Abstract
Diabetes is a profound disease that results in a severe lack of regulation of systemic salt and water balance. From our earlier work on the endocrine regulation of salt taste at the level of the epithelial sodium channel (ENaC), we have begun to investigate the ability of insulin to alter ENaC function with patch-clamp recording on isolated mouse taste receptor cells (TRCs). In fungiform and vallate TRCs that exhibit functional ENaC currents (e.g., amiloride-sensitive Na+ influx), insulin (5–20 nM) caused a significant increase in Na+ influx at −80 mV (EC50 = 7.53 nM). The insulin-enhanced currents were inhibited by amiloride (30 μM). Similarly, in ratiometric Na+ imaging using SBFI, insulin treatment (20 nM) enhanced Na+ movement in TRCs, consistent with its action in electrophysiological assays. The ability of insulin to regulate ENaC function is dependent on the enzyme phosphoinositide 3-kinase since treatment with the inhibitor LY294002 (10 μM) abolished insulin-induced changes in ENaC. To test the role of insulin in the regulation of salt taste, we have characterized behavioral responses to NaCl using a mouse model of acute hyperinsulinemia. Insulin-treated mice show significant avoidance of NaCl at lower concentrations than the control group. Interestingly, these differences between groups were abolished when amiloride (100 μM) was added into NaCl solutions, suggesting that insulin was regulating ENaC. Our results are consistent with a role for insulin in maintaining functional expression of ENaC in mouse TRCs.
Keywords: salt intake
taste receptor cells (TRCs) must recognize a vast array of different chemical structures, ranging from those that are small and ionic to compounds with complex tertiary structures. This chemosensory ability allow animals to distinguish compounds that may be either harmful to the organism or, alternatively, necessary for nutritional needs. In mammals, it is well established that salt taste transduction is mediated by sodium (Na+) ions through the apical amiloride-sensitive epithelial sodium channel (ENaC). The influx of Na+ ions directly depolarizes the taste cell, eventually leading to the release of neurotransmitter onto the afferent nerve fibers (10, 12). ENaC has been characterized both in TRCs and in other transporting epithelia, and it shares a number of common features across tissue types. Similarities include small conductance (∼5 pS), Na+ ∼ Li+ >> K+ ion selectivity, regulation by extracellular Na+ (self-inhibition) and intracellular Na+ (feedback inhibition), and regulation by hormones. These functional similarities between ENaC in the taste system and other transporting epithelia extend to the molecular level. Experiments in different rodent species have shown that ENaC channels expressed in TRCs have a high sequence homology with those channels expressed in other organs. Thus, in many regards, ENaC appears similar across organ types (11, 15, 23, 27, 39).
The regulation of ENaC is essential for salt and water balance in various Na+-transporting epithelia. Current evidence in taste cells and other transporting epithelia has demonstrated that ENaC expression and/or function may be altered by a number of hormones (e.g., aldosterone, vasopressin, atrial natriuretic peptide). Aldosterone (Aldo) and arginine8-vasopressin (AVP) appear to increase ENaC-mediated currents, while atrial natriuretic peptide and, perhaps, oxytocin reduce these Na+ currents. Hormones like Aldo and AVP can increase Na+ influx through ENaC by increasing the channel's open probability and/or decreasing the turnover of ENaC in the cell membrane, thus increasing functional ENaC expression (13, 19, 26). Additionally, the importance of ENaC regulation is well understood for sodium balance, blood pressure, and extracellular fluid volume (46).
Recently, research in kidney epithelia has suggested that insulin contributes to Na+ movement via ENaC. In general, both ENaC open probability and membrane expression of ENaC in the cell membrane can be increased by insulin stimulation acting through insulin/IGF-I receptors. Insulin-mediated Na+ reabsorption is believed to occur through the phosphoinositide 3-kinase OH (PI3-kinase) signaling pathway (38, 45). Activation of PI3-kinase by insulin leads to phosphorylation of two downstream signaling cascades: 1) a PI3-kinase signaling pathway leading to the phosphorylation of ENaC and/or 2) the activation of phosphoinositide-dependent kinase 1 (PDK1). Thus, synthesis of phosphatidylinositol 4,5-bisphosphate [PtdIns(3,4)P2 or PIP2]/phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3 or PIP3] by PI3-kinase directly affects ENaC activity and open probability (24, 40, 45). In addition, PtdIns(3,4,5)P3 phosphorylates PDK1 and then activates serum- and glucocorticoid-regulated kinase 1 (SGK1). SGK1 is considered a key regulator of Na+ reabsorption via ENaC in kidney epithelia since several hormones such as insulin, Aldo, and AVP regulate ENaC through this pathway (8, 47). SGK1 function may increase either the open probability of apical ENaCs or the number of active channels in the membrane, although the mechanism of ENaC trafficking by insulin-SGK1-mediated responses is not well understood (21, 25, 28).
Little is known about the importance of insulin in the peripheral gustatory system. In the present study, we use a combination of whole cell patch-clamp recording and ratiometric functional Na+ imaging to determine whether insulin influences Na+ transport via ENaC in taste cells. Our results showed that insulin stimulates Na+ movement through amiloride-sensitive channels (i.e., ENaC) in these cell-based assays. In agreement with earlier reports in other transporting epithelia, insulin/ENaC-mediated responses in the peripheral gustatory system occurred via PI3-kinase-mediated pathways and its phospholipid products (40, 48). Following our functional and molecular studies, we attempted to investigate the role of insulin in the regulation of salt taste. Our results showed that insulin modulation of ENaC activity is extended to the animals' ability to detect NaCl. These results reveal a novel insulin pathway that modulates ENaC function and salt taste in mouse TRCs.
METHODS
All experiments were performed on adult (2–4 mo old) male C57BL/6 mice that were maintained on a 12:12-h light-dark cycle with normal chow and water provided ad libitum. Mice lacking sgk (sgk−/− mice) were provided by Dr. A. Náray-Fejes-Tóth (9). All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Utah State University and were performed in accordance with American Veterinary Medical Association guidelines.
Isolation of Taste Receptor Cells
Individual taste buds were isolated from mouse tongues following protocols used in early reports (2, 6). Briefly, tongues were removed and immediately immersed in Tyrode's solution containing (in mM) 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10 glucose, and 10 Na+ pyruvate (osmolarity, 310 mosM). The pH was adjusted to 7.4 with NaOH. The anterior portion of the tongue containing the fungiform papilla was injected between the muscle layer and the lingual epithelium with ∼0.15 ml of physiological saline (Tyrode's) containing a mixture of collagenase A (1.1 mg/ml; Roche Applied Science, Indianapolis, IN), dispase II (2.4 mg/ml; Roche Applied Science), and trypsin inhibitor (1 mg/ml; type I-S; Sigma Chemical, St. Louis, MO). Approximately 0.1 ml of the same enzyme solution was also used to inject the area surrounding the circumvallate papilla. The injected tongue was incubated in Tyrode's solution and bubbled with O2 for 45 min. The lingual epithelium was then peeled from the underlying tissue and pinned out in Tyrode's in a Sylgard-lined petri dish with the mucosal side facing down. The epithelium was then incubated for 7 min with the same enzyme cocktail. Following the incubation, the epithelium was incubated in Ca2+-Mg2+-free Tyrode's containing 2 mM BAPTA (Invitrogen, Eugene, OR) for 5 min. Amiloride (10 μM; Sigma Chemical) was added to all solutions to help protect against enzymatic degradation of ENaCs (11). Taste buds were removed from the epithelium using a large bore (∼150–200 μm) pipette and plated either onto a charged microscope slide in a Tyrode's-containing Sylgard ring for patch-clamp recording or onto a Cell-Tak-coated coverslip in a laminar flow perfusion chamber for functional imaging. For isolation of single taste cells, this procedure was slightly modified by an additional incubation in Ca2+-Mg2+-free Tyrode's for 10 min before the second enzyme treatment.
Patch-Clamp Solutions and Recording Conditions
Extracellular saline solution (Tyrode's) contained (in mM) 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 10 Na+ pyruvate (osmolarity, 310 mosM). The pH was adjusted to 7.40 with NaOH. Na+-free solution was made by replacing NaCl with equimolar N-methyl-d-glucamine (NMDG; a large impermeant cation) in Tyrode's solution. Amiloride (30 μM) solutions were made up in normal Tyrode's. Insulin (1, 3, 5, 7.5, 10, and 20 nM) from porcine pancreas (Sigma) was dissolved in double-distilled H2O (ddH2O) at a concentration of 10 mM and then diluted to its final concentrations. To establish conditions whereby Na+ was the only ion contributing to the whole cell current through ENaC, cells were held at −80 mV and intracellular and extracellular solutions were used that set the chloride equilibrium potential (ECl) and potassium equilibrium potential (EK) at ∼−80 mV. In this experiment, recording pipettes were filled with a solution containing (in mM) 140 K-gluconate, 1 CaCl2, 2 MgCl2, 10 HEPES, 11 EGTA, and 3 ATP. The pH and osmolarity were adjusted to 7.2 and ∼310 mosM, respectively, with KOH. This low intracellular Cl− (10 mM) helped to eliminate most of the inward Cl− current, which facilitates the analysis of insulin-activated ENaC current and set the ECl near −80 mV.
Individual fungiform TRCs were recorded using conventional whole cell patch clamp. Patch pipettes were pulled to a resistance of 5–8 MΩ when filled with intracellular solution. Series resistance and capacitance were compensated optimally before recording. Currents were recorded in the presence and absence of test solutions in a continuous (gap-free) recording mode. The holding potential in all experiments was −80 mV. Current data were recorded and command potentials were delivered using pClamp software (version 10). This software was interfaced with an AxoPatch 200B amplifier and Digidata 1322A data acquisition system (Molecular Devices, Sunnyvale, CA). Current-time relationships (I-T curve) were used to determine whether test solutions significantly altered amiloride-sensitive currents in TRCs.
Functional Sodium (Na+) Imaging
Functional imaging of taste receptor cells was carried out on cells loaded with a Na+-sensitive dye, sodium-binding benzofuran isophthalate-acetoxymethyl ester (SBFI-AM; Invitrogen). Single taste cells were isolated as described above and plated onto charged coverslips attached to a laminar flow perfusion chamber (RC-25F Warner Instruments, Hamden, CT). TRCs were loaded with ∼4 μM SBFI-AM in Hanks' buffer salt solution with HEPES, sodium pyruvate, 1% pluronic acid F-127 (Invitrogen), and 2% fetal bovine serum for 60 min. The cells were perfused with Na+-free Tyrode's containing (in mM) 140 NMDG, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 10 glucose, and 10 Na+ pyruvate, adjusted to pH 7.4 with HCl. Increases in intracellular Na+ were recorded in Tyrode's solution, with and without insulin (20 nM) and/or amiloride (30 μM). The PI3-kinase inhibitor LY294002 (10 μM) and its inactive analog LY303511 (10 μM) were prepared from a stock solution of 30 mM (CalBiochem, San Diego, CA). Wortmannin (0.05 and 1 μM) was prepared from a 2 mM stock solution (Sigma). All inhibitors were diluted in Tyrode's solution and made fresh daily before use. Data collection and analyses were recorded by InCyt High Speed I/M imaging system (Intracellular Imaging, Cincinnati, OH). Briefly, images were acquired with a monochrome integrating charge-coupled device camera through a ×40 oil immersion objective lens of an inverted Nikon TE-2000s microscope. Excitation wavelengths of 340 nm and 380 nm were emitted by a Benthan FGS 150 fast changing monochromator (Intracellular Imaging) with an emission wavelength ∼510 nm. Images obtained were captured every 3 s by InCyt Im2 software (Intracellular Imaging). The SBFI ratio (340/380) was used to determine whether test solutions significantly altered Na+ influx on TRCs. Data analyses were carried out by measuring the area under the curve of the SBFI ratio in the presence and/or absence of both amiloride and/or insulin using Origin software (version 7; Northampton, MA).
RT-PCR
First-strand cDNA was synthesized using the iScript RT Kit (Bio-Rad, Hercules, CA). The maximum volume of taste RNA or 50 ng of kidney RNA was used for the reaction with the total volume being 20 μl. Reactions were also set up in which the reverse transcriptase enzyme was omitted as a control to detect genomic DNA contamination. After first-strand synthesis, 1 μl of cDNA was added to a PCR reaction mixture containing final concentrations of 50 mM KCl, 10 mM Tris·HCl (pH 8.3), 2.5 mM Mg2+, 200 μM dNTPs, ∼500 nM forward and reverse primers, and 1.25 units Taq polymerase. PCR products were amplified using an initial 5-min denaturation step followed by 40 cycles of a 3-step PCR (30-s denaturation at 95°C, 30 s annealing at optimal temperature, and 45 s extension at 72°C), and concluding with a 7-min final extension step. Amplified sequences were visualized by electrophoresis in 2% agarose gels using 1× TAE buffer (40 mM Tris-acetate and 1 mM EDTA). Primer sequences, accession numbers, expected product sizes, and corresponding nucleotide sequences are shown in Table 1. Purification of PCR products for sequencing was performed using the QIAquick PCR purification kit (Qiagen, Valencia, CA). Sequences were determined by the dye-terminator method using an ABI Model 3100 Automatic Sequencer (Foster City, CA). Partial sequences for each product were examined using the BLAST 2.0 search engine (National Center for Biotechnology Information;http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Table 1.
Nucleotide sequences for primers in the RT-PCR assays
| Target | GenBank Accession No. | Sense Primer/Antisense Primer | Corresponding Nucleotide Sequence |
|---|---|---|---|
| IR | NM_010568 | 5′-CTT TGG GAA ATC ACT AGC TTG-3′ | 3694–3715 |
| 5′-CCT TGT TCT CCT CGC TGT AG-3′ | 3929–3949 | ||
| IRS-1 | NM_010570 | 5′-CGC AAC TGC CGA AGA TTC-3′ | 3311–3329 |
| 5′-CCT ATT CTG CCC AAC TCA ACT-3′ | 3781–3802 | ||
| IRS-2 | NM_001081212 | 5′-GCG GAG ACA ATG ACC AGT ATG T-3′ | 2423–2445 |
| 5′-TCT TGG GCT CTG TGG GTA GA-3′ | 2699–2719 | ||
| SGK1 | NM_011361 | 5′-TAC CTT CTG TGG CAC GC-3′ | 833–849 |
| 5′-GCC GTA GAG CAT CTC ATA CA-3′ | 928–947 |
IR, insulin receptor; IRS-1, insulin receptor substrate 1; IRS-2, insulin receptor substrate 2; SGK-1, serum- and glucocorticoid-regulated kinase-1.
Animal Model of Acute Hyperinsulinemia
Male, 2- to 4-mo-old C57BL/6 mice from Charles River Laboratories (Wilmington, MA) were administered either vehicle or insulin (Novolin; Novo Nordisk, Princeton, NJ) at 0.75 U/kg body wt in sterile 0.9% NaCl as a single intraperitoneal injection. Both insulin-treated (n = 40) or vehicle-treated (n = 40) mice received an injection 15 min before the start of behavioral testing. At the end of the behavioral assay, blood glucose (mg/dl) was measured with a glucometer (BD Biosciences, San Diego, CA) using blood obtained from the saphenous vein using techniques previously described (18).
Behavioral Experiments
Lickometer training.
All behavioral experiments were performed using a computer-controlled stimulus delivery and a lick monitoring station (“Davis Rig”; model MS-180, Dilog Instruments, Tallahassee, FL). Mice were trained and tested under 22.5 h of water access restriction. To prevent excessive body mass losses, all animals received 1 h of water in their home cage after each section. Under this regimen, the mice were able to maintain a body mass between 85% and 90% of their initial body weight. Training consisted of 2 days: First, the mice were allowed one presentation of distilled water for 15 min and a 15-min time limit to first lick. The second day of training consisted of thirty 10- to 15-s presentations of distilled water with a 12-s intertrial interval and a 150-s time limit to first lick.
Testing procedure.
Following the training phase, mice were subjected to behavioral testing for three consecutive days. Each day, all of the mice received either insulin or vehicle injection 15 min before the trial, and any animal that exhibited signs of distress was excluded from the study. In addition, we performed insulin tolerance tests to decide the best time frame to perform our behavioral assays based on the animal's alertness and motivation to complete the task. All experiments began at the same time every morning, and each mouse from either insulin-treated or vehicle-injected group was presented with two 5-s presentations of each concentration in an ascending order from the lowest to the highest concentration followed by a 2-s rinse with distilled water. The intertrial interval was 10 s with a 150-s time limit for the first lick. Seven NaCl concentrations (30, 150, 270, 330, 450, 600, and 1,000 mM) were tested to determine relative preference for NaCl solutions using lick rates as the dependent variable. For experiments using orally administered amiloride, a 10 mM amiloride stock was prepared in ddH2O, and 100 μM amiloride was made by dilution in each of the seven NaCl concentrations including the ddH2O used for the water stimulus and rinses. All NaCl solutions were prepared fresh every day before testing. At the end of the behavioral experiments, data were analyzed by the average of the number of licks for each NaCl concentration divided by the average number of licks to water per trial. This lick ratio normalizes for individual differences and motivational state of the animal (16). The mean ± SE values of the tastant/water lick ratio were plotted to obtain concentration-response curves for each group.
RESULTS
Insulin Increases Amiloride-Sensitive Na+ Currents in Mouse Taste Cells
To determine whether insulin stimulates sodium (Na+) transport in the gustatory system, we recorded insulin effects in Na+ transport in taste cells using conventional whole cell patch-clamp recording. In all experiments, cells were held at −80 mV in a continuous (gap-free) recording mode with the pipette containing 140 mM K-gluconate and Tyrode's as the extracellular solution. This intracellular solution helped to eliminate any inward Cl− or K+ current since the ECl and EK were approximately −80 mV. In most TRCs, insulin increased inward Na+ currents, consistent with previous studies in other transporting epithelia that showed that the ability of insulin to enhance Na+ movement is through the activation of ENaC (38, 44). To determine whether the insulin-enhanced responses we recorded were attributable to ENaC, we recorded insulin-induced responses in the presence or absence of amiloride (30 μM) and/or benzamil (10 μM). Insulin dramatically increased inward Na+ current in taste cells, and the enhancing effects of insulin were sensitive to amiloride (Fig. 1A) or benzamil (Fig. S1; Supplemental Material for this article is available online at the Journal website), suggesting that insulin activates ENaC in the taste system. In contrast, insulin had no effect on taste cell conductance when Na+ was replaced with NMDG.
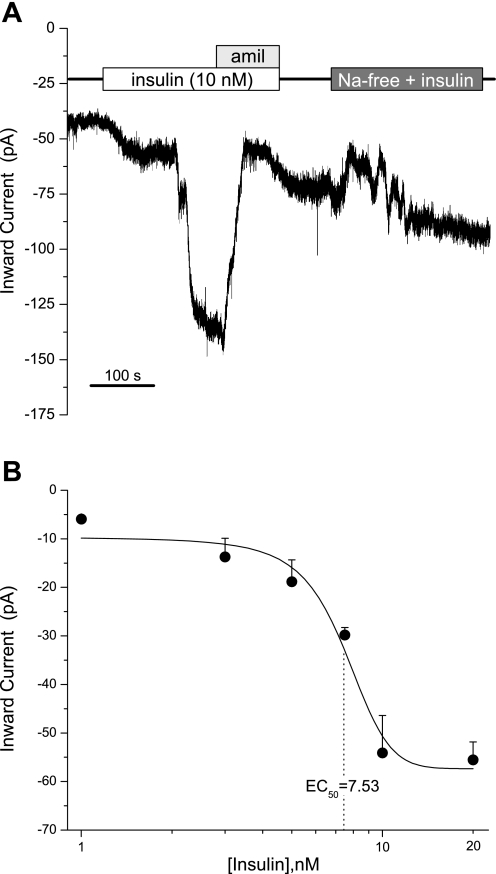
Fig. 1.
Effects of insulin on amiloride-sensitive cells. A: steady-state current in a mouse taste cell during whole cell patch-clamp recording. Shown is a continuous recording from a fungiform taste cell during the application of insulin (10 nM), insulin with amiloride (Amil; 30 μM), and insulin in Na+-free Tyrode's solution. Taste cells, like this one, showed a marked increase in sodium (Na+) inward current by the application of insulin. Solid line indicates timing of normal physiological saline. B: insulin dose-response curve. Insulin was applied by bath perfusion at 1, 3, 5, 7.5, 10, and 20 nM. Data points are means ± SE of the current enhancement as function of insulin concentration. Solid line is the statistically weighted best-fit logistic relation with EC50 = 7.53 nM.
To investigate whether insulin stimulation of inward Na+ currents occurred in a concentration-dependent manner, we characterized insulin responses in TRCs using whole cell patch clamp. In all fungiform cells, the enhancement of inward Na+ currents correlated with insulin concentration (Fig. 1B). The concentration-dependent changes in Na+ transport illustrated that the magnitude of Na+ current depends on insulin and that these responses occur within seconds. Interestingly, insulin (10–20 nM) has a maximum effect on Na+ influx in the taste system, and concentrations of insulin >20 nM did not show any difference in the magnitude of inward Na+ currents (data not shown). Concentration-response curves for insulin in the gustatory system reveal an EC50 of 7.53 nM. Clearly, the magnitude of sodium transport in taste epithelia appears to be regulated by insulin.
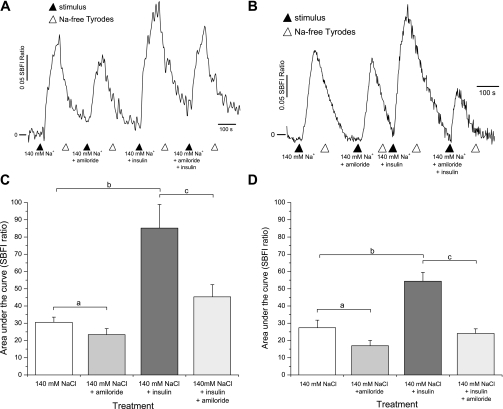
To confirm the ability of insulin to enhance Na+ movement in the gustatory system, we performed a series of experiments using functional sodium imaging in isolated taste cells. Using a Na+-sensitive dye, SBFI, we measured ratiometric changes of intracellular Na+ in taste cells from fungiform and circumvallate papillae. Taste cells loaded with SBFI were analyzed by obtaining the area under the curve (AUC) resulting from changes in the 340/380 ratio for each condition in a manner analogous to the patch-clamp experiments. Conservatively, we classified as amiloride-sensitive cells only those cells that showed a reversible decrease in SBFI ratio. In all cases, fungiform or circumvallate cells were perfused with Na+-free solution, and 140 mM NaCl was used as the prototypical salt stimulus. As shown in Fig. 2A, 140 mM NaCl evoked Na+ responses (AUC: 30.5 ± 2.9) in fungiform taste cells and these NaCl-induced responses were inhibited by amiloride (30 μM; AUC: 23.3 ± 3.4). Repeated-measures ANOVA showed statistically significant differences between NaCl and amiloride treatments (P < 0.01; Fig. 2B). Following from our electrophysiological results, we investigated whether insulin could stimulate Na+ movement into taste cells. Na+ movement in fungiform taste cells was dramatically increased from an AUC of 30.5 ± 2.9 to an AUC of 85.1 ± 13.6 when insulin (20 nM) was added into the solution (Fig. 2, A and B). Insulin-induced Na+ transport in TRCs is mostly through ENaC since Na+ responses were dramatically decreased by amiloride (30 μM; AUC: 45.2 ± 7). Statistical analysis with repeated-measures ANOVA revealed a significant difference between Na+ responses with and without insulin (P < 0.01); i.e., amiloride + insulin-mediated responses were significantly different from those with insulin treatment alone (P < 0.01).
Fig. 2.
Functional sodium imaging confirms insulin enhancement of epithelial sodium channel (ENaC). Fungiform and circumvallate taste cells were preloaded with sodium-binding benzofuran isophthalate (SBFI) and Na+ mobilization was measured. A: SBFI ratio elicited by 140 mM NaCl delivered to fungiform taste cells in the presence or absence of insulin (20 nM) and subsequent application of amiloride (30 μM). B: circumvallate taste cells evoke greater responses to 140 mM Na+ in the presence of insulin. These insulin-mediated Na+ responses were generally inhibited by amiloride (30 μM). C: fungiform taste cells summary graph comparing the area under the curve of 140 mM NaCl responses before and after insulin (20 nM) application, as well as comparing the effects of amiloride (30 μM) treatment. D: summary graph comparing insulin effects in the posterior mouse tongue before and after amiloride (30 μM) application. Data are shown as means ± SE. a,cSignificant reduction in SBFI ratio; bsignificant increase in SBFI ratio compared with 140 mM NaCl (P < 0.01, ANOVA).
The ability of insulin to regulate Na+ movement via ENaC is extended to other areas of the tongue. Circumvallate cells loaded with SBFI evoked similar Na+ responses (AUC: 27.3 ± 4.3; Fig. 2C). These responses were inhibited by amiloride (30 μM; AUC: 16.9 ± 3). Similar to the anterior taste buds, the posterior part of the mouse tongue exhibited amiloride sensitivity. Insulin treatment in circumvallate cells stimulated Na+ movement approximately twofold (AUC: 27.3 ± 4.3 to 54.3 ± 5.1). Amiloride (30 μM) diminished the insulin-mediated Na+ enhancement back to near control levels (AUC: 24.0 ± 2) in the posterior tongue, consistent with the action of insulin on ENaC-mediated Na+ movement. Repeated-measures ANOVA analysis revealed significant differences between NaCl and amiloride (30 μM; P < 0.01). Insulin treatment showed significant differences compared with NaCl (P < 0.01), and Na+ responses between insulin and insulin + amiloride-treated cells were significantly different (P < 0.01). Importantly, the insulin-mediated enhancement of Na+ influx in taste cells was rapid and reversible. However, we observed a difference in the amount of Na+ movement mediated by insulin between fungiform and circumvallate taste cells (Fig. 2, C and D).
Insulin-Mediated Na+ Transport Occurs via PI3-Kinase
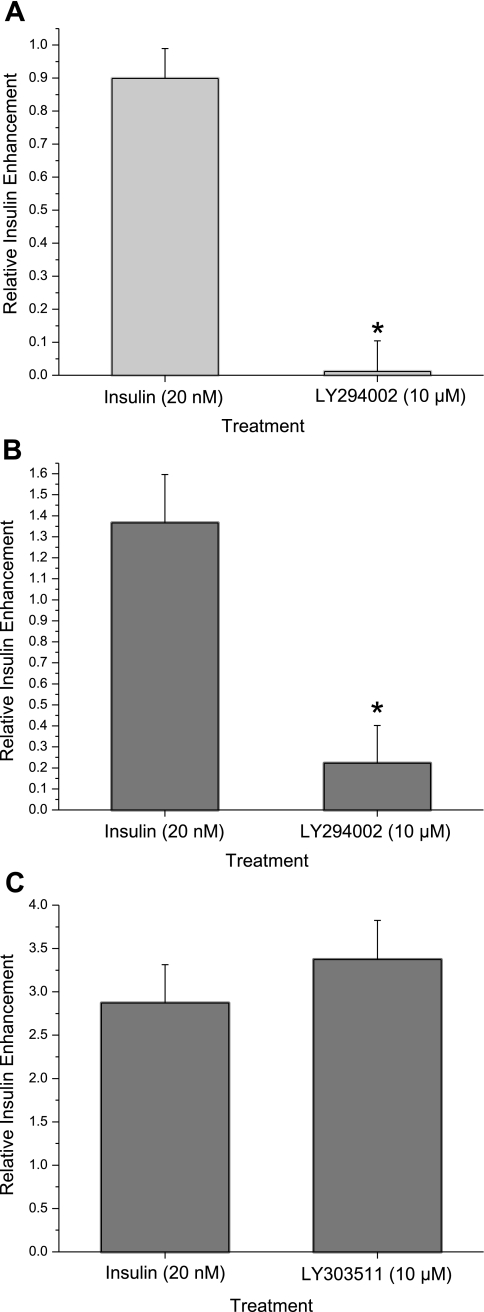
Recent studies in kidney epithelia have suggested that PI3-kinase is involved in the activation of ENaC by insulin (38, 40, 45). However, the role of PI3-kinase in the signaling pathway in taste transduction is unknown. Therefore, we tested the sensitivity of insulin-induced Na+ transport to applications of PI3-kinase inhibitors. LY294002 (10 μM), a specific pharmacological blocker of PI3-kinase, can decrease insulin's effects on Na+ responses in SBFI-loaded mouse taste cells (Fig. 3A). Insulin enhancement of Na+ movement dramatically decreases with LY294002 treatment from (0.89 ± 0.09) to (−0.1 ± 0.09). Paired Student's t-test statistical analysis revealed differences between insulin (20 nM) and insulin (20 nM) + LY294002 (10 μM) (P < 0.01).
Fig. 3.
Insulin enhancement of ENaC-mediated Na+ transport occurs via phosphoinositide 3-kinase (PI3-K) signaling pathway. A: effects of PI3-K inhibitor LY294002 (10 μM) on insulin-induced Na+ responses in mouse fungiform taste cells using functional Na+ imaging with SBFI. B: functional Na+ imaging from circumvallate taste receptor cells showing changes in insulin enhancement of Na+ influx in the absence and presence of LY294002 (10 μM). C: summary graph of insulin effects in Na+ influx before and after LY303511 treatment. Data are shown as means ± SE. *Significant reduction in insulin-mediated Na+ influx by the PI3-K inhibitor (P < 0.01, Student's t-test).
To determine whether PI3-kinase was involved in insulin-mediated responses in the posterior tongue, SBFI-loaded circumvallate taste cells were treated with insulin (20 nM) and LY294002 (10 μM). As shown in Fig. 3B, LY294002 blocked insulin's effects on relative Na+ influx from 1.36 ± 0.2 to 0.22 ± 0.18, and the difference in Na+ response between insulin and insulin + LY294002 was statistically significant (P < 0.01). In contrast, LY303511, an inactive analog of LY294002, had no effect on insulin stimulation of relative Na+ transport (from 2.87 ± 0.44 to 3.37 ± 0.45; Fig. 3C). Therefore, we observed reversible effects of LY294002 on insulin-stimulated Na+ movement within 10 min following the initial presence of the inhibitor (data not shown). Together, our observations showed that PI3-kinase is critical for insulin-enhanced Na+ transport in the taste system.
The Ability of Insulin to Enhance ENaC Function Is Mediated by Phosphatidylinositides
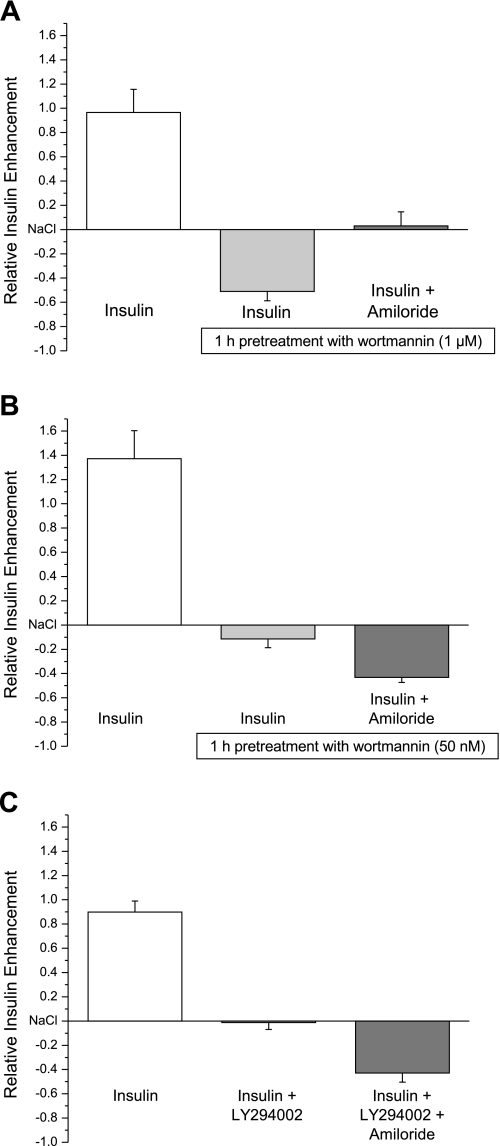
A number of phosphatidylinositides have been suggested to participate in ENaC activation. Several of these phosphatidylinositides are products of the PI4-kinase signaling pathway such as PtdIns(4,5)P2. Other phosphatidylinositides like PtdIns(3,4)P2/PtdIns(3,4,5)P3 are synthesized by PI3-kinase, which is directly activated by insulin (29, 31, 34, 36, 37, 45). On the basis of our imaging results in which PI3-kinase blockers abolished acute insulin-enhanced Na+ responses, we next wanted to investigate the role of phosphatidylinositides in insulin/ENaC-mediated responses by inhibiting the PI4-kinase signaling pathway. Using wortmannin (1 μM), which is an inhibitor of type III PI4-kinase and PI-related kinases at higher micromolar concentrations (1), we performed functional Na+ imaging in TRCs. The AUC obtained from SBFI ratio in the presence and absence of the test solution was analyzed by the relative amount of insulin enhancement (using the 140 mM Na+ response as baseline). Insulin-induced effects on amiloride-sensitive cells were abolished by wortmannin (1 μM) treatment (Fig. 4A). Inhibition of PI4-kinase decreased insulin-mediated Na+ responses from 0.96 ± 0.19 to −0.5 ± 0.07. Consistent with this response, amiloride sensitivity is absent in these cells, which suggests that PI4-kinase phospholipid products PtdIns(4)P/PtdIns(4,5)P2 are vital to maintain Na+ influx via ENaC.
Fig. 4.
Insulin-induced effects on amiloride-sensitive cells were abolished by both wortmannin and LY294002. A: insulin enhancement on Na+ influx was recorded with functional Na+ imaging in the absence and presence of wortmannin (1 μM) treatment for 1 h and before and after amiloride (30 μM). B: insulin-mediated responses in the absence and presence of pretreatment with wortmannin (50 nM) for 1 h and, subsequently, amiloride (30 μM). C: insulin-mediated responses in the absence and presence of acute treatment with LY294002 (10 μM) and, subsequently, amiloride (30 μM). All results obtained were compared with preceding 140 mM Na+ response. Values shown are means ± SE of between 8 and 10 cells per point.
Since PI3-kinase is a downstream signaling effector of PI4-kinase, we wanted to determine the significance of PtdIns(3,4)P2 and PtdIns(3,4,5)P3 on insulin/ENaC-mediated responses in TRCs. In these experiments, we used two pharmacological PI3-kinase blockers, wortmannin (50 nM) and LY294002 (10 μM). Insulin-evoked changes in taste cells were inhibited by both wortmannin, from 1.37 ± 0.23 to −0.11 ± 0.07, and LY294002, from 0.89 ± 0.09 to −0.01 ± 0.05 (Fig. 4, B and C). In contrast to what we found for PI4-kinase, inhibition of PI3-kinase does not affect 140 mM Na+ responses in the absence of insulin. Amiloride (30 μM) only reduces 140 mM Na+ responses, which suggests that there are functional ENaC channels in the plasma membrane but that PIP2 and PIP3 are necessary to increase insulin-mediated Na+ movement through ENaC. Clearly, our findings strongly suggest that ENaC function is regulated by phosphatidylinositol synthesis in the gustatory system and that both PI3-kinase and PI4-kinases are capable of enhancing and maintaining ENaC channel activity, respectively.
Physiological Role of SGK in Insulin/ENaC-Mediated Responses
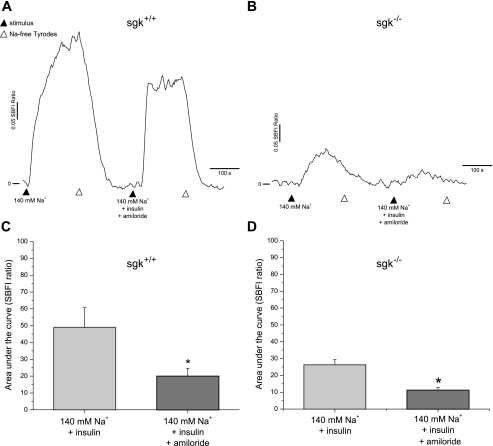
Having established that PI3-kinase is crucial for ENaC activation by insulin in TRCs, we performed experiments to explore other mechanisms of insulin action. PtdIns(3,4,5)P3, which is a product of PI3-kinase activation, is capable of interacting with the pleckstrin homology (PH) domain of PDK1 and PKB/Akt and then activating SGK (28, 31, 33). To discover whether insulin could enhance Na+ movement via SGK in the gustatory system, we first identified SGK expression in the taste system (Fig. S2). We performed functional Na+ imaging assays in the presence and absence of insulin (20 nM) in taste cells from transgenic SGK−/− mice (9). Insulin had no effect on Na+ transport in taste cells from SGK−/− mice (Fig. 5, B and D). In contrast, taste cells from SGK+/+ evoked greater Na+ responses to insulin (Fig. 5, A and C). Amiloride sensitivity was, however, found in taste cells from both SGK−/− and SGK+/+ mice. Student's t-test analysis of the AUC showed a significant difference (P < 0.01) between 140 mM Na+ and amiloride (30 μM) treatment for both SGK−/− and SGK+/+ TRCs (Fig. 5, C and D). Interestingly, insulin-induced Na+ influx was severely impaired in SGK−/− compared with SGK+/+ littermates. The magnitude of the insulin-stimulated Na+ transport was found to be less in SGK−/− (26.2 ± 3) than SGK+/+ (48.9 ± 11.7). Our observations imply that both SGK−/− and SGK+/+ taste cells have functional ENaC channels. However, functional ENaC activity seems dramatically reduced in SGK−/− taste cells. Consequently, our findings suggest that SGK is essential to maintain normal ENaC function and that the absence of SGK protein in the null mice severely inhibits insulin's effects in the gustatory system.
Fig. 5.
The importance of serum- and glucocorticoid-regulated kinase (SGK) in insulin/ENaC mediated responses in taste cells. A: functional Na+ imaging with SBFI from SGK+/+ wild-type mice in the presence of insulin (20 nM) and, subsequently, amiloride (30 μM). B: insulin effects on Na+ influx in SGK−/− mice in the absence and presence of amiloride (30 μM) treatment. C: summary graph showing insulin-mediated effects in Na+ transport in SGK+/+ wild-type animals. D: area under the curve graph from SGK−/− mice showing a dramatic reduction in the magnitude of insulin-mediated Na+ responses. Data are shown as means ± SE. *Significant reduction in insulin-mediated Na+ influx by amiloride (30 μM; P < 0.01, Student's t-test).
Expression of Insulin Receptor, Insulin Receptor Substrate 1, and Insulin Receptor Substrate 2 in Taste Cells
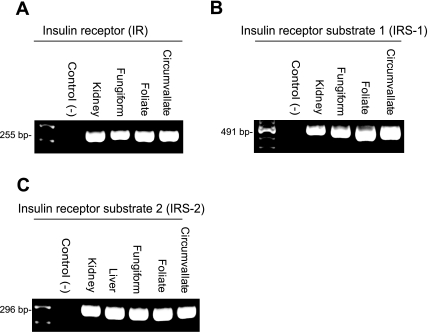
In most transporting epithelia, insulin reabsorption of NaCl is initiated when insulin binds to the basolateral insulin receptor (IR). Autophosphorylation of IR elicits a number of downstream events including phosphorylation of IRS and PI3- kinase (3, 44). To verify the expression of IR, insulin receptor substrate 1 (IRS-1), and insulin receptor substrate 2 (IRS-2) in mouse taste buds, a series of RT-PCR assays were performed using total RNA isolated from fungiform, foliate, and circumvallate taste buds. Expression of IR, IRS-1, and IRS-2 was found in all three lingual taste bud types in a minimum of three independent experiments (Fig. 6). All PCR products were sequenced using a PE Biosystem 377 automated DNA sequencer. The sequences for mouse taste bud IR, IRS-1, and IRS-2 were at least 99% homologous with sequences from GenBank.
Fig. 6.
RT-PCR reveals the presence of insulin receptor (IR; A), insulin receptor substrate 1 (IRS-1; B), and insulin receptor substrate 2 (IRS-2; C). Primers for IR, IRS-1, and IRS-2 amplify stained PCR products of expected sizes (IR 255 bp, IRS-1 491 bp, and IRS-2 296 bp). Positive controls (mouse kidney or liver RNA) are shown for IR, IRS-1, and IRS-2 with each set of primers. Negative control (−) lanes represent those in which cDNA was omitted from the PCR reaction.
Insulin Regulates Salt Intake in Mice
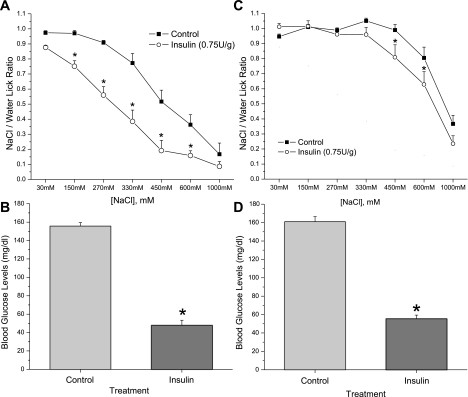
We next performed a series of brief access behavioral tests to determine whether insulin enhancement of Na+ movement via ENaC in mouse taste cells was correlated with any differences in behavioral responsiveness to NaCl. Since we hypothesized that insulin could act primarily as a regulator of salt appetite in the taste system, we tested the ability of insulin to alter NaCl preference in a hyperinsulinemic animal model. For all experiments, 24 C57BL/6 mice were trained for 2 days and then divided into two groups. Each group received either intraperitoneal injections of insulin (0.75 U/kg) or vehicle. Both insulin-treated and vehicle-treated groups were injected 15 min before the start of the behavioral test, and preference for seven different NaCl concentrations was tested. Insulin-treated mice exhibited significant avoidance of NaCl at lower concentrations than the control group (Fig. 7A). These changes in NaCl preference in hyperinsulinemic mice were more evident at concentrations between 150 mM to 600 mM NaCl. Simple effects ANOVA analysis revealed highly significant differences (P < 0.01) between insulin-treated and control groups at the following NaCl concentrations: 150, 270, 330, 450, and 600 mM. Blood glucose levels (mg/dl) were lower in insulin-treated mice (47.7 ± 5.6) compared with the vehicle-injected group (155.6 ± 3.8; P < 0.01 paired Student's t-test; Fig. 7B).
Fig. 7.
Behavioral effects of insulin on salt preference appear to be via ENaC channels. A: NaCl/water lick ratio (means ± SE) measured in short-term taste assays using the Davis Rig in two groups of 22 mice. Insulin-treated mice significantly avoid NaCl solutions between 150 nM and 600 mM. *Significant difference in NaCl preference compared with control mice (P < 0.01, simple effects ANOVA). B: blood glucose levels (mg/dl) in each group. Values are means ± SE. C: NaCl/water lick ratio. Each point represents 12 mice for each group. Avoidance of NaCl solutions was reduced in both control and insulin-treated mice due to the blocking of ENaC channel by amiloride (100 μM), which was present in all solutions. Values are means ± SE. *Significant difference in NaCl preference compared with control mice (P < 0.01, simple effects ANOVA). D: graph of blood glucose levels (mg/dl) in each group. Values are mean ± SE. *Significant reduction in blood glucose levels by insulin treatment compared with control (P < 0.01, Student's t-test).
To elucidate the functional role of ENaC in alterations of NaCl sensitivity by insulin treatment, we added amiloride (100 μM), a diuretic by means of its antagonism of ENaC, to all NaCl solutions (7). Additionally, amiloride was also added into the ddH2O used for the water stimulus and rinses to eliminate any possible taste cue from amiloride during the short-term taste assays. In contrast, behavioral effects of insulin in salt preference were abolished by amiloride (Fig. 7C). Thus, NaCl taste is diminished in both insulin-treated and control groups in the presence of amiloride (100 μM). The pharmacological effect of amiloride on ENaC channels attenuated any difference in NaCl sensitivity between hyperinsulinemic and control groups at concentrations below 330 mM NaCl. However, we observed differences between insulin-treated and control mice in the presence of amiloride (100 μM) at 600 mM NaCl (P = 0.01). Blood glucose levels differed between the control group (160.8 ± 5.6) and the insulin-treated group (55.3 ± 4.1; P < 0.01, paired Student's t-test; Fig. 7D).
DISCUSSION
The importance of ENaC in the transduction of sodium salts in taste cells is well established (4, 13, 14, 20, 39). Studies have shown ENaC in other tissues to be regulated by hormones, such as aldosterone, vasopressin, and insulin (13, 19, 26, 38, 43). However, there is little understanding about the mechanisms of ENaC regulation in the taste system. In the present study, we have identified a novel signaling pathway of ENaC regulation by insulin in mammalian taste buds. Our experiments show that insulin increases Na+ transport via ENaC. Both fungiform and circumvallate cells show increases in ENaC function by insulin that occurs through PI3-kinase signaling pathway. Moreover, we observed that enhancement of ENaC function by insulin at the cellular level is concomitant with changes in animals' behavior for NaCl preference.
Circulating insulin wields its effects by binding the insulin receptor (IR) on target tissues. IR activation by insulin binding creates both autophosphorylation of the receptor and phosphorylation of members of the insulin substrate (IRS) family. Phosphorylation of IRS results in the activation of signaling molecules such as PI3-kinase (32, 42, 49). Our RT-PCR assays were consistent with the expression of IR, IRS-1, and IRS-2 in mouse taste buds (Fig. 6). The presence of IR, IRS-1, and IRS-2 in all three papillae provides all the necessary machinery for insulin action in the taste system. Consistent with our molecular results, we found that the PI3-kinase signaling pathway is necessary for insulin-mediated Na+ influx through ENaC in TRCs (Fig. 3). Similar results in kidney epithelia studies have also suggested that insulin contributes to Na+ movement via ENaC (43, 44). Insulin-mediated Na+ reabsorption occurs through activation of PI3-kinase. Thus, PI3-kinase signaling via production of PtdIns-(3,4)P2/PtdIns-(3,4,5)P3 directly affects ENaC activity and open probability (30, 38, 40, 45). Studies in kidney epithelia have suggested that insulin also stimulates Na+ transport through the insulin growth factor receptor (IGF-R) (17). In the present study, we focused only on insulin action through IR. Future studies are needed to address whether the IGF-R signaling pathway plays a role in the taste system.
Recent studies indicate that ENaC activity can be increased directly by phosphatidylinositides (35, 37). In the present study, we revealed the importance of PI4-kinases in the taste system. Using wortmannin, which is a pharmacological blocker of PI4-kinases at high concentrations (i.e., 1 μM), we inhibited the synthesis of phosphatidylinositol 4-phosphate [PI(4)P]. In general, our results suggested that PtdIns(4)P/PtdIns(4,5)P2 are likely involved in Na+ transport (Fig. 4A). Though our results showed a reduction in 140 mM NaCl responses, we could not draw any meaningful conclusions about the role of PI4-kinase products since wortmannin (1 μM) also blocked PI3-kinases and PI-related kinases (1). To overcome the issue, we blocked PI3-kinase with a specific blocker, LY294002 (10 μM), then compared the effects with wortmannin (50 nM), which is considered a pharmacological blocker of PI3-kinase. As shown in Fig. 4, B and C, both LY294002 and wortmannin (50 nM) inhibited insulin's effect on Na+ influx, yet did not affect 140 mM NaCl responses. In addition, there was evidence of amiloride-sensitive ENaC activity. In agreement with our results, it has been reported in other transporting epithelia that phosphatidylinositides bind ENaC and increase its open probability (31, 34, 37, 45). Although we have provided the first evidence of the role of PI4-kinase and PI3-kinase in taste cells, further experiments are needed to elucidate the characteristics of PtdIns(3,4)P2/PtdIns(3,4,5)P3 interaction with ENaC.
Insulin stimulation of ENaC-mediated Na+ transport occurs via PI3-kinase signaling pathway cascade by one of two mechanisms: 1) increasing the open probability of apical ENaCs and/or 2) increasing the number of active channels in the membrane. Insulin-induced PI3-kinase signaling cascade leads to phosphorylation of PDK1 and SGK1 activation. In kidney epithelia, stimulation of SGK1 by insulin increases the number of apical ENaC. The interaction between SGK and ENaC occurs via phosphorylation of the ubiquitin ligase NEDD4–2. Thus, NEDD4–2 removes and degrades ENaC protein from the cell membrane (5, 8, 41, 50). In the present study we were interested in demonstrating the physiological role of SGK in mammalian taste cells. Our results show that TRCs from SGK−/− mice evoked alterations in the magnitude of insulin-mediated Na+ movement (Fig. 5). These findings illustrate the inability of insulin to increase Na+ influx in SGK−/− taste cells. Moreover, the present observations are also consistent with a reduction in functional ENaC expression in SGK−/− taste cells. It could be possible that absence of SGK1 protein allows a greater degradation of ENaC in the peripheral taste system. Similar results have been shown in kidney epithelia in which insulin-mediated Na+ retention was abolished in SGK−/− mice (22). More studies are needed to investigate the link between SGK and insulin-mediated salt appetite.
Our results showed insulin-mediated enhancement of Na+ influx through ENaC in taste cells, and we hypothesize that insulin plays a physiological role in the regulation of salt taste. To test this, we characterized insulin's effects on behavioral responses to NaCl using a mouse model of acute hyperinsulinemia. NaCl concentrations were presented in ascending order to both groups to maintain the behavioral momentum of the animal. We showed that insulin-treated mice displayed a strong avoidance of NaCl solutions (Fig. 7, A and B). Given this, we performed a separate experiment with five vehicle-injected mice. NaCl concentrations were presented in random order, and no differences in NaCl lick ratio were found between the two experiments (data not shown). We considered the possibility that low glucose levels may create distress in the animals during behavior assays. However, this is unlikely because there were no differences in latency to the first lick for all NaCl concentrations between control or insulin-treated mice (Fig. S3).
Our results show evidence that insulin-mediated effects on Na+ movement extend to the animal's behavior. Using amiloride to block ENaC function in taste cells, we attempted to determine the specificity of insulin's effect on ENaC. If insulin was targeting Na+ movement through ENaC, it would be expected that NaCl preference will be similar in both hyperinsulinemic and control groups. Clearly, the presence of amiloride in all solutions was very effective at suppressing insulin's effects on NaCl responses (Fig. 7, C and D). These data suggest that insulin activates ENaC channels expressed in the apical membrane of taste cells, causing these animals to experience a change in their NaCl taste preference. However, more studies are needed in this area to understand in greater detail the effects of insulin in the animal's taste behavior.
In conclusion, the present study has demonstrated a new signaling pathway for maintaining functional ENaC expression in the taste system. Thus, insulin leads to PI3-kinase activation which either increases ENaC activity with its phospholipid products or increases the number of apical ENaCs by the activation of SGK. Our results are consistent with the emerging idea that the gustatory system is capable of responding to nutritional challenges and may play a central role in the restoration of nutritional balance.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant DC02507 and by Project 630 from the Utah Agricultural Experiment Station (to T. A. Gilbertson). This project was also supported in part by NIH Grants RO1 DC006021 and P30 DC04657.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Anikó Náray-Fejes-Tóth for providing the breeding pairs of the SGK knockout mice and Dr. Sue Kinnamon for helpful discussions and comments on the manuscript. We also thank the Rocky Mountain Taste and Smell Center for use of core facilities and Cathy Anderson for technical assistance.
REFERENCES
- 1. Balla T. Pharmacology of phosphoinositides, regulators of multiple cellular functions. Curr Pharm Des 7: 475–507, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Béhé P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca2+ currents and inhibition of K+ currents by saccharin. J Gen Physiol 96: 1061–1084, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blazer-Yost BL, Vahle JC, Byars JM, Bacallao RL. Real-time three-dimensional imaging of lipid signal transduction: apical membrane insertion of epithelial Na+ channels. Am J Physiol Cell Physiol 287: C1569–C1576, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doolin RE, Gilbertson TA. Distribution and characterization of functional amiloride-sensitive sodium channels in rat tongue. J Gen Physiol 107: 545–554, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eylam S, Spector AC. The effect of amiloride on operantly conditioned performance in an NaCl taste detection task and NaCl preference in C57BL/6J mice. Behav Neurosci 116: 149–159, 2002 [PubMed] [Google Scholar]
- 8. Faletti CJ, Perrotti N, Taylor SI, Blazer-Yost BL. sgk: an essential convergence point for peptide and steroid hormone regulation of ENaC-mediated Na+ transport. Am J Physiol Cell Physiol 282: C494–C500, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Fejes-Toth G, Frindt G, Naray-Fejes-Toth A, Palmer LG. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol 294: F1298–F1305, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol 10: 519–527, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Gilbertson TA, Fontenot DT. Distribution of amiloride-sensitive sodium channels in the oral cavity of the hamster. Chem Senses 23: 495–499, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Gilbertson TA, Kinnamon SC. Making sense of chemicals. Chem Biol 3: 233–237, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Gilbertson TA, Roper SD, Kinnamon SC. Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: enhancement by vasopressin and cAMP. Neuron 10: 931–942, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Gilbertson TA, Zhang H. Characterization of sodium transport in gustatory epithelia from the hamster and rat. Chem Senses 23: 283–293, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Gilbertson TA, Zhang H. Self-inhibition in amiloride-sensitive sodium channels in taste receptor cells. J Gen Physiol 111: 667–677, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses 27: 461–474, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Rodriguez E, Gaeggeler HP, Rossier BC. IGF-1 vs insulin: respective roles in modulating sodium transport via the PI-3 kinase/Sgk1 pathway in a cortical collecting duct cell line. Kidney Int 71: 116–125, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hem A, Smith AJ, Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim 32: 364–368, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Herness MS. Aldosterone increases the amiloride-sensitivity of the rat gustatory neural response to NaCl. Comp Biochem Physiol 103: 269–273, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Herness MS, Gilbertson TA. Cellular mechanisms of taste transduction. Annu Rev Physiol 61: 873–900, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Hills CE, Squires PE, Bland R. Serum and glucocorticoid regulated kinase and disturbed renal sodium transport in diabetes. J Endocrinol 199: 343–349, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Huang DY, Boini KM, Friedrich B, Metzger M, Just L, Osswald H, Wulff P, Kuhl D, Vallon V, Lang F. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am J Physiol Regul Integr Comp Physiol 290: R935–R944, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47: 51–64, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19: 142–143, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4–2. J Biol Chem 282: 29866–29873, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405: 406–420, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Lindemann B, Barbry P, Kretz O, Bock R. Occurrence of ENaC subunit mRNA and immunocytochemistry of the channel subunits in taste buds of the rat vallate papilla. Ann NY Acad Sci 855: 116–127, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Loffing J, Flores SY, Staub O. Sgk kinases and their role in epithelial transport. Annu Rev Physiol 68: 461–490, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflügers Arch 455: 169–180, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol 16: 3182–3187, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Markadieu N, Blero D, Boom A, Erneux C, Beauwens R. Phosphatidylinositol 3,4,5-trisphosphate: an early mediator of insulin-stimulated sodium transport in A6 cells. Am J Physiol Renal Physiol 287: F319–F328, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Miura A, Sajan MP, Standaert ML, Bandyopadhyay G, Kahn CR, Farese RV. Insulin substrates 1 and 2 are corequired for activation of atypical protein kinase C and Cbl-dependent phosphatidylinositol 3-kinase during insulin action in immortalized brown adipocytes. Biochemistry 43: 15503–15509, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Pearce D, Kleyman TR. Salt, sodium channels, and SGK1. J Clin Invest 117: 592–595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem 280: 37565–37571, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol 130: 399–413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol 290: F949–F957, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Pochynyuk O, Tong Q, Staruschenko A, Stockand JD. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J Physiol 580: 365–372, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Record RD, Froelich LL, Vlahos CJ, Blazer-Yost BL. Phosphatidylinositol 3-kinase activation is required for insulin-stimulated sodium transport in A6 cells. Am J Physiol Endocrinol Metab 274: E611–E617, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Shigemura N, Ohkuri T, Sadamitsu C, Yasumatsu K, Yoshida R, Beauchamp GK, Bachmanov AA, Ninomiya Y. Amiloride-sensitive NaCl taste responses are associated with genetic variation of ENaC alpha-subunit in mice. Am J Physiol Regul Integr Comp Physiol 294: R66–R75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol 18: 1652–1661, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Staub O, Verrey F. Impact of Nedd4 proteins and serum and glucocorticoid-induced kinases on epithelial Na+ transport in the distal nephron. J Am Soc Nephrol 16: 3167–3174, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab 17: 72–78, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol 293: F974–F984, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem 279: 22654–22663, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Vallon V, Blantz R, Thomson S. The salt paradox and its possible implications in managing hypertensive diabetic patients. Curr Hypertens Rep 7: 141–147, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Vallon V, Huang DY, Grahammer F, Wyatt AW, Osswald H, Wulff P, Kuhl D, Lang F. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am J Physiol Regul Integr Comp Physiol 289: R395–R401, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Wang J, Knight ZA, Fiedler D, Williams O, Shokat KM, Pearce D. Activity of the p110-α subunit of phosphatidylinositol-3-kinase is required for activation of epithelial sodium transport. Am J Physiol Renal Physiol 295: F843–F850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wing SS. The UPS in diabetes and obesity. BMC Biochem 9, Suppl 1: S6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou R, Snyder PM. Nedd4–2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J Biol Chem 280: 4518–4523, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.