Abstract
Central and peripheral neurons as well as neuroendocrine cells express a variety of neurotransmitters/modulators that play critical roles in regulation of physiological systems. The synthesis of several neurotransmitters/modulators is regulated by O2-requiring rate-limiting enzymes. Consequently, hypoxia resulting from perturbations in O2 homeostasis can affect neuronal functions by altering neurotransmitter synthesis. Two broad categories of hypoxia are frequently encountered: continuous hypoxia (CH) and intermittent hypoxia (IH). CH is often seen during high altitude sojourns, whereas IH is experienced in sleep-disordered breathing with recurrent apneas (i.e., brief, repetitive cessations of breathing). This article presents what is currently known on the effects of both forms of hypoxia on neurotransmitter levels and neurotransmitter synthesizing enzymes in the central and peripheral nervous systems.
Keywords: biogenic amines, bioactive peptides, glutamate, γ-amino butyric acid, gasotransmitters
the mammalian brain represents 2% of the total body weight and consumes 20% of the total body oxygen for its normal function (108). This high level of oxygen consumption makes the brain an extremely sensitive tissue to changes in arterial blood oxygen levels. Many physiological systems are regulated by the nervous system, and neurotransmitters are critical for neuronal function. The rate-limiting enzymes associated with the synthesis of several neurotransmitters require molecular oxygen for their activity. Consequently, hypoxia (i.e., reduced oxygen availability) resulting from perturbations in O2 homeostasis can profoundly impact neurotransmitter synthesis resulting in altered neuronal functions and consequently impact physiological systems.
Hypoxia is experienced in a variety of physiological and pathological conditions. Two broad categories of hypoxia are most frequently encountered; continuous hypoxia (CH) and intermittent hypoxia (IH). For instance, CH is experienced by healthy humans during high altitude sojourns, whereas IH is encountered during sleep-disordered breathing with recurrent apneas; i.e., brief repetitive cessations of breathing wherein each episode lasts for tens of seconds. The purpose of this article is to provide a brief review of the effects of both forms of hypoxia on neurotransmitter levels, rate-limiting enzymes associated with neurotransmitter synthesis and the underlying mechanisms. Several of these molecules, which function as transmitters/modulators in the nervous system, are also expressed in nonneuronal tissues. However, because of space constraints, this review will focus on the effects of hypoxia on neurotransmitters in the central and peripheral nervous systems.
Based on the chemical properties, neurotransmitters are divided into various groups as shown in Table 1. This review focuses on the impact of CH and IH on the synthesis of biogenic amines, acetylcholine, excitatory and inhibitory amino acids, bioactive peptides, and gasotransmitters (for examples see Table 1).
Table 1.
Neurotransmitter classification
| Transmitter Type | Examples |
|---|---|
| Biogenic amines | Dopamine, norepinephrine, epinephrine, 5-hydroxytryptamine, histamine |
| Cholinergic | Acetylcholine |
| Excitatory and inhibitory amino acids | Glutamate, aspartate, γ-amino butyric acid, glycine, taurine |
| Bioactive peptides | α-Amidated peptides: Adrenomedullin, substance P, neuropeptide Y, vasoactive intestinal peptide, cholecystokinin, galanin, gastrin, vasopressin, calcitonin, oxytosin |
| Non-α-amidated peptides: angiotensin II, endothelin-1, atrial natriuretic peptide, neurotensin, enkephalin, β-endorphin | |
| Gasotransmitters | Nitric oxide, carbon monoxide, hydrogen sulfide |
BIOGENIC AMINES
Biogenic amines constitute an important class of neurotransmitters containing amine functional group. Examples of some well-studied biogenic amines include catecholamines and 5-hydroxytryptamine (5-HT; Table 1).
Impact of Hypoxia on Catecholamines
Catecholamines comprising dopamine (DA), norepinephrine (NE), and epinephrine (Epi) are expressed in many regions of the brain, adrenal medulla, and the carotid body, which is a peripheral neuronal sensory organ specialized for detecting changes in arterial blood O2. In addition to their roles in development and energy metabolism, catecholamines play important roles in regulation of cardio-respiratory functions both centrally and peripherally (16, 109). Catecholamines are synthesized enzymatically from l-tyrosine through a sequence of reactions as outlined in Table 2. Tyrosine hydroxylase (TH) expressed in the cytosol catalyzes hydroxylation of l-tyrosine to produce l-dihydroxyphenylalanine (l-Dopa). l-Dopa is then enzymatically converted to DA, NE, and Epi by the sequential actions of cytosolic l-aromatic amino acid decarboxylase (AADC), vesicular dopamine-β-hydroxylase (DBH), and cytosolic phenylethanolamine-N-methyltransferase (PNMT), respectively.
Table 2.
Enzymatic reactions associated with the synthesis of biogenic amines
| Transmitters | Synthesizing Enzyme(s) | Enzymatic Reactions | Requirement for Activity |
|---|---|---|---|
| Dopamine | Tyrosine hydroxylase (rate-limiting) | Hydroxylation:l-Tyrosine → 3,4-dihydroxyphenylalanine + H2O | Tetrahydrobiopterin (BH4), Fe2+, and O2 |
| l-Aromatic amino acid decarboxylase (AADC) | Decarboxylation: 3,4-dihydroxyphenylalanine → Dopamine + H2O | Pyridoxal l-phosphate (PLP) | |
| Norepinephrine | Dopamine-β-hydroxylase | Dopamine → Norepinephrine + H2O | Cu2+, O2, and ascorbic acid |
| Epinephrine | Phenylethanolamine N-methyl transferase | Norepinephrine → Epinephrine | S-Adenosylmethionine |
| 5-Hydroxy tryptamine (5-HT; serotonin) | Tryptophan hydroxylase (rate-limiting) | Hydroxylation:l-Tryptophan → 5-hydroxytryptophan | BH4 + O2 |
| AADC | Decarboxylation: 5-hydroxytryptophan → 5-HT | PLP |
The effects of CH, lasting minutes to hours, on catecholamine synthesis were investigated in various brain regions, adrenal medulla, and carotid bodies. In rats, short-term exposure (30 min) to either moderate (10 or 12% O2) or severe hypoxia (6% O2) during different stages of development (postnatal days P0 to P28) produced an initial decrease in brain catecholamine synthesis, which returned to control levels despite continuing the hypoxic challenge (11, 12, 14, 41, 42). A similar biphasic response of catecholamine synthesis to CH (7.5% O2 for 4–12 h) was also reported in the adrenal medulla of guinea pigs and rats (110). On the other hand, CH (10% O2 for 3 h) facilitates DA and NE synthesis in rabbit and rat carotid bodies, an effect seen only with tyrosine but not with DOPA as substrate, suggesting a role for altered TH activity in CH-induced facilitation of catecholamine synthesis (22, 23). These studies suggest that CH exerts differential effects on catecholamine synthesis in the central (brain) versus peripheral nervous system (i.e., carotid body).
The impact of more prolonged form of CH, lasting days to weeks, on catecholamine levels was investigated in the rat carotid body. Exposure to either normobaric (10% O2 for up to 28 days) or hypobaric (0.45 atm; high altitude simulation for 7 days) hypoxia elevated DA and NE levels (37, 84, 88, 113). After 28 days of normobaric CH, there was a 27- and 51-fold increase in DA and NE levels, respectively (88).
The effects of IH, consisting of alternating cycles of 5% O2 for 15 s followed by 21% O2 for 5 min for 8 h/day (86) on catecholamine levels in rat brainstem and adrenal medulla were studied. Exposure to 10 days of IH significantly increased DA levels in rat brainstem (98) and NE levels in adrenal medulla (57) compared with normoxic controls. Similarly, IH (1% O2 for 15 s and 21% O2 for 4 min; 60 cycles) also increased DA levels in pheochromocytoma 12 (PC12) cell cultures (56). These studies demonstrate that both CH and IH elevate tissue and cellular levels of DA and NE.
Effects of Hypoxia on Catecholamine Synthesizing Enzymes
TH is the rate-limiting enzymes of catecholamine synthesis and DBH is the NE-synthesizing enzyme (Table 2). Both enzymes require molecular oxygen for their catalytic activity (24). Consequently, several studies examined the effects of hypoxia on TH and DBH.
Tyrosine hydroxylase.
CH (10% O2 for 1–14 days) increased TH activity in the rat carotid body (36, 45) and brain cortex (34). The CH-induced increase in TH activity in the carotid body was associated with an upregulation of TH protein expression (45, 47, 117, 118). CH also elevated TH mRNA levels in the rat carotid body, which can be seen as early as 6 h of CH treatment (9). Studies on PC12 cell cultures showed that CH increases the TH gene transcription as well as the stability of TH mRNA. The increased TH transcription by CH requires activation of hypoxia-inducible transcription factors (HIFs) that interact with a specific hypoxia-responsive element (HRE) in the TH promoter region (10, 103).
In addition to its effect on transcription, CH (10% O2 for 30 days) affects TH activity via posttranslational mechanisms. Phosphorylation of serine-19, serine-31, and serine-40 at the NH2-terminal regulatory domain of TH alters the Km and maximal velocity (Vmax) leading to more active form of TH with increased activity (19, 25). CH increased phosphorylation of serine-19, serine-31 and serine-40 residues in TH in rat carotid bodies but not in adrenal medullae and superior cervical ganglion (45). A similar increase in TH phosphorylation was also reported in the cortex and brainstem of rats exposed to CH (10% O2 for 14 days) (34). The mechanisms underlying increased phosphorylation of TH by CH, however, are not known.
Studies on PC12 cell cultures revealed that IH (1% O2 for 15 s and 21% O2 for 4 min; 60 cycles) progressively increased TH activity (56). Although IH increased TH mRNA levels via HIF-1 activation (123), TH protein levels were unaffected in IH-treated PC12 cells (56, 123). Despite unaltered TH protein, IH markedly increased TH activity. The IH-induced increase in TH activity is primarily due to its effects on posttranslational modification involving phosphorylation of TH at serine-40 mediated by calcium/calmodulin-dependent protein kinase (CaMK) and protein kinase A (PKA) (56). Studies in rats showed that IH (5% O2 for 15 s followed by 21% O2 for 5 min for 8 h/day; termed as “15 s IH”) also increases TH activity in the dorsal and ventral medullary but not in the pontine regions of the brainstem (98). IH increased TH activity in brainstem regions via phosphorylation at serine-31 and serine-40 residues without altering TH protein expression (98). However, the effects of IH are critically dependent on the severity of hypoxia and duration of hypoxia-reoxygenation of the IH paradigm. Thus exposing rats to 10% O2 and 21% O2 for 90 s each for 12 h/day (termed as “90 s IH”; 34) had virtually no effect either on TH activity or on TH phosphorylation in the brainstem regions as opposed to robust activation observed with 15 s IH (98).
Raghuraman et al. (98) examined the mechanisms underlying IH-induced TH phosphorylation. These authors found that 15 s IH increases reactive oxygen species (ROS) generation and the ensuing ROS signaling facilitates PKA, CaMK, and ERK activation with a simultaneous inhibition of protein phosphatase 2A activity in the brainstem. The resulting imbalance between kinase and phosphatase activities mediates sustained phosphorylation of TH at multiple serine residues. The effects of IH on TH phosphorylation, like its effect on TH activity, depend on IH paradigm. Thus 90 s IH paradigm leads to decreased TH phosphorylation in the brainstem with a modest augmentation in the cerebral cortex (34). A comparison of ROS levels revealed that ROS generation is significantly lower in 90 s IH than with 15 s IH paradigm in rat brainstem (98), suggesting that 90 s IH is less potent than 15 s IH paradigm in stimulating ROS generation in the brainstem. However, 90 s IH increased TH phosphorylation at serine-19, serine-31, and serine-40 in the rat carotid body, without affecting TH phosphorylation levels in other catecholamine expressing peripheral tissues including superior cervical ganglion and adrenal medulla (45). It is likely that the stimulatory effects of 90 s IH on the carotid body can be attributed to greater hypoxic sensitivity of the carotid body relative to other tissues. Nonetheless, the above results suggest that IH, depending on the duration and severity of hypoxia and period of reoxygenation, induces oxidative stress, which via increased serine phosphorylation activates TH and augments catecholamine synthesis. Thus the effects of IH on TH activity primarily involve posttranslational modifications rather than transcriptional mechanisms. Since IH-mediated activation of TH is coupled to elevated DA levels in the medullary brainstem regions, it is conceivable that IH-evoked changes in DA may, in part, contribute to cardiorespiratory abnormalities associated with recurrent apneas.
Dopamine-β-hydroxylase.
Exposure of ewes to high altitude (3, 820 m) from 30 to 138 days gestation reduced DBH protein and mRNA expression in the adrenal medulla (17). In contrast, exposure to 15 s IH paradigm (96) but not 90 s IH paradigm (45) increased DBH protein in the rat adrenal medulla with a concomitant increase in NE levels (57). Future studies are, however, needed to determine whether IH-induced increase in NE levels in the adrenal medulla is due to upregulation of DBH activity and changes in DBH mRNA.
Effects of Hypoxia on 5-HT Synthesis
5-HT, another well-established monoamine neurotransmitter, is synthesized from l-tryptophan via two enzymatic reactions involving tryptophan hydroxylase (TPH) and AADC (Table 2). The TPH-mediated reaction is the rate-limiting step in 5-HT biosynthesis and requires molecular oxygen (48). Two isoforms of TPH, i.e, TPH1 and TPH2, are known. While TPH1 is expressed in several tissues, TPH2 is expressed only in the brain (115).
As short as 30 min of CH decreased TPH activity in many brain regions in neonatal and adult rats (13, 40). On the other hand, prolonged exposures to CH affected TPH activity in the brain in a region-dependent manner. Thus CH (10% O2 for 14 days) decreased TPH activity in the dorsal and median raphe, striatum, dorsomedian medulla oblongata, the locus coeruleus, and the anterior hypothalamic nucleus, whereas increased TPH activity in the ventrolateral medulla oblongata and the preoptic area (91). The effects of CH on TPH 1 and TPH 2 isoforms, however, remain to be studied.
ACETYLCHOLINE
Acetylcholine (ACh) functions as an important neurotransmitter in the autonomic nervous system, central nervous system (CNS), and at the neuromuscular junction. ACh is synthesized in the cytosol from acetyl-coenzyme A (formed via mitochondrial oxidation of pyruvate and transported via an acyl carrier into the cytosol) and choline (produced via lipid metabolism) by the catalytic action of choline acetyl transferase. ACh synthesis was assessed by monitoring the incorporation of [2H]choline and [14C]glucose into ACh.
CH (10% O2), as short as 15 min, decreases ACh synthesis in adult and developing rat brains (5, 27, 28, 30 55). The mechanisms and functional consequences of CH-evoked decrease in ACh synthesis have not been elucidated. Since the acetyl group of ACh is derived from pyruvate oxidation and pharmacological inhibition of pyruvate oxidation with either bromopyruvate or 2-keto acids reduced ACh synthesis (29), it is conceivable that reduction in precursor availability might have contributed to the decreased ACh synthesis by CH. Also, little is known on the consequences of prolonged CH (for days) on ACh synthesis. Such studies, especially in the brain will be of value in understanding the effects of CH associated with stroke on cholinergic transmission.
On the other hand, IH (consisting of alternating cycles of 1% O2 for 15 s and 21% O2 for 4 min for 60 cycles) had no significant effect on ACh levels in PC12 cell cultures, an oxygen-sensitive cell line derived from rat adrenal medullary tumors (50). Whether IH affects ACh synthesis in intact animals remains to be studied.
EXCITATORY AND INHIBITORY AMINO ACIDS
Amino acids such as glutamate and γ-amino butyric acid (GABA) are synthesized in the central and peripheral nervous systems, and they function as fast-acting neurotransmitters. Glutamate provides the major excitatory drive to neurotransmission, whereas GABA plays an inhibitory role in the CNS. GABA and glutamate synthesis involves intermediates of the citric acid cycle that are regulated by O2 availability (3, 18, 72). Consequently, hypoxia is expected to affect the synthesis of GABA and glutamate. The following sections summarize the effects of CH and IH on the GABA and glutamate synthesis.
Effects of Hypoxia on GABA Synthesis
GABA is synthesized via enzymatic decarboxylation of l-glutamate involving glutamic acid decarboxylase (GAD) and pyridoxal-l-phosphate (PLP) as cofactor. Two forms of GAD, i.e, GAD65 (vesicle associated) and GAD67 (localized in the cytosol) with molecular masses of 65 and 67 kDa, respectively, are known (49). GAD67, which has a greater affinity for PLP than GAD65, exists in vivo in a PLP-bound active form (74). The activities of GAD67 and GAD65 are regulated in vivo via reversible phosphorylation and dephosphorylation reactions (119).
CH (10% O2 for days) increased GABA levels in neurons but not in glial cells (35, 121). Although the effects of CH on GAD activity were not assessed in intact animals, studies on PC12 cells showed that CH (10% O2 for 24 h) increased GAD activity, which was in part due to increases in GAD65 and GAD67 mRNA as well as protein (53). In striking contrast, GAD activity markedly decreased in IH-treated PC12 cells (97). This decrease in GAD activity by IH was due to cAMP-protein kinase A (PKA)-dependent phosphorylation of GAD67 involving activation of dopamine 1 receptor but not as a result of changes in either GAD67 mRNA or protein expression (97). Thus studies on PC12 cell cultures revealed striking differences in the regulation of GAD activity by CH and IH, wherein the former form of hypoxia stimulates, whereas the later inhibits the enzyme activity. Furthermore, studies by Raghuraman et al. (97) suggest a cross-talk between dopaminergic and GABA-ergic systems contributing to IH-induced GAD inhibition and GABA synthesis.
GABA is involved in the regulation of blood pressure and sympathetic activity (104) and endocrine functions (32, 76, 78, 89). Since GABA is involved in regulation of cardiorespiratory functions, further studies on intact animals might unravel the role of GABAergic system in CH-evoked adapatation versus IH-induced maladaptation of cardiorespiratory systems.
Effects of Hypoxia on Glutamate Synthesis
Glutamate is primarily produced from glutamine by the action of phosphate-activated glutaminase (PAG), a mitochondrial enzyme. Alternatively, glutamate can also be generated in the cytosol via alanine aminotransferase-catalyzed reaction involving α-ketoglutarate and alanine. Glutamate generated in the cytoplasm is transported into vesicles by vesicular glutamate transporters.
Studies in PC12 cell cultures showed that CH (10% O2 for 24 h) decreased PAG activity in a time-dependent manner with a concomitant reduction in extracellular glutamate levels (53). The hypoxia-evoked reduction in PAG activity was in part due to decreased expressions of PAG mRNA and protein. In striking contrast, IH increased PAG activity and glutamate levels in PC12 cell cultures as well as in dorsal and ventral brainstem regions in rats (Kumar and Raghuraman; unpublished observations). This increase in PAG activity was associated with elevated PAG protein levels.
BIOACTIVE PEPTIDES
A variety of bioactive peptides are expressed in the central and peripheral nervous systems. The peptides expressed in neuronal tissues require α-amidation of the carboxy-terminus for their biological activity (21, 77). Examples of α-amidated peptides include neuropeptide Y (NPY) and substance P (SP). These peptides are often referred to as neuropeptides and exert powerful modulatory effects on neuronal transmission.
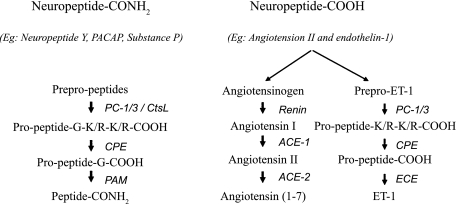
α-Amidated peptides are synthesized initially as prepro-peptide precursor molecules, which then undergo posttranslational endoproteolytic processing inside the secretory vesicles involving prohormone convertase (PC), cathepsin L (CtsL), carboxypeptidase E (CPE), and peptidyl glycine α-amidating monooxygenase (PAM) as shown in Fig. 1 (left). In the final step of α-amidated peptides synthesis, PAM catalyzes the conversion of the COOH-terminal glycine of glycine-extended peptides to a carboxy terminal amide group.
Fig. 1.
Pathways for the synthesis of mature bioactive peptides. Left: α-amidated peptide synthesis; middle: angiotensin II synthesis; right: endothelin-1 (ET-1) synthesis. PC, prohormone convertase; CtsL, cathepsin L; CPE, carboxypeptidase E; PAM, peptidyl glycine α-amidating monooxygenase; ECE, endothelin-converting enzyme; ACE, angiotensin-converting enzyme.
Effects of Hypoxia on α-Amidated Peptide Synthesis
Neuropeptide Y.
NPY is expressed in specific cell bodies and processes of the central and peripheral nervous systems (102, 111). In addition to being a potent vasoconstrictor, NPY also plays important roles in the regulation of food intake (via paraventricular nucleus in the hypothalamus), energy balance (120), and sympathetic neurotransmission (69).
The effects of CH (10% O2 for 14 days) on NPY expression were investigated in the rat brain and carotid bodies (58, 59, 92). CH selectively increased NPY-like immunoreactivity in the ventrolateral medulla oblongata, striatum, and anterior pituitary without affecting NPY expression in other brain regions (92). A similar increase in NPY-like immunoreactivity was also reported in rat carotid bodies exposed to CH (10% O2 for 3 mo) (58, 59, 92). It has been suggested that CH-evoked changes in NPY-like immunoreactivity in the carotid body and specific brain regions may contribute to adaptative mechanisms involving morphological changes in carotid bodies and alterations in sympathetic control and neuroendocrine function, respectively (92).
IH (15 s paradigm) augmented NPY-like immunoreactivity in the rat brainstem (105) and markedly upregulated NPY expression in DBH-expressing adrenal chromaffin cells (96). IH-induced increase in NPY in the adrenal medulla was associated with ROS-dependent upregulation of prepro-NPY mRNA and protein as well as increased prepro-NPY processing. Furthermore, IH-induced increase in blood pressure (57, 87) was reversed following in vivo inhibition of NPY amidation suggesting an important role for amidated peptides including NPY in blood pressure regulation during IH.
Substance P.
SP, a member of the tachykinin neuropeptide family, is involved in the regulation of pain and nociception (125), mood disorders, anxiety, stress (20), and respiratory rhythm (4).
CH (10–12% O2 for 1 h) increased SP content in the cat carotid body in vivo compared with hyperoxic and normoxic controls (94). On the other hand, severe CH (5% O2 for 1 h) reduced SP content in the rabbit carotid body (38). Since peptide secretion requires stronger stimulus than that required for biogenic amines (26), the reduced SP content by severe CH reported by Hanson et al. (38) is likely due to enhanced release of SP. Mechanisms underlying the elevated SP content in the cat carotid body by moderate CH, however, have not been examined.
Longer exposures to CH exert diverse effects on SP levels in the central and peripheral nervous systems. For instance, CH (10% O2) exposure for 3 mo leads to increased density of SP-immunoreactive nerve fibers in the nasal mucosa, especially in the intra- and subepithelial and lamina propria regions (75). Given that SP is one of the predominant signal peptides of primary sensory neurons, the increased density of SP expressing fibers may represent enhanced sensory mechanisms in the hypoxic nasal mucosa. On the other hand, long-term CH (10% O2 for 2–12 wk) decreased SP-like immunoreactivity in the cat (118) and rat (58, 59) carotid bodies. Based on these findings, Kusakabe et al. (58, 59) suggested that SP might play a role in carotid body adaptations to chronic hypoxia. In the central nervous system, 2 wk of CH (10% O2) increased SP expression in the fetal rabbit brainstem (33) but not in the adult rat brainstem (92). A recent study showed that IH increases SP expression in various regions of the rat brainstem via ROS-mediated endoproteolytic activation of PAM, the rate-limiting enzyme in the synthesis of α-amidated peptides (105).
Adrenomedullin.
Adrenomedullin (ADM) is an important regulator in the renal and cardiovascular systems, where it exerts a dose-dependent increase in vasodilation (51). Although ADM is expressed in several brain regions including the brainstem and hypothalamus (46), the effects of hypoxia on ADM expression in the central nervous system have not been studied. However, studies on non-neuronal cells showed that CH (1% O2 for 3–12 h) stimulated ADM expression in cardiac myocytes (8) and human coronary artery endothelial cells (82). The CH-induced ADM expression was associated with increases in ADM mRNA levels (81) and activation of HIF-1 transcription factor (8). The effects of IH on ADM expression have not been determined.
Effects of Hypoxia on Non-α-Amidated Peptides
Although bioactive peptides such as endothelin 1 (ET-1) and angiotensin II (ANG II) undergo prepro-peptide processing as illustrated in Fig. 1 (right and middle, respectively), the mature peptide formation in the final step is not catalyzed by PAM but involves a converting enzyme that is specific for a given type of bioactive peptide. For example, ET-converting enzyme (ECE) is the rate-limiting enzyme in ET-1 synthesis, whereas ANG-converting enzyme (ACE) catalyzes the conversion of ANG I to ANG II (Fig. 1, right and middle, respectively). The following section summarizes hypoxia-evoked changes on ET-1 and ANG II.
Endothelin-1.
ET-1, a 21-amino acid peptide with potent vasoconstrictor property, is synthesized and secreted primarily by vascular endothelial cells and several cell types in the lung. ET-1 is implicated in the regulation of vascular and airway tone and has mitogenic properties (107). In addition, ET-1 is also expressed in the central nervous system and has been shown to play important roles in the central neural control of circulation and respiration (60). Available evidence suggests that ET-1 synthesis in vascular endothelium and central nervous system is regulated by hypoxia.
Prolonged exposure to hypobaric CH (380 Torr for 14 days) increased ET-1 levels in type I cells of rat carotid bodies, which was in part due to marked upregulation of prepro-ET-1 mRNA (39). A similar increase in prepro-ET-1 mRNA was also reported in lungs of rats exposed to 4 wk of CH (10% O2; 65). CH decreases ECE expression, the rate-limiting enzyme in ET-1 synthesis, in the rat brain cortex and striatum (83).
IH significantly increased ET-1-like immunoreactivity in the carotid body of adult cats (100) and rats (15). Although ET-1 levels were not altered in IH-treated neonatal rat carotid body, its release was augmented by acute hypoxia and the ensuing ET-1 signaling mediates the sensitization of the carotid body response to hypoxia (85), a finding that is similar to that previously reported in adult cats (100). Studies in the rat heart and renal medulla suggested that IH-induced increase in ET-1 levels in adult rats may in part be due to increase in ET-1 mRNA (2). Although the molecular mechanism by which CH elevates ET-1 levels has not been delineated, the human ET-1 promoter region contains a consensus HIF-1 binding site that may contribute to the upregulation of prepro-ET-1 mRNA expression in endothelial cells (43). In addition to the HIF-1 binding site, a flanking sequence containing binding sites for the factors activator protein-1 (AP-1), GATA-2, and CAAT-binding factor (NF-1) are also indentified in prepro-ET-1 promoter, which may also contribute to enhanced ET-1 mRNA expression in response to hypoxia (122).
Angiotensin II.
Angiotensins, a family of peptide hormones with potent vasoregulatory properties, contribute to the pathogenesis of hypertension. They are formed via sequential proteolytic processing of angiotensinogen, a protein precursor, as shown in Fig. 1, middle. Among angiotensins, ANG II, the principal effector of the renin-angiotensin cascade, is primarily synthesized by a pathway requiring ACE.
Acute lowering of oxygen levels attenuated ACE activity in dog cerebral vasculature (90). Prolonged CH (10% O2 for 4 wk) increased ACE activity in rat carotid bodies, which was associated with significant increase in ACE mRNA (62, 63).
GASOTRANSMITTERS
Gasotransmitters such as nitric oxide (NO) and carbon monoxide (CO) are membrane-permeable, low-molecular-weight signaling molecules (31, 112, 116). They differ from classical neurotransmitters in that they are not stored in secretory vesicles but are enzymatically generated on demand as outlined in Table 3. The synthesis of NO and CO requires molecular oxygen and, therefore, their cellular production can be altered by hypoxia.
Table 3.
Enzymatic reactions associated with the synthesis of gasotransmitters
| Transmitters | Synthesizing Enzyme(s) | Enzymatic Reactions | Requirement for Activity |
|---|---|---|---|
| Nitric oxide (NO) | Nitric oxide synthase | l-arginine → l-citrulline + NO | NADPH and O2 |
| Carbon monoxide (CO) | Heme oxygenase | Heme → Biliverdin + Fe2+ + CO | NADPH and O2 |
| Hydrogen sulfide (H2S) | Periphery: Cystathionine γ-lyase (CSE) | l-Cysteine → Pyruvate + NH3 + H2S | PLP |
| Central nervous system: Cystathionine β-synthase (CBS) | l-Cysteine → Cystathionine + H2S | PLP |
Effects of Hypoxia on NO Synthase
NO, a freely diffusible gas, is involved in regulation of blood vessel homeostasis and neuronal cell function. NO is synthesized from l-arginine via NO synthase (NOS) involving molecular O2, NADPH (cosubstrate), and several cofactors including calmodulin, tetrahydrobiopterin (BH4), flavin adenine dinucleotide, flavin adenine mononucleotide, and heme (1). Three isoforms of NOS are known. Two isoforms are constitutively expressed in neuronal tissues (neuronal NOS; nNOS) and in endothelial cells (endothelial NOS; eNOS). They produce in a Ca2+-dependent manner low levels of NO, which play important roles in neurotransmission, vasodilatation, and inhibition of platelet and leukocyte adhesion (6, 7). The third Ca2+-independent isoform (inducible NOS; iNOS) is induced by inflammatory cytokines and LPS in many cell types but mostly in monocytes/macrophages (54). All three isoforms catalyze the conversion of arginine and O2 into NO and citrulline. eNOS is also capable of releasing NO from nitrite (79). The effects of NO on target cells are mediated via activation of the soluble guanylate cyclase (sGC) isoform, generating cGMP.
The apparent Km values for O2 for nNOS, eNOS, and iNOS were 23.2 ± 2.8, 7.7 ± 1.6, and 6.3 ± 0.9 μM, respectively (99). The regulation and mechanisms of action of NOS have been well characterized (52, 67, 68). NOS activity and the ensuing NO production by NOS in addition to substrate and cofactor availability also depends on dimeric state of NOS, which facilitates the formation of high-affinity binding sites for BH4 and l-arginine.
Neuronal NOS.
Short-term exposure (40 mmHg for 1 h) to CH decreased nNOS activity in the cat carotid body in vitro (93). Since NO inhibits carotid body activity, it was proposed that the hypoxia-induced decrease in NO results in sensory excitation via “disinhibition” of the carotid body. A similar decrease in nNOS activity was also reported in immature rat cerebellum in vivo (114) and in bovine cerebellum extracts in vitro (99). The inhibitory effect of short-term CH on nNOS activity was due to reduced oxygen availability (99) but not as a result of either lack of BH4, arginine, and NADPH availability or reduction in the amount of NOS dimers (101, 114).
On the other hand, Prabhakar et al. (95) demonstrated that prolonged CH increased nNOS activity and mRNA in rat cerebellum and nodose ganglion, a finding that is confirmed later in the rat brain (61). These observations suggest that hypoxia induces nNOS, which is constitutively expressed, similar to inducible NOS isoform; i.e., iNOS.
IH (90 s paradigm) had no significant effect on nNOS expression in the cortex of the adult rat brain (66). However, another pattern of IH comprising 5 s 7% O2 and 115 s 21% O2 for 35 days reduced nNOS mRNA and protein expression in rat paraventricular and periventricular hypothalamic nuclei and in the subfornical organ (44). These results suggest that IH-induced alterations in nNOS expression may depend on IH paradigm and brain regions under investigation.
Inducible NOS.
The effects of IH on iNOS activity and expression have been studied in mice and rats. IH increased iNOS activity in wake-active brain regions in mice and was associated with increased sleep times and shortened sleep latencies (124). The IH-induced alteration in sleep behavior was absent in mice treated with iNOS inhibitor or with genetic absence of iNOS activity, suggesting a critical role for iNOS activity in IH-evoked disturbances in sleep behavior (124). Also, IH transiently elevated iNOS expression and activity in the cortex of adult rat brains (66). These authors further showed that IH-induced neurobehavioral deficits were absent in iNOS knockout mice (66), suggesting that NO generated by iNOS is an important mediator of neurobehavioral deficits evoked by IH.
Effects of Hypoxia on Heme Oxygenase
Heme oxygenase (HO) catalyzes the cleavage of the heme ring via oxidation at the α-meso-carbon with the formation of biliverdin, gaseous carbon monoxide, and free iron (80). Three isoforms of HO have been identified: the inducible HO-1, also known as Hsp32, and the constitutive HO-2 and HO-3, which are products of individual genes (71, 73, 106). HO-2 and HO-3 share a very high level of homology, whereas they differ from HO-1 in their amino acid sequence (71). HO-1 gene expression is induced by transcription factors, such as the NF-E2-related factor-2 (Nrf2), which binds anti-oxidant response elements (ARE) in the gene promoter region (70) The ARE is critical to enzyme induction by several stimuli including LPS, cytokines, heat shock, hyperoxia, oxidants, and hypoxia-ischemia. Furthermore, HO-1 is also induced by hypoxia via hypoxia-inducible factor-1 (HIF-1; 64).
Very little is known on the effects of hypoxia on HO expression in the nervous system. However, studies on rat aortic smooth muscle cells (SMC) and rat pulmonary artery SMCs showed that CH (Po2 of 18–20 Torr for up to 12 h) augmented CO production and HO activity, which was inhibited by SnPP-9, an inhibitor of HO in a dose-dependent manner (80). The CH-induced upregulation of HO activity was associated with increased HO-1 mRNA expression. This study further showed that hypoxia increased cGMP levels, an important regulator of vascular tone in SMC, and SnPP-9 inhibited hypoxia-evoked cGMP. Based on these findings, it has been suggested that hypoxia-induced CO production regulates SMC function and vascular tone (80). No information on the effects of CH or IH on other HO-isoforms (i.e, HO-2 and HO-3) is available.
SUMMARY AND CONCLUSIONS
In summary, the above-described studies demonstrate that both CH and IH, depending on the duration and severity, profoundly impact neurotransmitter levels and neurotransmitter-synthesizing enzymes. The effects of CH and IH often show regional variations in the central and peripheral nervous system and often differ between neonates and adults. Studies on TH and GAD indicate that CH primarily utilizes transcriptional mechanisms as opposed to posttranslational regulation by IH. It would be interesting to explore whether similar such differential mechanisms also operate with other transmitters under the conditions CH and IH. Although much attention has been focused in delineating the effects of CH and IH on transmitter levels, very little is known on the functional significance of altered neurotransmitters on physiological responses to both forms of hypoxia.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants PO1HL-90554 and RO1HL-89616.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The author thanks Drs. Gayatri Raghuraman, Vandana Rai, and Suresh Sharma for their scientific contributions to studies related to glutamic acid decarboxylase, tyrosine hydroxylase, and peptidyl glycine α-amidating monooxygenase, respectively reported in this review. The author also expresses gratitude to Dr. Nanduri R. Prabhakar for several decades of constant support and exciting scientific collaborations.
REFERENCES
- 1. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, Kanagy NL. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol 295: H434–H440, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachelard HS, Lewis LD, Pontén U, Siesjö BK. Mechanisms activating glycolysis in the brain in arterial hypoxia. J Neurochem 22: 395–401, 1974 [DOI] [PubMed] [Google Scholar]
- 4. Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol 101: 219–230, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Booth RF, Harvey SA, Clark JB. Effects of in vivo hypoxia on acetylcholine synthesis by rat brain synaptosomes. J Neurochem 40: 106–110, 1983 [DOI] [PubMed] [Google Scholar]
- 6. Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Synder SH. 450 reductase. Nature 351: 714–718, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Busse R, Feming I. Regulation and functional consequences of endothelial nitric oxide formation. Ann Med 27: 331–340, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Cormier-Regard S, Nguyen SV, Claycomb WC. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem 273: 17787–17792, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Czyzyk-Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem 58: 1538–1546, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. J Biol Chem 269: 760–764, 1994 [PubMed] [Google Scholar]
- 11. Davis JN, Carlsson A. Effect of hypoxia on tyrosine and tryptophan hydroxylation in unanesthetized rat brain. J Neurochem 20: 913–915, 1973a [DOI] [PubMed] [Google Scholar]
- 12. Davis JN, Carlsson A. The effect of hypoxia on monoamine synthesis levels and metabolism in rat brain. J Neurochem 21: 783–790, 1973b [DOI] [PubMed] [Google Scholar]
- 13. Davis JN, Carlsson A, MacMillan V, Siesjö BK. Brain tryptophan hydroxylation: dependence on arterial oxygen tension. Science 182: 72–74, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Davis JN. Adaptation of brain monoamine synthesis to hypoxia in the rat. J Appl Physiol 39: 215–220, 1975 [DOI] [PubMed] [Google Scholar]
- 15. Di Giulio C, Verratti V, Artese L, Petruccelli G, Walski M, Pokorski M. Aging and expression of heme oxygenase-1 and endothelin-1 in the rat carotid body after chronic hypoxia. J Physiol Pharmacol 60, Suppl 5: 41–44, 2009 [PubMed] [Google Scholar]
- 16. Díaz-Cabiale Z, Parrado C, Fuxe K, Agnati L, Narváez JA. Receptor-receptor interactions in central cardiovascular regulation. Focus on neuropeptide/alpha2-adrenoreceptor interactions in the nucleus tractus solitarius. J Neural Transm 114: 115–125, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Ducsay CA, Hyatt K, Mlynarczyk M, Root BK, Kaushal KM, Myers DA. Long-term hypoxia modulates expression of key genes regulating adrenomedullary function in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 293: R1997–R2005, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Duffy TE, Nelson SR, Lowry OH. Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem 19: 959–977, 1972 [DOI] [PubMed] [Google Scholar]
- 19. Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91: 1025–1043, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids 31: 251–272, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Eipper BA, Mains RE. Peptide alpha-amidation. Annu Rev Physiol 50: 333–344, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Fidone S, Gonzalez C. Catecholamine synthesis in rabbit carotid body in vitro. J Physiol 333: 69–79, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fidone S, Gonzalez C, Yoshizaki K. Effects of hypoxia on catecholamine synthesis in rabbit carotid body in vitro. J Physiol 333: 81–91, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher DB, Kaufman S. The inhibition of phenylamine and tyrosine hydroxylases by high oxygen levels. J Neurochem 19: 1359–1365, 1972 [DOI] [PubMed] [Google Scholar]
- 25. Fujisawa H, Okuno S. Regulatory mechanism of tyrosine hydroxylase activity. Biochem Biophys Res Commun 338: 271–276, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosc 25: 7324–7332, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gibson GE, Blass JP. Impaired synthesis of acetylcholine in brain accompanying mild hypoxia and hypoglycemia. J Neurochem 27: 37–42, 1976 [DOI] [PubMed] [Google Scholar]
- 28. Gibson GE, Duffy TE. Impaired synthesis of acetylcholine by mild hypoxic hypoxia or nitrous oxide. J Neurochem 36: 28–33, 1981 [DOI] [PubMed] [Google Scholar]
- 29. Gibson GE, Jope R, Blass JP. Decreased synthesis of acetylcholine accompanying impaired oxidation of pyruvic acid in rat brain minces. Biochem J 148: 17–23, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gibson GE, Peterson C, Sansone J. Decreases in amino acids and acetylcholine metabolism during hypoxia. J Neurochem 37: 192–201, 1981 [DOI] [PubMed] [Google Scholar]
- 31. Gillman MA. Nitrous oxide as neurotransmitter. Lancet 339: 307, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC. The influence of γ-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 129: 2521–2529, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Gingras JL, Long WA, Segreti T, Wasserstein M. Pre- and postnatal effects of chronic maternal hypoxia on substance-P immunoreactivity in rabbit brainstem regions. Dev Neurosci 17: 350–356, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Gozal E, Shah ZA, Pequignot J-M, Pequignot J, Sachleben LR, Czyzyk-Krzeska MF, Li RC, Guo S-Z, Gozal D. Tyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol 99: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Hagberg H, Lehmann A, Sandberg M, Nystrom B, Jacobson I, Hamberger A. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab 5: 413–419, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Hanbauer I. Regulation of tyrosine hydroxylase in carotid body. Adv Biochem Psychopharmacol 16: 275–280, 1977 [PubMed] [Google Scholar]
- 37. Hanbauer I, Karoum F, Hellstrom S, Lahiri S. Effects of hypoxia lasting up to one month on the catecholamine content in rat carotid body. Neuroscience 6: 81–86, 1981 [DOI] [PubMed] [Google Scholar]
- 38. Hanson G, Jones L, Fidone S. Physiological chemoreceptor stimulation decreases enkephalin and substance P in the carotid body. Peptides 7: 767–769, 1986 [DOI] [PubMed] [Google Scholar]
- 39. He L, Chen J, Dinger B, Stensaas L, Fidone S. Endothelin modulates chemoreceptor cell function in mammalian carotid body. Adv Exp Med Biol 410: 305–311, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Hedner T, Lundborg P. Regional changes in monoamine synthesis in the developing rat brain during hypoxia. Acta Physiol Scand 106: 139–143, 1979 [DOI] [PubMed] [Google Scholar]
- 41. Hedner T, Lundborg P, Engel J. Effect of hypoxia on monoamine synthesis in brains of developing rats. Biol Neonate 31: 122–126, 1977 [DOI] [PubMed] [Google Scholar]
- 42. Hedner T, Lundborg P, Engel J. Effect of hypoxia on monoamine synthesis in brains of developing rats. III. Various O2 levels. Biol Neonate 34: 55–60, 1978 [DOI] [PubMed] [Google Scholar]
- 43. Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun 245: 894–899, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Huang J, Tamisier R, Ji E, Tong J, Weiss W.J. Chronic intermittent hypoxia modulates nNOS mRNA and protein expression in the rat hypothalamus. Resp Physiol Neurobiol 158: 30–38, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Hui AS, Striet JB, Gudelsky G, Soukhova GK, Gozal E, Beitner-Johnson D, Guo SZ, Sachleben LR, Jr, Haycock JW, Gozal D, Czyzyk-Krzeska MF. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension 42: 1130–1136, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Hwang IS, Tang F. The distribution and gene expression of adrenomedullin in the rat brain: peptide/mRNA and precursor/active peptide relationships. Neurosci Lett 267: 85–88, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Kato K, Yamaguchi-Yamada M, Yamamoto Y. Short-term hypoxia increases tyrosine hydroxylase immunoreactivity in rat carotid body. J Histochem Cytochem 58: 839–846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katz IR. Oxygen dependence of dopamine-beta-hydoxylase activity and lactate metabolism in synaptosomes from rat brain. Brain Res 231: 399–409, 1982 [DOI] [PubMed] [Google Scholar]
- 49. Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem 56: 720–723, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim DK, Natarajan N, Prabhakar NR, Kumar GK. Facilitation of dopamine and acetylcholine release by intermittent hypoxia in PC12 cells: involvement of calcium and reactive oxygen species. J Appl Physiol 96: 1206–1215, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Kitamura K, Matsui E, Kato J, Katoh F, Kita T, Tsuji T, Kangawa K, Eto T. Adrenomedullin (11–26): a novel endogenous hypertensive peptide isolated from bovine adrenal medulla. Peptides 22: 1713–1718, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J 298: 249–258, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kobayashi S, Millhorn DE. Hypoxia regulates glutamate metabolism and membrane transport in rat PC12 cells. J Neurochem 76: 1935–1948, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Kroncke KD, Feshel K, Kolb-Bachofen V. Inducible nitric oxide synthase and its product nitric oxide, a small molecule with complex biological activities. Biol Chem Hoppe Seyler 376: 327–337, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Ksiezak HJ, Gibson GE. Oxygen dependence of glucose and acetylcholine metabolism in slices and synaptosomes from rat brain. J Neurochem 37: 305–314, 1981 [DOI] [PubMed] [Google Scholar]
- 56. Kumar GK, Kim DK, Lee MS, Ramachandran R, Prabhakar NR. Activation of tyrosine hydroxylase by intermittent hypoxia: involvement of serine phosphorylation. J Appl Physiol 95: 536–544, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kusakabe T, Hirakawa H, Matsuda H, Kawakami T, Takenaka T, Hayashida Y. Peptidergic innervation in the rat carotid body after 2, 4, and 8 weeks of hypocapnic hypoxic exposure. Histol Histopathol 18: 409–418, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Kusakabe T, Hirakawa H, Matsuda H, Yamamoto Y, Nagai T, Kawakami T, Takenaka T, Hayashida Y. Changes in the peptidergic innervation in the carotid body of rats chronically exposed to hypercapnic hypoxia: an effect of arterial CO2 tension. Histol Histopathol 17: 21–29, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Kuwaki T, Ling GY, Onodera M, Ishii T, Nakamura A, Ju KH, Cao WH, Fukuda Y. Endothelin in the central control of cardiovascular and respiratory functions. Clin Exp Pharmacol Physiol 26: 989–994, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Lacza Z, Puskar M, Figueroa JP, Zhang J, Rajapakse N, Busija DW. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free Radic Biol Med 31: 1609–1615, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Lam SY, Leung PS. Chronic hypoxia activates a local angiotensin-generating system in rat carotid body. Mol Cell Endocrinol 203: 147–153, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Lam SY, Fung M-L, Leung PS. Regulation of the angiotensin-converting enzyme activity by a time-course hypoxia in the carotid body. J Appl Physiol 96: 809–813, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272: 5375–5381, 1997 [PubMed] [Google Scholar]
- 65. Li H, Chen S-J, Chen Y-F, Meng QC, Durand J, Oparil S, Elton TS. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol 77: 1451–1459, 1994 [DOI] [PubMed] [Google Scholar]
- 66. Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis 17: 44–53, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med 120: 227–237, 1994 [DOI] [PubMed] [Google Scholar]
- 68. Luiking YC, Engelen MP, Deutz NE. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care 13: 97–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lundberg JM, Franco-Cereceda A, Lacroix JS, Pernow J. Neuropeptide Y and sympathetic neurotransmission. Ann NY Acad Sci 611: 166–174, 1990 [DOI] [PubMed] [Google Scholar]
- 70. Maines MD. The heme oxygenase system: update 2005. Antioxid Redox Signal 7: 1761–1766, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Maines MD, Trakshel GM, Kutty PK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem 261: 411–419, 1986 [PubMed] [Google Scholar]
- 72. MacMillan V, Siesjö BK. Brain energy metabolism in hypoxemia. Scand J Clin Lab Invest 30: 127–136, 1972 [DOI] [PubMed] [Google Scholar]
- 73. McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem 247: 725–732, 1997 [DOI] [PubMed] [Google Scholar]
- 74. Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem 60: 395–407, 1993 [DOI] [PubMed] [Google Scholar]
- 75. Matsuda H, Kusakabe T, Hayashida Y, Furukawa M, Kawakami T, Takenaka T, Tsukuda M. Substance P- and calcitonin gene-related peptide-containing nerve fibers in the nasal mucosa of chronically hypoxic rats. Brain Res Bull 45: 563–569, 1998 [DOI] [PubMed] [Google Scholar]
- 76. Matsuoko H, Harada K, Endo Y, Warashina A, Doi Y, Nakamura J, Inoue M. Molecular mechanisms supporting a paracrine role of GABA in rat adrenal medullary cells. J Physiol 20: 4825–4842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Merkler DJ. C-terminal amidated peptides: production by the in vitro enzymatic amidation of glycine-extended peptides and the importance of the amide to bioactivity. Enzyme Microb Technol 16: 450–456, 1994 [DOI] [PubMed] [Google Scholar]
- 78. Metzeler K, Agoston A, Gratzl M. An intrinsic γ-aminobutyric acid (GABA)ergic system in the adrenal cortex: findings from human and rat adrenal glands and the NCI-H295R cell line. Endocrinology 145: 2402–2411, 2004 [DOI] [PubMed] [Google Scholar]
- 79. Mikula I, Durocher S, Martasek P, Mutus B, Slama-Schwok A. Isoform-specific differences in the nitrite reductase activity of nitric oxide synthases under hypoxia. Biochem J 418: 673–682, 2009 [DOI] [PubMed] [Google Scholar]
- 80. Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 92: 1475–1479, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nagata D, Hirata Y, Suzuki E, Kakoki M, Hayakawa H, Goto A, Ishimitsu T, Minamino N, Ono Y, Kangawa K, Matsuo H, Omata M. Hypoxia-induced adrenomedullin production in the kidney. Kidney Int 55: 1259–1267, 1999 [DOI] [PubMed] [Google Scholar]
- 82. Nakayama M, Takahashi K, Murakami O, Shirato K, Shibahara S. Induction of adrenomedullin by hypoxia in cultured human coronary artery endothelial cells. Peptides 20: 769–772, 1999 [DOI] [PubMed] [Google Scholar]
- 83. Nalivaeva NN, Fisk L, Kochkina EG, Plesneva SA, Zhuravin IA, Babusikova E, Dobrota D, Turner AJ. Effect of hypoxia/ischemia and hypoxic preconditioning/reperfusion on expression of some amyloid-degrading enzymes. Ann NY Acad Sci 1035: 21–33, 2004 [DOI] [PubMed] [Google Scholar]
- 84. Olson EB, Jr, Vidruk EH, McCrimmon DR, Dempsey JA. Monoamine neurotransmitter metabolism during acclimatization to hypoxia in rats. Respir Physiol 54: 79–96, 1983 [DOI] [PubMed] [Google Scholar]
- 85. Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 296: R735–R742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pequignot JM, Cottet-Emard JM, Dalmaz Y, Peyrin L. Dopamine and norepinephrine dynamics in rat carotid body during long-term hypoxia. J Auton Nerv Syst 21: 9–14, 1987 [DOI] [PubMed] [Google Scholar]
- 89. Peters JA, Lambert JJ, Cottrell GA. An electrophysiological investigation of the characteristics and function of GABAA receptors on bovine adrenomedullary chromaflfin cells. Pflügers Arch 415: 95–103, 1989 [DOI] [PubMed] [Google Scholar]
- 90. Pitt BR, Lister G, Dawson CA, Linehan JH. Effect of hypoxia and hypercapnia on ACE activity in the cerebral microcirculation of anesthetized dogs. Am J Physiol Heart Circ Physiol 250: H19–H24, 1986 [DOI] [PubMed] [Google Scholar]
- 91. Poncet L, Denoroy L, Dalmaz Y, Pequignot JM. Activity of tryptophan hydroxylase and content of indolamines in discrete brain regions after a long-term hypoxic exposure in the rat. Brain Res 765: 122–128, 1997 [DOI] [PubMed] [Google Scholar]
- 92. Poncet L, Denoroy L, Dalmaz Y, Pequignot JM, Jouvet M. Alteration in central and peripheral substance P- and neuropeptide Y-like immunoreactivity after chronic hypoxia in the rat. Brain Res 733: 64–72, 1996 [DOI] [PubMed] [Google Scholar]
- 93. Prabhakar NR, Kumar GK, Chang CH, Agani FH, Haxhiu MA. Nitric oxide in the sensory function of the carotid body. Brain Res 625: 16–22, 1993 [DOI] [PubMed] [Google Scholar]
- 94. Prabhakar NR, Landis SC, Kumar GK, Mullikin-Kilpatrick D, Cherniack NS, Leeman S. Substance P and neurokinin A in the cat carotid body: localization, exogenous effects and changes in content in response to arterial pO2. Brain Res 481: 205–214, 1989 [DOI] [PubMed] [Google Scholar]
- 95. Prabhakar NR, Pieramici SF, Premkumar DR, Kumar GK, Kalaria RN. Activation of nitric oxide synthase gene expression by hypoxia in central and peripheral neurons. Brain Res Mol Brain Res 43: 341–346, 1996 [DOI] [PubMed] [Google Scholar]
- 96. Raghuraman G, Kalari A, Dhingra R, Prabhakar NR, Kumar GK. Enhanced neuropeptide Y synthesis during intermittent hypoxia in the rat adrenal medulla: role of reactive oxygen species-dependent alterations in precursor peptide processing. Antioxid Redox Signal. 2011, February 6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Raghuraman G, Prabhakar NR, Kumar GK. Post-translational modification of glutamic acid decarboxylase 67 by intermittent hypoxia: evidence for the involvement of dopamine D1 receptor signaling. J Neurochem 115: 1568–1578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Raghuraman G, Rai V, Peng YJ, Prabhakar NR, Kumar GK. Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases. Antioxid Redox Signal 11: 1777–1789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rengasamy A, Johns RA. Characterization of endothelium-derived relaxing factor/nitric oxide synthase from bovine cerebellum and mechanism of modulation by high and low oxygen tensions. J Pharmacol Exp Ther 259: 310–316, 1991 [PubMed] [Google Scholar]
- 100. Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res 1086: 152–159, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Robinson MA, Tuttle SW, Otto CM, Koch CJ. pO2-dependent NO production determines OPPC activity in macrophages. Free Radic Biol Med 48: 189–195, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schalling M, Franc-Cereceda A, Hemsen A, Dagerling A, Seroogy K, Persson H, Hokfelt T, Lundberg JM. Neuropeptide Y and catecholamines synthesizing enzymes and their mRNAs in rat sympathetic neurons and adrenal gland: studies on expression, synthesis and axonal transport after pharmacological and experimental manipulations using hybridisation techniques and radioimmunoassay. Neuroscience 41: 753–766, 1991 [DOI] [PubMed] [Google Scholar]
- 103. Schnell PO, Ignacak ML, Bauer AL, Striet JB, Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase promoter activity by the von Hippel-Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J Neurochem 85: 483–491, 2003 [DOI] [PubMed] [Google Scholar]
- 104. Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002 [DOI] [PubMed] [Google Scholar]
- 105. Sharma SD, Raghuraman G, Lee M, Prabhakar NR, Kumar GK. Intermittent hypoxia activates peptidylglycine α-amidating monooxygenase in rat brain stem via reactive oxygen species-mediated proteolytic processing. J Appl Physiol 106: 12–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shibahara S, Muller R, Taguchi H, Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci USA 82: 7865–7869, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shimoda LA, Sham JSK, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 49: 549–560, 2000 [PubMed] [Google Scholar]
- 108. Sokoloff L. Circulation and energy metabolism of the brain. In: Basic Neurochemistry edited by Siegel G, Albers R, Katzman R, Agranoff B. Boston, MA: Little Brown, 1976, p. 388 [Google Scholar]
- 109. Soulier V, Dalmaz Y, Cottet-Emard JM, Kitahama K, Pequignot JM. Delayed increase of tyrosine hydroxylation in the rat A2 medullary neurons upon long-term hypoxia. Brain Res 674: 188–195, 1995 [DOI] [PubMed] [Google Scholar]
- 110. Steinsland OS, Passo SS, Nahas GG. Biphasic effect of hypoxia on adrenal catecholamine content. Am J Physiol 218: 995–998, 1970 [DOI] [PubMed] [Google Scholar]
- 111. Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y–a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296: 659–660, 1982 [DOI] [PubMed] [Google Scholar]
- 112. Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science 259: 381–384, 1993 [DOI] [PubMed] [Google Scholar]
- 113. Verna A, Schamel A, Pequignot JM. Long-term hypoxia increases the number of norepinephrine-containing glomus cells in the rat carotid body: a correlative immunocytochemical and biochemical study. J Auton Nerv Syst 44: 171–177, 1993 [DOI] [PubMed] [Google Scholar]
- 114. Wainwright MS, Arteaga E, Fink R, Ravi K, Chace DH, Black SM. Tetrahydrobiopterin and nitric oxide synthase dimer levels are not changed following hypoxia-ischemia in the newborn rat. Dev Brain Res 156: 183–192, 2005 [DOI] [PubMed] [Google Scholar]
- 115. Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299: 76, 2003 [DOI] [PubMed] [Google Scholar]
- 116. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Wang Z, Bisgard GE. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech 59: 168–177, 2002 [DOI] [PubMed] [Google Scholar]
- 118. Wang ZZ, Dinger B, Fidone SJ, Stensaas LJ. Changes in tyrosine hydroxylase and substance P immunoreactivity in the cat carotid body following chronic hypoxia and denervation. Neuroscience 83: 1273–1281, 1998 [DOI] [PubMed] [Google Scholar]
- 119. Wei J, Wu JY. Post-translational regulation of l-glutamic acid decarboxylase in the brain. Neurochem Res 33: 1459–1465, 2008 [DOI] [PubMed] [Google Scholar]
- 120. White JD. Neuropeptide Y: a central regulator of energy homeostasis. Regul Pept 49: 93–107, 1993 [DOI] [PubMed] [Google Scholar]
- 121. Wood JD, Watson WJ, Drucker AJ. The effect of hypoxia on brain gamma-aminobutyric acid levels. J Neurochem 15: 603–608, 1968 [DOI] [PubMed] [Google Scholar]
- 122. Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, and p300/CBP. J Biol Chem 276: 12645–12653, 2001 [DOI] [PubMed] [Google Scholar]
- 123. Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem 280: 4321–4328, 2005 [DOI] [PubMed] [Google Scholar]
- 124. Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: Hypersomnolence and brain injury. Am J Respir Crit Care Med 171: 1414–1420, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zubrzycka M, Janecka A. Substance P: transmitter of nociception. Endocr Regul 34: 195–201, 2000 [PubMed] [Google Scholar]