Abstract
The consumption of cacao-derived (i.e., cocoa) products provides beneficial cardiovascular effects in healthy subjects as well as individuals with endothelial dysfunction such as smokers, diabetics, and postmenopausal women. The vascular actions of cocoa are related to enhanced nitric oxide (NO) production. These actions can be reproduced by the administration of the cacao flavanol (−)-epicatechin (EPI). To further understand the mechanisms behind the vascular action of EPI, we investigated the effects of Ca2+ depletion on endothelial nitric oxide (NO) synthase (eNOS) activation/phosphorylation and translocation. Human coronary artery endothelial cells were treated with EPI or with bradykinin (BK), a well-known Ca2+-dependent eNOS activator. Results demonstrate that both EPI and BK induce increases in intracellular calcium and NO levels. However, under Ca2+-free conditions, EPI (but not BK) is still capable of inducing NO production through eNOS phosphorylation at serine 615, 633, and 1177. Interestingly, EPI-induced translocation of eNOS from the plasmalemma was abolished upon Ca2+ depletion. Thus, under Ca2+-free conditions, EPI can stimulate NO synthesis independent of calmodulin binding to eNOS and of its translocation into the cytoplasm. We also examined the effect of EPI on the NO/cGMP/vasodilator-stimulated phosphoprotein (VASP) pathway activation in isolated Ca2+-deprived canine mesenteric arteries. Results demonstrate that under these conditions, EPI induces the activation of this vasorelaxation-related pathway and that this effect is inhibited by pretreatment with nitro-l-arginine methyl ester, suggesting a functional relevance for this phenomenon.
Keywords: cocoa flavanols, nitro-l-arginine methyl ester, bradykinin
cardiovascular diseases (CVD) are a leading cause of morbidity and mortality, and the pathogenesis of these diseases is frequently linked to endothelial cell dysfunction (33). The consumption of cacao-derived products, particularly from dark chocolate (referred to herein as cocoa) has provided beneficial cardiovascular effects in normal individuals (Kuna Indians) and in those with endothelial (i.e., vascular) dysfunction such as smokers, diabetics, and postmenopausal women (7). In a cohort of 470 elderly men, cocoa intake was inversely associated with blood pressure as well as 15-year cardiovascular and all-cause mortality (4). In 19,357 participants of the Potsdam arm of the European Prospective Investigation into Cancer, 6 g of chocolate consumption appeared to lower CVD risk (stroke and myocardial infarction), in part by lowering blood pressure (5). Recently, it has been shown that cocoa intake can improve vascular function in coronary artery disease patients (16).
The actions of cocoa on the vasculature are related to its ability to activate endothelial nitric oxide (NO) synthase (eNOS), and thus enhance NO production (7, 32). These actions can be reproduced by the administration of (−)-epicatechin (EPI) to animals or humans, which is the most abundant flavanol present in cacao (32). It is well accepted that the activation of eNOS can exert cardioprotective effects (32, 18) and thus, agents that stimulate its activity may be of therapeutic interest. In support of this, we recently reported on the cardioprotective effects of EPI on animals subjected to ischemia-reperfusion or permanent coronary occlusion (35, 36).
Recently, we demonstrated that EPI is capable of inducing the synthesis of NO via eNOS activation in human coronary artery endothelial cells (HCAEC) (31). These effects are similar to those exerted by endogenous stimulators such as bradykinin (BK). In HCAEC, both EPI and BK are capable of inducing eNOS activation and NO production via the PI3K/AKT/PKA and Ca2+-CaM/CaMKII pathways (31). Furthermore, there is evidence of a possible acceptor-effector-like molecule on the plasmalemma. It is well established that eNOS activation can be both Ca2+-dependent and Ca2+-independent (28). Most ligands, including BK and acetylcholine, stimulate eNOS by raising the intracellular calcium concentration ([Ca2+]i), which in turn forms a Ca2+/calmodulin (Ca2+-CaM) complex that binds to eNOS (26). eNOS function is also modulated by its interaction with proteins such as caveolin-1 (Cav-1) or heat shock protein 90 (HSP90) (14, 29). Under basal conditions, the majority of eNOS present in the caveolae is bound to Cav-1, while its enzymatic activity remains repressed (20, 25). Enzymatic inhibition is removed by eNOS translocation from the caveolae into the cytoplasm. The Ca2+-CaM complex displaces eNOS from Cav-1 through the action of Ca2+-mobilizing agonists (20).
In our previous study, we demonstrated that under the pharmacological inhibition of intracellular signaling pathways that completely block BK-induced effects on eNOS, EPI was still capable of partially stimulating NO production (∼27%) (25). These results suggested that EPI may be uniquely able to stimulate eNOS in a Ca2+-independent manner. The results from the present study demonstrate that in HCAEC, EPI can induce eNOS activation in a novel manner that is both [Ca2+]i and translocation independent.
METHODS
Materials.
HCAEC and HCAEC growth medium were purchased from Cell Applications, and Dulbecco's Modified Eagle's Media (DMEM) and Hank's phenol red free as well as Hank's phenol red and Ca2+ free were from GIBCO. BRL, EPI, protease, and phosphatase inhibitor cocktails caffeine, EGTA, catechin, cholera toxin subunit B peroxidase conjugate (CTB-HRP), amlodipine besylate, NG-nitro-l-arginine methyl ester hydrochloride (l-NAME), and sildenalfil citrate salt were obtained from Sigma Chemicals. Quercetin was from Indofine Chemical. BAPTA was from Enzo-Life Sciences. Trichloroacetic acid and ethyl ether were from Fisher Scientific. Phospho-eNOS-Ser-615 primary antibody was from Upstate. eNOS-Ser-1177, phospho-eNOS-Ser-633, eNOS, Cav-1, phospho vasodilator-stimulated phosphoprotein (VASP)-Ser-239, VASP, and GAPDH primary antibodies, normal rabbit IgG control, and horseradish peroxidase (HRP)-conjugated secondary antibodies were from Cell Signaling Technology. Protein G-agarose, phospho-eNOS Thr-495, CaMI, and transferrin receptor (TfR) primary antibodies were obtained from Santa Cruz Biotechnologies. Ultracentrifuge tubes (catalog no. 347356) were from Beckman Coulter, polyvidinil fluoride (PVDF) transfer membrane was from Millipore, and calcium green TM2 was from Invitrogen. BK (used as a positive control) was from EMD Biosciences. ECL Plus Western Blot detection kit was from Amersham. The nitrite/nitrate fluorometric assay kit and cGMP EIA kit were from Cayman Chemical.
Cell culture.
Commercially available (Cell Applications) HCAEC from 14-, 40-, and 60-year-old healthy males were maintained in a humidified atmosphere at 37°C with 5% CO2 and 95% O2 in HCAEC growth medium. HACAEC between passages 12–14 were used for all experiments. Treatments were applied to confluent cell cultures.
[Ca2+]i measurements.
HCAEC cultures were trypsinized and resuspended in HCAEC growth media. One milliliter of cell suspension (2 × 105 cells/ml) was placed in each well of a 24-well plate, and the cells were allowed to attach and settle for 24 h. To maintain cells in a steady state, they were incubated with DMEM plus 0.5% fetal bovine serum (FBS) 24 h before experiments. Cells were incubated with M-199 without phenol red or FBS and were supplemented with 200 mol/l of glutamine 6 h before experiments. Two experimental groups of HCAEC were generated: regular calcium and calcium deprived. Each group was subdivided for EPI or BK treatments. HCAEC were deprived of Ca2+ by washing them (3 × 1 min) with Epilife media (pH 7.4) without Ca2+ or phenol red and were supplemented with 0.1 mmol/l EGTA, 25 μmol/l BAPTA (Ca2+ chelators), and 0.1 mmol/l caffeine to deplete intracellular Ca2+ concentration ([Ca2+]i) deposits. Cells were washed with either regular MOPS-Krebs-Henseleit solution (Krebs 1) composed of (in mmol/l) 137 NaCl, 6 KCl, 1.8 CaCl2, 1.2 NaH2PO4, 1.2 MgSO4·7H2O, 5 dextrose, 2 sodium pyruvate, and 10 MOPS or with Ca2+-free Krebs (Krebs 2). Cells were then incubated for 2 h at 37°C with 500 μl of 3 μmol/l calcium green TM2 diluted in the respective Krebs solution. Cells were washed with 500 μl Krebs 1 or Krebs 2 (whichever applicable) 3 × 1 min. Cells were allowed to settle for 1 h, and then the plate was inserted into a Synergy HT Fluorometer (BioTek). Either EPI or BK [0.1 nmol/l to 1 μmol/l] were automatically applied to the wells to measure intracellular dose-response increases in [Ca2+]i (calcium kinetics from 0 to 10 s) at excitation and emission wavelengths of 503 and 536 nm.
NO measurements.
NO levels were measured using a fluorescent kit and a fluorometer (FLx800 Bio-Tek Instruments). EPI was diluted in water and BK (used as positive control) in DMSO (water or DMSO were used as vehicle for control cells). EPI and BK-induced NO dose-response curves were generated. For these experiments, cells were treated with either EPI or BK [0.1 nmol/l to 1 μmol/l], and culture media samples were collected at 10 min (peak time of NO response) as end point to measure extracellular NO indirectly (31).
Immunoprecipitation.
Immunoprecipitation assays were performed as described previously (30). Briefly, cells were lysed with 50 μl of nondenaturing extraction buffer (0.5%, Triton X-100, 50 mmol/l Tris·HCl, pH 7.4, 0.15 mol/l NaCl, and 0.5 mmol/l EDTA) and supplemented with protease and phosphatase inhibitor cocktail, plus 1 mmol/l PMSF, 2 mmol/l Na3VO4, and 1 mmol/l NaF. Homogenates were incubated on ice for 10 min and passed through an insulin syringe five times. The homogenate was incubated on ice with shaking for 10 min and centrifuged (10 min) at 12,000 g at 4°C. A total of 0.5 mg protein was precleared by adding 1 μg of normal rabbit IgG control and 20 μl prot-G-agarose with mixing for 30 min (4°C) and subsequent centrifugation at 12,000 g for 10 min at 4°C. The supernatant was recovered and incubated at 4°C under mild agitation with 3 μg of immunoprecipitating anti-eNOS antibody. Twenty microliters of protein G-sepharose were added, and the mixture was incubated at 4°C for 3 h with shaking. The immunoprecipitation mixture was centrifuged at 12,000 g for 15 min at 4°C, and the supernatant was recovered and stored at 4°C. The pellet was washed three times with extraction buffer and centrifuged at 12,000 g for 15 min at 4°C. The immunoprecipitated proteins in the pellet and those remaining in the supernatant were applied to a 5% or 10% SDS-PAGE for immunoblotting. Coimmunoprecipitation was also performed with anti Cav-1 or anti-CaMI antibodies to confirm results. The assay was carried out at least three times with each immunoprecipitating antibody.
Immunoblotting.
Cells grown on 10-cm dishes were homogenized in 50 μl lysis buffer (1% Triton X-100, 20 mmol/l Tris, 140 mmol/l NaCl, 2 mmol/l EDTA, and 0.1% SDS) with protease and phosphatase inhibitor cocktails supplemented with 1 mmol/l PMSF, 2 mmol/l Na3VO4, and 1 mmol/l NaF. Homogenates were passed through an insulin syringe five times, sonicated for 30 min at 4°C, and centrifuged (12,000 g) for 10 min. The total protein content was measured in the supernatant. A total of 40 μg of protein was loaded onto a 5% or 10% SDS-PAGE, electrotransferred, incubated for 1 h in blocking solution (5% nonfat dry milk in TBS plus 0.1% Tween 20 [TBS-T]), and followed by either 3-h incubation at room temperature or overnight incubation at 4°C with primary antibodies. Primary antibodies were typically diluted 1:1,000 or 1:2,000 in TBS-T plus 5% bovine serum albumin. Membranes were washed (3× for 5 min) in TBS-T and incubated 1 h at room temperature in the presence of HRP-conjugated secondary antibodies diluted 1:10,000 in blocking solution. Membranes were again washed three times in TBS-T, and the immunoblots were developed using an ECL detection kit. The band intensities were digitally quantified.
Detergent-resistant membrane isolation.
Detergent-resistant membrane (DRM) (lipid rafts and caveolae) isolation was performed as previously described (24). Briefly, ∼4.5 × 106 cells were lysed with 300 μl of cold TNE buffer (20 mmol/l Tris, 140 mmol/l NaCl, and 2 mmol/l EDTA) containing 0.05% Triton X-100 plus protease and phosphatase inhibitors. Lysates were mixed with 375 μl of 80% sucrose in TNE-Triton X-100 buffer and transferred to ultracentrifuge tubes. Cell lysates, placed in 45% sucrose, were gently overlaid with 1 ml of 35% sucrose in TNE Triton X-100 buffer, while the latter fraction was overlaid with 400 μl of 5% sucrose in TNE-Triton X-100 buffer. Samples were centrifuged at 4°C for 16 h at 170,000 g in an Optima TLX ultracentrifuge using the TLS 55 rotor (Beckman Coulter) to form a 45–5% sucrose gradient. After centrifugation, eight fractions were collected. Five microliters of each sucrose gradient fraction were placed onto a PVDF membrane. The drop was allowed to dry, and the PVDF membrane was incubated 1 h at room temperature (RT) in blocking solution. The PVDF membrane was subsequently incubated with 1:2,000 CT-B-HRP [used as ganglioside M1 (GM1) marker] dilution in blocking solution and developed using an ECL detection kit.
NO/cGMP pathway analysis.
Mesenteric arteries (1A branch) from male dogs (n = 3) were isolated in Hank's solution, after removing blood, fat, and surrounding tissue, the arteries were washed 3 × 10 min either with phenol red-free Hank's solution (group 1) or with phenol red and Ca2+-free Epilife media supplemented with 1 mmol/l of the Ca2+ chelator EGTA, 1 mmol/l caffeine to deplete [Ca2+]i deposits (17), 10 μmol/l sildenafil, and with or without 0.1 mmol/l l-NAME (group 2). Subsequently, arteries from each group were incubated in the presence of 1 μmol/l EPI or 1 μmol/l BK in their respective media for 5 min. One gram of artery was frozen in liquid nitrogen and homogenized in 5% TCA for cGMP measurement following cGMP EIA Kit instructions, and 500 mg of artery were homogenized with 500 μl of lysis buffer for protein extraction, which was in turn used for Western blot analysis of VASP/p-VASP.
The experimental protocols were approved by the UCSD animal care and use committee (IAICUC).
Data analysis.
A minimum of three experiments were performed (each in triplicate) unless otherwise noted. Statistical analysis was performed using t-test or ANOVA and Tukey post hoc tests for multiple comparisons. Significance is noted at P < 0.05.
RESULTS
EPI-induced NO production is partially Ca2+ independent.
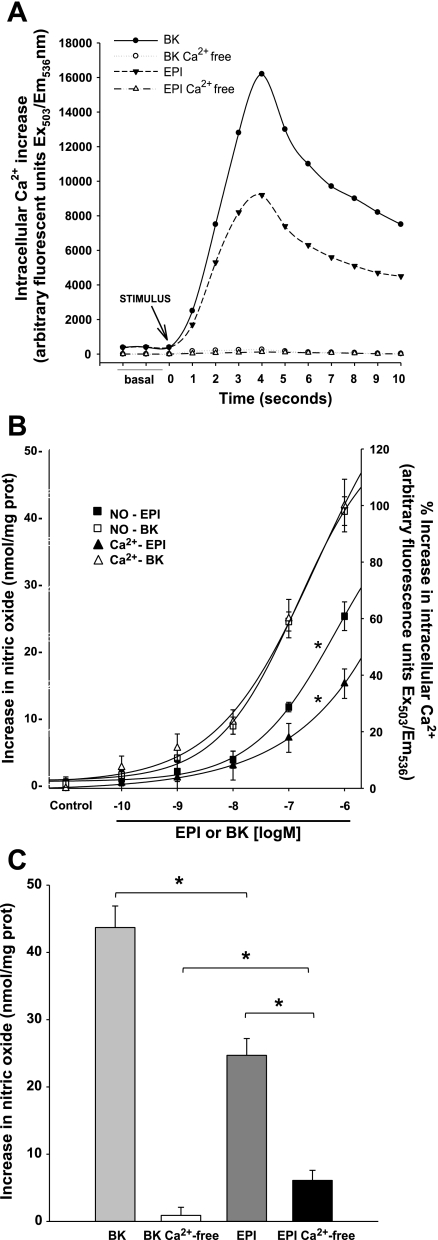
To examine the capacity of EPI to increase [Ca2+i] levels, we measured Cai2+ kinetics after treatment of cells with either BK or EPI (1 μmol/l). Cai2+ kinetics induced by both agonists follows the same pattern. However, EPI induced a lower Cai2+ release than BK. Under calcium-free conditions, neither EPI nor BK stimulated Cai2+ release, demonstrating the effectiveness of extra and intracellular Ca2+ deprivation (Fig. 1A).
Fig. 1.
A: (−)-Epicatechin (EPI) and bradykinin (BK) treatment of human coronary artery endothelial cells (HCAEC) induces increases in intracellular Ca2+ concentration ([Ca2+]i) as measured by changes in relative fluorescence intensity (Ex/Em 503/536). Calcium-free HCAEC treated with 1 EPI or 1 μmol/l BK do not show increases in fluorescence regardless of treatment. B: nitric oxide (NO) production and [Ca2+]i increases were separately measured in HCAEC treated with BK or EPI. Increases in NO production and [Ca2+]i in cells treated with increasing concentrations of BK or EPI were assessed. EPI induced a dose-response increase in NO of ∼60% compared with BK-induced effects. However, increases in [Ca2+]i (as compared with BK) do not match increases in NO, suggesting the possibility of EPI-induced calcium independent increases in NO. Data are expressed as means ± SD (n = 3) and analyzed by ANOVA, *P < 0.05. C: in calcium-free conditions, BK induced no increases in NO nor in [Ca2+]i. However, EPI is able to stimulate NO (∼25% of production in presence of calcium). Data are expressed as means ± SD (n = 3) and analyzed by t-test, *P < 0.05.
Given the known dependence of agonist-induced NO production on increases in [Ca2+]i (1), we measured [Ca2+]i increases and NO synthesis in BK- and EPI-treated cells. Increasing concentrations of BK and EPI (0.1 nmol/l-1 μmol/l) were used. NO production and [Ca2+]i reached maximum levels at 1 μmol/l for both BK and EPI treatments (Fig. 1B). BK-induced increases in [Ca2+]i were paralleled by increases in NO synthesis. However, in cells treated with EPI, the relationship between NO production and [Ca2+]i (normalized to BK effects) was not comparable; NO production was higher than corresponding [Ca2+]i increases. In other words, the ratio of NO production to [Ca2+]i is higher from EPI-induced effects than for BK-induced effects, with this being particularly evident between 10 nmol/l-1 μmol/l. These results suggest that the activation of eNOS by EPI is partially Ca2+ independent. In Ca2+-free conditions, BK-induced NO synthesis was completely abrogated. However, EPI-treated HCAEC with Ca2+ deprivation were still capable of producing NO ∼25% of that synthesized under normal calcium conditions (Fig. 1C).
EPI induces phosphorylation of eNOS amino acid activation residues under Ca2+-free conditions.
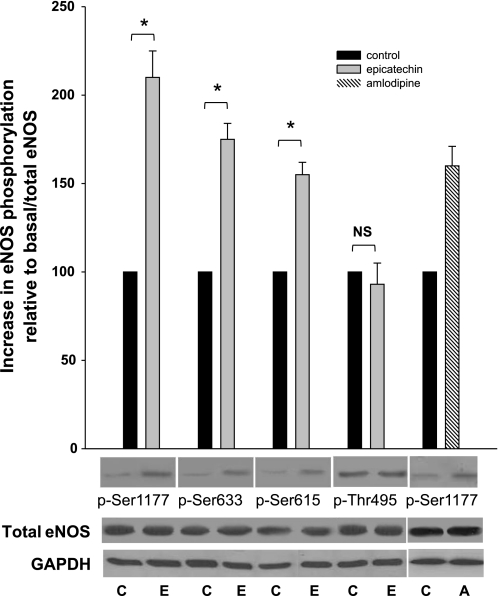
The phosphorylation status of Ser-1177, Ser-633, Ser-615 (stimulation sites), and Thr-495 (inhibition site) reflect eNOS activity. To assess eNOS activation under Ca2+-free conditions, we evaluated the phosphorylation of these residues in EPI-treated cells (Fig. 2). Changes in phosphorylation status were only observed in activation residues Ser-1177, Ser-633, and Ser-615. Overall, phosphorylation status was increased compared with control (unstimulated cells). Interestingly, the status of the inactivation residue Thr-495 was not altered, indicating its Ca2+ dependency. The results indicate that EPI-induced NO production under Ca2+-free conditions is mediated by changes in the phosphorylation of Ser-1177, Ser-633, and Ser-615 but not in Thr-495. Amlodipine, a known calcium independent inducer of eNOS Ser-1177 phosphorylation (22), was used as a positive control.
Fig. 2.
EPI activates endothelial NO synthase (eNOS) through Ser-1177, -633, and -615 phosphorylation in the absence of Ca2+. The relative phosphorylation of serine residues to total basal eNOS phosphorylation increased in 1 μmol/l EPI-treated HCAEC. Phosphorylation of Ser-1177 increased by 100%, Ser-633 75%, and Ser-615 by 65% vs. phosphorylation in controls. No changes in Thr-495 phosphorylation were observed. Amlodipine was used as a positive control for eNOS Ser-1177 phosphorylation in calcium-deprived endothelial cells. Data are expressed as means ± SD (n = 3, and analyzed by t-test, *P < 0.05). C, control; E, EPI; A, amlodipine.
EPI treatment activates eNOS without dissociation from caveolin-1 (Cav-1).
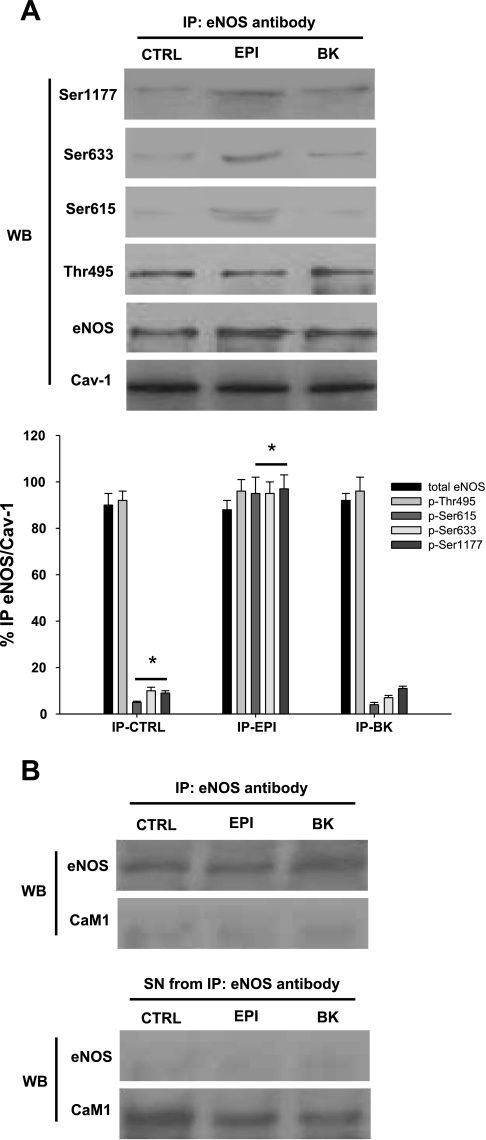
It has been reported in endothelial cells that after an agonist induces increases in [Ca2+]i, eNOS activates and disengages from Cav-1 translocating to the cytoplasm (20). Because we observed eNOS activation in EPI-treated HCAEC under Ca2+-free conditions, we decided to investigate whether eNOS disengages from Cav-1 under this condition. We used specific antibodies to immunoprecipitated eNOS, Cav-1, and CaMI in control, BK- and EPI-treated HCAEC under Ca2+-free conditions. The immunoprecipitated phase (IP) was then used for immunoblots analysis of total eNOS, eNOS Ser-615, Ser-633, Ser-1177, and Thr-495 residues as well as Cav-1 (Fig. 3A). In control cells, as well as in BK- and EPI-treated cells, eNOS did not detach from Cav-1, suggesting that Ca2+ is essential for the detachment of eNOS from caveolae. In the absence of Ca2+, BK-treated HCAEC responses resembled those from control conditions because BK did not induce phosphorylation changes in eNOS amino acid residues, nor its dissociation from Cav-1 (Fig. 3A). In contrast, the IP phase of EPI-treated HCAEC demonstrated enhanced phosphorylation of Ser-1177, Ser-633, and Ser-615 without dissociating from Cav-1. Furthermore, changes in Thr-495 phosphorylation were not observed, indicating that under calcium-free conditions its dephosphorylation is not required for eNOS activation. The Western blot for the supernatant phase of the IP does not show total eNOS or eNOS phosphorylation residues, which indicates that the enzyme is still bound to caveolae after treatment (data not shown). IP assays also show the lack of association between eNOS and CaM after treatment, which indicates that CaM is not necessary for eNOS activation under calcium-free conditions (Fig. 3B).
Fig. 3.
eNOS is activated by EPI in Ca2+-free HCAEC without detachment from caveloin-1 (Cav-1). A: total protein from EPI- or BK-treated HCAEC was precipitated with either Cav-1 or eNOS antibodies. Western blots were performed on the immunoprecipitated phase against phosphorylated eNOS residues,\ eNOS and Cav-1. In control HCAEC, eNOS was not activated nor disengaged from Cav-1. BK treatment did not activate eNOS as observed by the phosphorylation status of Ser-1177, Ser-633, and Ser-615. Also eNOS did not disengage from Cav-1. B: eNOS does not associate with CaMI in Ca2+-free HCAEC treated with EPI or BK as controls. HCAEC were lysed and precipitated with either eNOS or CaMI antibodies. The supernatant phase displayed only CaM1 presence but not eNOS. Data are expressed as means ± SD (n = 3, *P < 0.05 by ANOVA).
EPI-activated eNOS is found in DRM cell fractions.
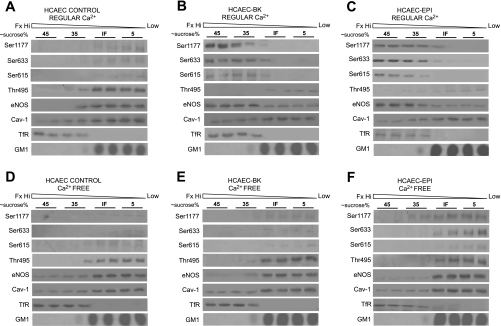
To further determine eNOS localization under Ca2+-free conditions, we used a subcellular fractionation (based on a sucrose gradient with concentrations from 45 to 5%) that allows us to establish the migration pattern of proteins enriched in DRM (such as Cav-1 and eNOS). Each of the subcellular fractions was used to measure total eNOS and phosphorylation status of Ser-1177, Ser-633, Ser-615, and Thr-495. An antibody to Cav-1 and CT-B-HRP-conjugated (which binds specifically to ganglioside GM1) were used as markers for DRM (lipid rafts and caveolae) found in low-density fractions. An antibody for the TfR was employed as a migration control for membranes not containing lipid rafts or caveolae, where TfR shifts to high-density fractions. In control HCAEC exposed to regular Ca2+, Ser-1177, Ser-633, and Ser-615 were not phosphorylated, whereas Thr-495 was phosphorylated, reflecting eNOS inactivity (Fig. 4A). eNOS was found in the low-density sucrose fraction, along with Cav-1, while TfR was contained in the 45% sucrose fraction (Fig. 4A). Sucrose gradient of BK-treated HCAEC with regular Ca2+ yield phosphorylation of Ser-1177, Ser-633, and Ser-615 and dephosphorylation of Thr-495, characteristics of eNOS activation (Fig. 4B). eNOS was present mostly in the 35% sucrose fraction, suggesting its translocation from low-density membrane lipids to the high-density fraction corresponding to no lipid rafts or caveolae domains (Fig. 4B). Similar to BK, the sucrose gradient of EPI-treated HCAEC with regular Ca2+ showed activation of eNOS, evidenced by the phosphorylation of Ser-1177, Ser-633, and Ser-615, and dephosphorylation of Thr-495 (Fig. 4C). Furthermore, eNOS was localized to sucrose fractions 45–35% along with TfR (Fig. 4C). Once we defined the activity and subcellular location of eNOS under regular Ca2+ conditions, we repeated the experiments under Ca2+-free conditions.
Fig. 4.
eNOS is activated by EPI and remains attached to caveolae/lipid rafts in Ca2+-free HCAEC. Total protein extracts from HCAEC were arranged in a sucrose gradient. Sucrose gradients of 45, 35, and 5% were used for the detection of eNOS residues, eNOS, Cav-1, transferrin receptor (TfR), and ganglioside M1 (GM1). A: control HCAEC in the presence of Ca2+ showed an inactive eNOS, located in the low-sucrose density fraction, along with Cav-1 and GM1. B: BK-treated HCAEC in the presence of regular Ca2+ had activated eNOS located in the high-sucrose density fraction as evidenced by the presence of TfR. C: EPI treatment of HCAEC in the presence of regular Ca2+-activated and localized eNOS to high-sucrose density fractions. D: control HCAEC free of Ca2+ had inactive eNOS in low-sucrose density fractions. E: BK was unable to activate and translocate eNOS to high-sucrose density fractions in Ca2+-free HCAEC. F: EPI activated eNOS without translocation to high-sucrose density fractions in Ca2+-free HCAEC.
Control HCAEC contained inactive eNOS localized to the low-density region of the sucrose gradient (Fig. 4D). Ca2+-free HCAEC treated with BK did not show phosphorylation of Ser-1177, Ser-633, and Ser-615 or dephosphorylation of Thr-495, indicating eNOS inactivation (Fig. 4E). Moreover, eNOS did not translocate to denser sucrose fractions as it was found in the 5% sucrose region along with Cav-1 (Fig. 4E). This result is consistent with our other experiments where BK-induced effects were noted as being Ca2+ dependent. Treatment of HCAEC with EPI under Ca2+-free conditions confirmed our previous results showing activation of eNOS. Of interest is the fact that phosphorylated eNOS at Ser-1177, Ser-633, and Ser-615 residues were localized in the low-density sucrose fraction (IF-5%) (Fig. 4F). These results indicate that under Ca2+-free conditions, the activation of eNOS takes place without translocation from the low-density region of membrane lipids and disengagement from caveolae.
EPI induces NO/cGMP/VASP pathway activation in Ca2+-deprived canine mesenteric arteries.
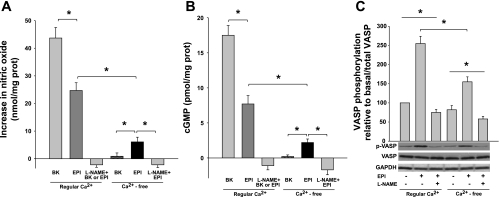
To determine whether EPI is able to induce the activation of the NO/cGMP/VASP vasorelaxation pathway through eNOS activation in vascular tissue in the absence of Ca2+, we used canine mesenteric arteries as an ex vivo functional vascular model. Vessels were deprived of Ca2+ and incubated with either 1 μmol/l EPI or BK, 10 μmol/l sildenafil, in the absence or presence of 0.1 mmol/l l-NAME. NO production was measured in vessel incubation media. EPI was able to induce increases in NO concentration in Ca2+-deprived arteries (∼25% of NO production with respect to the production in vessels in presence of Ca2+), EPI-induced NO production, either in presence or absence of Ca2+ was abolished by pretreatment with l-NAME (Fig. 5A). Meanwhile, BK under regular calcium conditions induced NO increase in the vessels but it is not capable to induce NO increases in Ca2+-free conditions (Fig. 5A). To further explore a possible physiological relevance of NO production in the Ca2+-deprived blood vessels, we measured the accumulation of cGMP (Fig. 5B). Results demonstrate a significant increase in cGMP production in EPI-treated Ca2+-deprived arteries, that represents ∼22% of total EPI-induced cGMP increases in Ca2+-containing arteries. Increases in cGMP were abolished by pretreatment with l-NAME. Similar results were found in the phosphorylation/activation of VASP at residue Ser-239 (p-VASP) (Fig. 5C), which is a biomonitor of PKG activity as a consequence of NO effects on vascular smooth muscle (27).
Fig. 5.
NO/cGMP/vasodilator-stimulated phosphoprotein (VASP) pathway activation in isolated Ca2+-deprived canine mesenteric arteries. A: NO production was measured in vessel incubation media. BK- and EPI-treated mesenteric arteries under regular Ca2+ conditions showed substantial amounts of NO production. In contrast, in the Ca2+-deprived vessels, BK is unable to induce NO increase; however, EPI is still cable to induce a discrete but sustained NO amount in the vessel under these conditions, in both conditions the induced NO production was blocked by pretreatment of the blood vessels with NG-nitro-l-arginine methyl ester (l-NAME). B: cGMP accumulation was measured in homogenized vessels. BK as well as EPI-treated mesenteric arteries under regular Ca2+ conditions showed a large amount of cGMP. In Ca2+-deprived vessels, EPI is able to induce discrete increases in cGMP but BK does not. There is no cGMP increase in vessels pretreated with l-NAME. C: protein extracts from EPI or l-NAME plus EPI-treated and untreated mesenteric arteries under regular Ca2+ and Ca2+-deprived conditions were assessed by Western blot for VASP and p-VASP. Data are expressed as means ± SD (n = 3, and analyzed by t-test *P < 0.05).
Catechin and quercetin effects on NO production in HCAEC in Ca2+-free conditions.
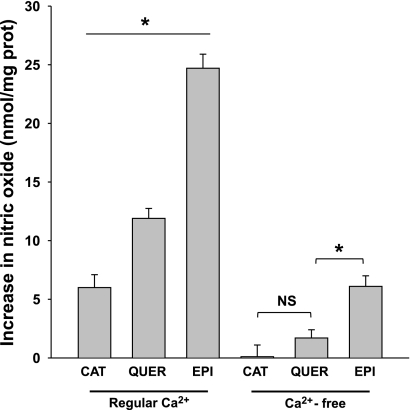
Figure 6 summarizes the effects of 1 μmol/l of catechin (a structural stereoisomer of EPI) or quercetin (a related flavonoid) compared with EPI-induced effects on NO production either in the presence or absence of Ca2+. Under regular Ca2+ conditions the stimulation of cells with catechin induced ∼25% and quercetin ∼50% of EPI-induced effects on NO production in HCAEC in culture. When the cells are Ca2+-deprived, catechin induced no effect on NO production, whereas quercetin induced ∼27% of the EPI effect.
Fig. 6.
Increase in NO concentration in HCAECs under regular Ca2+ and Ca2+-deprived conditions, treated with either catechin (CAT), quercetin (QUER), or EPI (1 μmol/l). In regular Ca2+ conditions EPI induced an increase of approximately fourfold in NO vs. catechin, and twofold vs. quercetin. Whereas in Ca2+-free conditions, EPI is able to induce more than threefold increase NO production vs. quercetin, while catechin induced no effect. Data are expressed as means ± SD (n = 3). *P < 0.05.
DISCUSSION
Strategies aimed at increasing NO bioavailability are of promise in the prevention and therapy of CVD. We recently reported that EPI is capable of stimulating NO production via eNOS activation and that the inhibition of phospholipase C (PLC) (a major intracellular calcium pathway activator) does not completely abolish EPI effects (31). These results prompted us to examine whether EPI is able to induce eNOS activation in a calcium-independent manner.
The molecular mechanisms that regulate eNOS activity in vascular tissue have been extensively studied. In response to physiological stimuli, eNOS activity regulation is mediated through phosphorylation at key residues (3, 8, 12). The phosphorylation of eNOS-Ser-1177, Ser-633, and Ser-615 (human sequence) is associated with increases in enzymatic activity (23, 13), whereas the phosphorylation at Thr-495 decreases its activity (6, 15).
It has been reported that a number of dihydropyridine Ca2+ antagonists, including nifedipine (21) and amlodipine (37), enhance NO production in endothelial cells independently of increases in intracellular calcium inducing in turn, relaxation of coronary arteries by a mechanism involving eNOS-Ser-1177 phosphorylation (22). In this study, we demonstrate that EPI induces a moderate but notable increase in NO production through eNOS Ser-615, Ser-633, and Ser-1177 phosphorylation in the absence of cytosolic Ca2+ showing similar patterns of phosphorylation of eNOS-Ser-1177.
Another way to regulating eNOS activity is trough modulation of intracellular calcium concentration. For example, shear stress activates eNOS independently of large increases in [Ca2+]i, but this stimulation results in rather low NO production (9) compared with agonist-evoked effects, where large increases in [Ca2+]i are induced.
Typically, agonist-induced eNOS activation follows its dissociation from caveolin-1 and subsequent translocation into the cytosol, which requires increases in [Ca2+]i and binding to CaMI (10). With nonreceptor-mediated stimuli such as shear stress (11) or ceramides (19), the activation of eNOS can take place independently of CaM binding and enzyme translocation while requiring small increases in [Ca2+]i. In the present study, we demonstrate that eNOS translocation was abolished after depletion of cytosolic Ca2+. Under these conditions, immunoprecipitation assays and sucrose gradient results demonstrate no decoupling of eNOS from caveolae, no association with CaMI, and no eNOS migration from the DRM to dense fractions, as observed for GM1 and Cav-1, proving that eNOS does not detach from caveolae, while EPI was still able to induce eNOS activation.
In our previous study, we demonstrated that under normal calcium conditions, EPI-induced eNOS activation in HCAEC is dependent of the PI3K/AKT/PKA pathway (31). Other studies have shown that calcium and receptor-independent eNOS phosphorylation at Ser-1177 is a process mediated through AKT/HSP90 activity (2, 34). However, Ca2+-independent eNOS phosphorylation has not been previously reported to occur as a consequence of the stimulation of receptors by commonly known agonists.
Our results demonstrate that EPI-induced NO production in human endothelial cells can be obtained through both Ca2+-dependent and Ca2+-independent eNOS phosphorylation. The relevance of the EPI-induced, Ca2+-independent eNOS activation in endothelial cells was demonstrated via the activation of the NO/cGMP/p-VASP vasorelaxation-related pathway in an ex vivo system using mesenteric arteries, an effect that BK cannot trigger. We do not have, at the moment, proof of any significant physiological relevance; however, it can be related with protective effects of cells under high stress conditions like ischemia where the homeostasis of calcium is lost.
Results from calcium-depleted NO measurements indicated ∼25% production of NO (vs. regular calcium) and ∼22% in cGMP coupled to the phosphorylation/activation of VASP. These EPI-triggered effects on Ca2+-deprived HCAEC were blocked by pretreatment of the vessels with l-NAME, demonstrating the relevant role of EPI in eNOS activation under these conditions. The magnitude of NO changes are comparable with those observed in calcium-depleted EPI-treated HCAEC thus, validating the physiological significance of these observations.
To evaluate the specificity on EPI-evoked responses in Ca2+-deprived HCAEC, we treated cells with catechin, a stereoisomer of EPI, and with the flavonoid quercetin. Catechin has the same chemical composition as EPI but differs in the spatial orientation of one of its rings. Quercetin has the same number of rings but different chemical substitutions.
Our results indicate that under Ca2+-free conditions catechin is not capable of inducing NO production and that quercetin, at a similar concentration, induces ∼27% of maximal EPI-induced effects. These results suggest specificity and/or higher potency of EPI compared with either catechin or quercetin.
The results presented herein serve to further understand the mechanisms by which EPI may act to retain vascular function in diseases where NO production is limited. These unique actions on eNOS may also serve to partly explain EPI cardioprotective effects. Our results also support those of Heiss et al. (16) where the cardiovascular effects of cocoa flavanols in patients with coronary artery disease are mediated by increases in the bioavailability of NO as reflect of endothelial function improvement. However, caution must be exercised when comparing in vitro EPI-induced effects versus those obtained after an oral intake of cocoa products or its flavanols, since EPI goes through extensive metabolism (GI, liver) and the bioavailability of free EPI is low and some of its metabolites may also be vasoactive (32).
Additional work and alternative forms of administration are necessary to further explore the vasoactive effects of EPI.
GRANTS
The work was partially funded by National Institutes of Health Grants HL-43617, AT004277 and NIH/NIMHD-sponsored (P60 MD000220) to F. Villarreal. I. Ramirez-Sanchez received postdoctoral fellowship no. 93759, and G. Ceballos received a sabbatical fellowship from The National Council for Science and Technology (CONACYT, Mexico).
DISCLOSURES
F. Villarreal is a cofounder of Cardero Therapeutics, Inc.
REFERENCES
- 1. Blatter LA, Taha Z, Mesaros S, Shacklock PS, Wier WG, Malinski T. Simultaneous measurements of Ca2+ and nitric oxide in bradykinin-stimulated vascular endothelial cells. Circ Res 76: 922–924, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Boo YC, Sorescu GP, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, Jo H. Endothelial NO synthase phosphorylated at SER635 produces NO without requiring intracellular calcium increase. Free Radic Biol Med 35: 729–741, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med 166: 411–417, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J 31: 1616–1623, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation 119: 1433–1441, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflügers Arch 459: 793–806, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Fleming I, Bauersachs J, Fisslthaler B, Busse R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ Res 82: 686–695, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem 274: 30101–30108, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 276: 16587–16591, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Heiss C, Jahn S, Taylor M, May Real W, Angeli F, Wong ML, Amabile N, Prasad M, Rassaf T, Ottaviani JI, Mihardja S, Keen Cl, Springer ML, Boyle A, Grossman W, Glantz SA, Schroeter H, Yeghiazarians Y. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol 56: 218–224, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Henkel CC, Asbun J, Ceballos G, del Carmen Castillo M, Castillo EF. Relationship between extra and intracellular sources of calcium and the contractile effect of thiopental in rat aorta. Can J Physiol Pharmacol 79: 407–414, 2001 [PubMed] [Google Scholar]
- 18. Hirata Y, Nagata D, Suzuki E, Nishimatsu H, Suzuki, Nagai RJ. Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J 51: 1–6, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Igarashi J, Thatte HS, Prabhakar P, Golan DE, Michel T. Calcium-independent activation of endothelial nitric oxide synthase by ceramide. Proc Natl Acad Sci USA 96: 12583–12588, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Kitakaze M, Asamuma H, Takashima S, Minamino T, Ueda Y, Sakata Y, Asakura M, Sanada S, Kuzuya T, Hori M. Nifedipine-induced coronary vasodilation in ischemic hearts is attribuible to bradykinin- and NO-dependent mechanisms in dogs. Circulation 101: 311–317, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Lenasi H, Kohlstedt K, Fichtlscherer B, Mülsch A, Busse R, Fleming I. Amlodipine activates the endothelial nitric oxide synthase by altering phosphorylation on Ser 1177 and Thr495. Cardiovasc Res 59: 844–853, 2003 [DOI] [PubMed] [Google Scholar]
- 23. McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem 274: 3910–3917, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 272: 15583–15586, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest 100: 2146–2152, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T. Vasodilator-stimulated phosphoprotein Serine 239 phosphorylation as a sensitive monitor fo defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res 87: 999–1005, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Omura M, Kobayashi S, Mizukami Y, Mogami K, Todoroki-Ikeda N, Miyake T, Matsuzaki M. Eicosapentaenoic acid (EPA) induces Ca(2+)-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett 487: 361–366, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Pritchard KA, Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Ramirez-Sanchez I, Ceballos-Reyes G, Rosas-Vargas H, Cerecedo-Mercado D, Zentella-Dehesa A, Salamanca F, Coral-Vazquez RM. Expression and function of utrophin associated protein complex in stretched endothelial cells: dissociation and activation of eNOS. Front Biosci 12: 1956–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. Epicatechin activation of endothelial cell eNOS, NO and related signaling pathways. Hypertension 55: 1398–1405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 103: 1024–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimbo D, Graham-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di Tullio M, Boden-Albala B, Sacco R, Homma Shunichi. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis 192: 197–203, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem 278: 30821–30827, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, Ceballos G, Villarreal F. Short- and long-term effects of (−)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 295: H761–H767, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamazaki KG, Taub PR, Barraza-Hidalgo M, Rivas M, Zambon AC, Ceballos G, Villarreal FJ. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling following permanent coronary occlusion. J Am Coll Cardiol 55: 2869–2876, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang XP, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels: An unexpected mechanism of action of a calcium channel-blocking agent. Circulation 97: 876–580, 1998 [DOI] [PubMed] [Google Scholar]