially close to the to LTCC currents in these neurons (24). A similar situation might exist in hippocampal neurons: in CA3 pyramidal neurons LTCC-mediated Ca2+-influx was found to activate long-lasting apamin-sensitive, hence SK-mediated potassium currents under ischemic conditions (39). Therefore one hypothesis would be the following: SK channels are rapidly activated by Ca2+-influx via N and/or P/Q-type voltage-gated Ca2+ channels (VGCCs) to mediate an mAHP. Ca2+-influx via LTCCs activates SK channels less effectively, probably because LTCCs are not so closely associated. Whenever other HVA-VGCCs were active, the activation of SK channels may be rapid, generating an mAHP. But if LTCCs are preferentially activated (e.g., by depolarizations up to −25 mV, as in this study, rather than above 0 mV), a slow apamin-sensitive AHP may be evoked. The question remains then, why apamin-insensitive sAHPs were only observed sparsely in our experiments, although their presence in hippocampal neurons has been repeatedly documented (2, 9, 19). It should be noted that the contribution of LTCC-mediated Ca2+-influx to the apamin-insensitive AHP may not be particularly prominent at least in some preparations. Shah and Haylett (36), for example, reported that about 70% of such AHPs are induced via non-LTCCs. Hence, considering that we activated only a selection of calcium channels (preferentially, if not exclusively, LTCCs) rather than the whole battery of VGCCs, only low levels of apamin-insensitive AHPs could be expected. Indeed, as can be seen in Fig. 2D, the longest pulses did induce a residual AHP even in the presence of apamin.
Implication of a bimodal regulation of neuronal activity by LTCCs.
What could be the role of the excitation-dependent activation of depolarizing and hyperpolarizing Ca2+-dependent conductances? Excitatory coupling was preferentially evoked by weak stimulation and can be envisaged to play a role in burst initiation and/or in carrying the depolarizing wave underlying burst discharge. Since LTCCs can activate at relatively mild depolarizations (see Fig. 5), excitatory LTCC coupling may also play a role in shaping excitatory postsynaptic potentials (EPSPs). Inhibitory coupling, on the other hand, was preferentially evoked by strong stimulation and may therefore play a role in burst termination, or more generally, act as an excitation break to avoid excess depolarization and probably concomitant Ca2+ overload. In such a scenario, LTCCs together with coupling to Ca2+-dependent conductances would operate as a bimodal regulator. Since both, KCa2.x channels and CAN channels, have been found to be tightly controlled by neurotransmitter systems (11, 26, 27, 34, 40), this bimodality would be subject to physiological alterations. Importantly, the stimulus-dependent activation of excitatory or inhibitory LTCC-mediated effects was shifted by application of BayK to weaker excitation, for example, in that inhibitory coupling prevailed at comparably shorter pulse durations (see Fig. 1B). BayK was used in this study to pharmacologically upregulate LTCC activity (mode 2 gating). However, it should be noted that a similar functional upregulation may take place physiologically. For example, mode 2 gating was also elicited by repetitive or strong membrane depolarization and neurotransmitter receptor activation (15, 16, 44). Hence, the voltage range of LTCC activity as well as the coupling mode may be dynamically modulated. In primary culture, hippocampal neurons are devoid of extrinsic innervation. BayK may thus depict the range of actions LTCCs can exert in mode 2 gating. When mode 2 gating is down, LTCC activation during physiological stimuli will preferentially have excitatory effects, e.g., driving bursts and promoting EPSPs. However, during long-lasting depolarizing voltage shifts, e.g., those that are thought to occur in neurons under ischemic conditions (8) or under conditions promoting plateau potentials (12, 20, 38), the coupling of LTCCs to KCa2.x channels may be activated, potentially supporting the counteraction of excitotoxicity (39).
On the other hand, when mode 2 gating is induced, LTCC activity will be excitatory only in initial phase of depolarizing stimuli and will trigger a hyperpolarizing drive thereafter, eventually in concert with other VGCCs. With regard to Cav2.1 and Cav2.2 channels, we hypothesized above that coupling of Ca2+ influx via these channels to KCa2.x channels occurred more rapidly than the one provided by LTCCs. If that was correct, then the LTCC-mediated activation of KCa2.x channels may be occluded. However, considering probable spatial differences, it was also possible that the contribution of LTCCs to KCa2.x channels serves to shape the AHP (e.g., increasing its duration) and/or to carry it to certain dendritic compartments. Alternatively, because of activation already at moderate depolarizations, LTCC-induced apamin-sensitive AHPs may operate at voltages where other VGCCs are not activated (17, 18, 24, 42). It will be interesting to test these potential roles in the future.
Conclusion.
We report on profound LTCC-mediated effects on membrane voltage and provide evidence that weak stimuli favor excitatory LTCC-mediated events, whereas strong stimuli induce LTCC-mediated deceleration of excitation. The abundance of LTCCs activating at relatively hyperpolarized potentials may enable LTCCs to operate even in modest synaptic stimulation and to contribute for example to the depolarizing wave of burst discharge activity. Additionally, LTCCs in combination with inhibitory Ca2+-dependent conductances can be envisaged to provide an excitation break. The excitation-dependent activation of Ca2+-dependent conductances by neuronal L-type calcium channels may have important implications for the usability of LTCC inhibitors in the treatment of various forms of abnormal neuronal electrical activities.
GRANTS
This work was supported by a grant from the Austrian Science Fund (FWF) to H. Kubista.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Alexandra Koschak (University of Innsbruck, Austria) for helpful comments during the preparation of the manuscript.
REFERENCES
- 1. Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC). Br J Pharmacol 153, Suppl 2: S1–S209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreasen M. Inhibition of slow Ca(2+)-activated K(+) current by 4-aminopyridine in rat hippocampal CA1 pyramidal neurones. Br J Pharmacol 135: 1013–1025, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boehm S, Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci 17: 4066–4075, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24: 5301–5306, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calin-Jageman I, Lee A. Ca(v)1 L-type Ca2+ channel signaling complexes in neurons. J Neurochem 105: 573–583, 2008 [DOI] [PubMed] [Google Scholar]
- 6. D'Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci 23: 935–944, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Das P, Bell-Horner CL, Huang RQ, Raut A, Gonzales EB, Chen ZL, Covey DF, Dillon GH. Inhibition of type A GABA receptors by L-type calcium channel blockers. Neuroscience 124: 195–206, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther 105: 229–266, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Disterhoft JF, Wu WW, Ohno M. Biophysical alterations of hippocampal pyramidal neurons in learning, ageing and Alzheimer's disease. Ageing Res Rev 3: 383–406, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol 2: 173–177, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 33: 615–624, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci 16: 4113–4128, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gruol DL, Netzeband JG, Quina LA, Blakely-Gonzalez PK. Contribution of L-type channels to Ca2+ regulation of neuronal properties in early developing purkinje neurons. Cerebellum 4: 128–139, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol 566: 689–715, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirano Y, Yoshinaga T, Murata M, Hiraoka M. Prepulse-induced mode 2 gating behavior with and without beta-adrenergic stimulation in cardiac L-type Ca channels. Am J Physiol Cell Physiol 276: C1338–C1345, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Hoogland TM, Saggau P. Facilitation of L-type Ca2+ channels in dendritic spines by activation of beta2 adrenergic receptors. J Neurosci 24: 8416–8427, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joux N, Chevaleyre V, Alonso G, Boissin-Agasse L, Moos FC, Desarmenien MG, Hussy N. High voltage-activated Ca2+ currents in rat supraoptic neurones: biophysical properties and expression of the various channel alpha1 subunits. J Neuroendocrinol 13: 638–649, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J Biol Chem 276: 22100–22106, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Krause M, Offermanns S, Stocker M, Pedarzani P. Functional specificity of G alpha q and G alpha 11 in the cholinergic and glutamatergic modulation of potassium currents and excitability in hippocampal neurons. J Neurosci 22: 666–673, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuzmiski JB, MacVicar BA. Cyclic nucleotide-gated channels contribute to the cholinergic plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci 21: 8707–8714, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109: 397–407, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Lawrence JJ, Saraga F, Churchill JF, Statland JM, Travis KE, Skinner FK, McBain CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci 26: 12325–12338, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci 27: 6531–6541, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lirk P, Poroli M, Rigaud M, Fuchs A, Fillip P, Huang C.Y, Ljubkovic M, Sapunar D, Hogan Q. Modulators of calcium influx regulate membrane excitability in rat dorsal root ganglion neurons. Anesth Analg 107: 673–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magistretti J, Ma L, Shalinsky M.H, Lin W, Klink R, Alonso A. Spike patterning by Ca2+-dependent regulation of a muscarinic cation current in entorhinal cortex layer II neurons. J Neurophysiol 92: 1644–1657, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Maingret F, Coste B, Hao J, Giamarchi A, Allen D, Crest M, Litchfield DW, Adelman JP, Delmas P. Neurotransmitter modulation of small-conductance Ca2+-activated K+ channels by regulation of Ca2+ gating. Neuron 59: 439–449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKinney BC, Sze W, Lee B, Murphy GG. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol Learn Mem 92: 519–528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25: 9883–9892, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci 19: 7309–7316, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol 68: 2100–2109, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Nguyen T, Di Giovanni S. NFAT signaling in neural development and axon growth. Int J Dev Neurosci 26: 141–145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nowycky MC, Fox AP, Tsien RW. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc Natl Acad Sci USA 82: 2178–2182, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pedarzani P, Stocker M. Molecular and cellular basis of small–and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci 65: 3196–3217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ping HX, Shepard PD. Blockade of SK-type Ca2+-activated K+ channels uncovers a Ca2+-dependent slow afterdepolarization in nigral dopamine neurons. J Neurophysiol 81: 977–984, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Shah M, Haylett DG. Ca(2+) channels involved in the generation of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurons. J Neurophysiol 83: 2554–2561, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Striessnig J, Hoda JC, Koschak A, Zaghetto F, Mullner C, Sinnegger-Brauns MJ, Wild C, Watschinger K, Trockenbacher A, Pelster G. L-type Ca2+ channels in Ca2+ channelopathies. Biochem Biophys Res Commun 322: 1341–1346, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki T, Kodama S, Hoshino C, Izumi T, Miyakawa H. A plateau potential mediated by the activation of extrasynaptic NMDA receptors in rat hippocampal CA1 pyramidal neurons. Eur J Neurosci 28: 521–534, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Tanabe M, Mori M, Gahwiler BH, Gerber U. Apamin-sensitive conductance mediates the K(+) current response during chemical ischemia in CA3 pyramidal cells. J Neurophysiol 82: 2876–2882, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci 18: 3155–3166, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21: 5944–5951, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci 20: 1–13, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol 95: 3480–3495, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci USA 87: 753–757, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. Ca1.2 and CaV1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur J Neurosci 23: 2297–2310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.