Abstract
Advances in medical and surgical treatments in the last two to three decades have resulted in quantum leaps in the overall survival of patients with many types of non-central nervous system (CNS) malignant disease, while survival of patients with malignant gliomas (WHO grades 3 and 4) has only moderately improved. Surgical resection, external fractionated radiotherapy and oral chemotherapy, during and after irradiation, remain the pillars of malignant glioma therapy and have shown significant benefits. However, numerous clinical trials with adjuvant agents, most of them administered systemically and causing serious complications and side effects, have not achieved a noteworthy extension of survival, or only with considerable deterioration in patients’ quality of life. Significant attention was focussed in the last decades on the cell biology and molecular genetics of gliomas. Improved understanding of the fundamental features of tumour cells has resulted in the introduction and increasing clinical use of local therapies, which employ spatially defined delivery methods and tumour-selective agents specifically designed to be used in the environment of a glioma-invaded brain. This review summarises the key findings of some of the most recent and important clinical studies of locally administered novel treatments for malignant glioma. Several such therapies have shown considerable anti-tumour activity and a favourable profile of local and systemic side effects. These include biodegradable polymers for interstitial chemotherapy, targeted toxins administered by convection enhanced delivery, and intra- and peritumourally injected genetically modified viruses conferring glioma-selective toxicity. Areas of possible improvement of these therapies and essential future developments are also outlined.
Keywords: Astrocytoma, Convection-enhanced delivery, Glioma, Immunotoxin, Virus
Glia-derived neoplasms of the brain (gliomas) of the histological grade 3 (anaplastic astrocytoma, anaplastic oligoastrocytoma, and anaplastic oligodendroglioma) and grade 4 (glioblastoma multiforme, [GBM]) according to the morphology-based classification of the World Health Organization (WHO),1 represent different stages of the same fatal neoplastic disease of the central nervous system (CNS). WHO grade 3 and 4 gliomas are also known as malignant gliomas and are the most frequent intrinsic brain tumours in adults.2 According to the US central brain tumour registry, the annual rate of primary tumours of the CNS is 14.1 per 100,000 persons, of which more than 36% are malignant gliomas.3,4
Malignant gliomas typically arise in the lobar white matter or in the deep grey matter of the brain and are characterised by diffusely infiltrating growth.2,5 These gliomas consist of highly proliferative and exceptionally migratory tumour cells with a variety of acquired genetic alterations which make them extremely resistant to antiproliferative therapies.6,7,8 There is no clear demarcation of tumour from normal surrounding brain tissue to allow the surgeon to completely remove a glioma. Neuroradiological imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) can only visualise areas with a high percentage of tumour cells and strong neoangiogenesis, but not those with a low density of invading glioma cells and neoplastic capillaries.9 Gliomas expand both by mitotic activity of tumour cells and by migration of these cells away from the initial tumour mass and into the surrounding brain.10 It is now generally accepted that widespread migration of glioma cells is a very early feature of these tumours, and by the time of clinical manifestation and histological diagnosis cells have spread throughout the CNS and are located beyond the extent of any feasible surgical resection.11,12 There is evidence to suggest that the density of infiltrating malignant cells decreases according to the distance from the initial tumour mass, and there is a tendency of tumour cells to migrate along defined anatomical structures such as white matter tracts.5,13 Supporting the notion that malignancy declines with distance from the main tumour, the fact is well known that most malignant gliomas will recur within a few centimetres of the walls of the resection cavity or the visible tumour mass, respectively.6,10,14 Simple diffusion of cells could potentially explain this observation. On the other hand, recent insights in glioma cell biology suggest that more malignant and actively proliferating cells might be less migratory and less invasive, while less malignant cells may tend to invade more remote areas of the brain which surround the tumour mass.10,15
Survival of patients with malignant glioma has remained essentially the same in the last three decades, despite a quantum leap in medical and surgical technology and major advancements in understanding the natural history and biology of the disease, and the molecular genetics of glioma cells.11,16 Median survival time for malignant gliomas varies according to different studies, but is generally agreed to be 18–20 months for anaplastic astrocytomas and 8–14 months for GBM.2,3,11 There are no curative treatments for malignant glioma at present and no treatment able to prevent tumour progression or recurrence. Even the most radical surgical resection is unable to cure a malignant glioma, although it may significantly prolong survival and improve quality of life.6,12,14,17
The current standard therapy of newly diagnosed malignant gliomas consists of surgery, preferably a gross total resection of contrast-enhancing areas, subsequent fractionated external radiotherapy up to a total dose of 60 Gy, and concomitant and adjuvant chemotherapy in WHO grade 4 tumours, with various combinations of these three approaches.6,8,16,18 The use of chemotherapy, in addition to surgery and radiation, was considered somewhat controversial until recent studies demonstrated significantly improved outcome for patients with previously untreated GBM receiving radiotherapy plus concomitant and adjuvant chemotherapy after surgery.18 However, in all clinical studies, significant improvements in overall survival are demonstrated mostly in selected subpopulations of patients with somewhat better prognostic factors, such as a higher Karnofsky Performance Status (KPS), younger age, specific molecular markers, complete surgical resection of tumour, and others.4,11,12,16–18
Perhaps the most prominent feature, which renders malignant glioma a preferred target for tumour selective drugs and local treatments, is the unique combination of high mitotic activity of tumour cells against the background of the mostly post-mitotic environment of the adult brain.9,10 In addition, malignant gliomas do not metastasise systemically, but are extremely aggressive in their local environment within the closed compartment of the CNS. Multidrug and radiation resistance genes are upregulated in primary and recurrent glioma and allow tumour cells to escape the toxicity of the standard treatment modalities and to continue growing even during radio- or chemotherapy.6,10,16
Distinct biological features common in malignant gliomas, but unusual in normal brain tissue, have been the subject of many research studies. Differential expression of specific proteins in malignant glioma versus the normal brain and aberrant activation of signal transduction cascades in tumours seems to provide an opportunity for selective molecular targeting of tumour cells despite the molecularly targeted agents being given mostly systemically, thus associated with less toxicity to the patient and with a higher rate of tumour cell killing.6,8,10,16 All these features make gliomas a model disease for the design and testing of novel treatments with tumour-selective toxicity and a local rather than systemic mode of administration.
Several novel approaches have been introduced into clinical practice in a continuous effort to improve the outcome of malignant glioma. In general, such approaches can be categorised as systemic or local, the latter being intratumoural, peritumoural, intracerebral, or combined.19,20 Novel systemic approaches demonstrating potential in early clinical studies are chemotherapy drugs with molecular selectivity mechanisms (such as receptor tyrosine kinase inhibitors), and active (e.g. dendritic cells) or passive (e.g. antibodies) immunotherapies. Novel local intracranial approaches include intracerebral delivery of standard chemotherapy drugs (interstitial chemotherapy) by slow release polymers, recombinant targeted toxins administered by convection enhanced delivery (CED), or genetically modified viruses with molecular selectivity for glioma cells.20,21
This review will summarise the most recent and clinically relevant approaches to local intracerebral therapy of malignant gliomas. All of these are currently employed in an adjuvant setting, in addition to the above mentioned and well established standard modalities of surgery and fractionated external radiotherapy with chemotherapy, and not as their replacements. We will focus mostly on therapeutic approaches that have demonstrated safety, feasibility, and potential anti-tumour efficacy in previous and ongoing clinical studies.
INTERSTITIAL CHEMOTHERAPY WITH BIODEGRADABLE POLYMER WAFERS (GLIADEL®)
Sustained and controlled local delivery of therapeutic agents to a brain glioma is one of the recent strategies for increasing toxicity to tumour cells while reducing systemic side effects. Gliadel® (MCI Pharma Inc., Bloomington, MN, USA) wafers were developed as a means for controlled release of a chemotherapeutic agent from biodegradable polymer wafers to the surroundings of surgically resected malignant glioma.21,22 Their efficacy was investigated in several preclinical and clinical studies.23–26 Gliadel® wafers are currently approved for use in patients with primary and recurrent glioblastoma as an adjunct to standard treatment modalities.
Clinical studies investigating local intracerebral delivery of the well-known and frequently used chemotherapy drug, 1,3-bis-(2-chloroethyl)-1-nitrosourea (BCNU or carmustine) began in the early 1990s.23 BCNU was initially chosen due to proven efficacy against glioma cell lines. For local delivery of BCNU, biodegradable polymer wafers were used so as to deliver the drug to the tumour resection cavity in a controlled-release fashion over time, which should circumvent the short in vivo half-life of the drug, increase the local concentration of BCNU reaching glioma-invaded areas of normal brain, and avoid the concomitant toxicities of systemic drug application.21,22
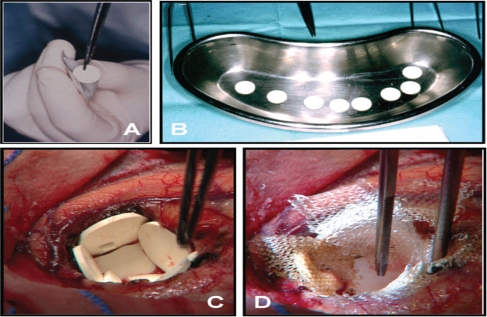
Polymer (prolifeprosan 20) wafers containing 3.85% (w/w) BCNU, which are also known by the brand name Gliadel®, are currently the only type of interstitial chemotherapy wafers licensed by U.S. and European agencies for treatment of de novo and recurrent malignant gliomas [Figure 1, A and B]. Wafers are placed directly into the resection cavity of a malignant glioma at the end of surgical resection of the tumour [Figure 1, B and C].22 This may result in local BCNU levels approximately 100-fold of those obtained with systemic delivery.27 Gliadel® wafers have been shown to release BCNU in vivo over a period of approximately 5 days. When in continuous contact with interstitial fluid, wafers have been shown to degrade completely over a period of 6–8 weeks. Polymer degradation products are excreted as expired CO2 or through the urine. BCNU degradation products are also excreted primarily through the urine.21,27
Figure 1:
Handling and implantation of Gliadel® wafers during brain tumour surgery. A: A single wafer is removed from the sterile aluminium foil protective packaging. B: All 8 Gliadel® wafers belonging to a complete pack are removed from the packaging and prepared for intracerebral implantation. C: Gliadel® wafers lining the wall of a glioblastoma (GBM) resection cavity. Note that the relatively small tumour resection cavity can only accommodate 5 wafers. Slight overlapping of wafers is acceptable. D: Implanted wafers are covered with oxidised cellulose (Surgicel®, Ethicon, Hamburg, Germany) to prevent dislocation and intracavitary movement.
No direct pharmacokinetic measurements have been made in humans after the implantation of Gliadel® wafers; however, drug distribution and clearance have been extensively studied in rodents and primates. In addition, degradation of the polymer matrix, the release kinetics of BCNU, and drug and polymer metabolism have been studied both in vitro and in vivo.27 Pharmacokinetic studies have demonstrated the capability of Gliadel® wafers to deliver high (mM) concentrations of BCNU within a few millimetres of the polymer implant and with a limited penetration distance. The limited interstitial spread of the drug is presumably due to the high transcapillary permeability of this lipophilic molecule.22 However, convective flow in the existing peritumoural oedema may augment diffusion.27,28
In 1991, Brem et al. established a significant survival advantage of Gliadel® wafers over placebo in patients with recurrent malignant glioma.23 They conducted a randomised, placebo-controlled, prospective phase 3 clinical study to evaluate the effectiveness of Gliadel® wafers. Their multicentre study enrolled 222 patients with recurrent malignant brain tumours requiring re-operation. Cases were randomly assigned to surgically implanted wafers with or without BCNU. Median survival of the 110 patients who received Gliadel® wafers was 31 weeks compared with 23 weeks for the 112 patients who received placebo wafers (P = 0.006). Among GBM patients, 6-month survival in the treated group was 50% greater than in the placebo group. There were no clinically important adverse reactions related to the carmustine polymer, either in the brain or systemically.23,24
A phase 1 trial was carried out in patients with newly diagnosed GBM.28 Twenty-two mostly elderly patients (mean age 60 years) with newly diagnosed malignant glioma (21 with GBM) were treated with Gliadel® wafers. Postoperatively, all patients received standard radiation therapy, but no additional chemotherapy in the first 6 months. The neurotoxicity of this regimen was equivalent to that occurring in other series of patients undergoing craniotomy and radiation. No significant bone marrow suppression occurred and there were no wound infections. Median survival of the whole group was 42 weeks, 8 patients survived 1 year, and 4 patients survived more than 18 months. The authors concluded that interstitial chemotherapy with Gliadel® wafers with subsequent radiation appears to be safe as an initial therapy for malignant glioma.28
In 2004, Kleinberg et al. described, in a retrospective review, the clinical course, toxicity, and pathologic findings after Gliadel® wafer implantation for newly diagnosed malignant glioma.29 Forty-five consecutive patients, most of them with GBM, received Gliadel® wafer implants followed by radiotherapy and were available for follow-up. Postoperative infection, or the need for reoperation within 30 days was uncommon after Gliadel® wafer placement. Full-dose external radiotherapy was well tolerated after Gliadel® implantation; however, 13 patients developed neurological symptoms during radiotherapy, but responded to medication (dexamethasone and/or anticonvulsants). Fifteen of these 45 patients underwent re-operation or biopsy for a new local contrast-enhancing lesion. In 5 of these, histological analysis revealed necrosis or post-treatment scars without active tumour. Median survival was 12.8 months for all GBM patients.29
To corroborate the evidence from early clinical trials, a randomised controlled trial planned to include 100 cases was carried out in patients undergoing surgery for newly diagnosed gliomas, but was terminated prematurely after enrolment of 32 patients because the Gliadel® implants became unavailable.30 The median overall survival time was 58.1 weeks for the active treatment group versus 39.9 weeks for the placebo group (P = 0.012). At the end of the study, 6 patients were still alive, 5 of whom belonged to the active treatment group. Results were, however, not corrected for the early termination and thus a possible bias may have been introduced in the statistical evaluation.30
Westphal et al. reported the results of a further multicentre phase 3 study investigating Gliadel® wafer treatment in 240 patients with newly diagnosed GBM.25 Patients were randomised to receive either BCNU or placebo wafers at the time of primary surgical resection. All patients were given identical standard treatment consisting of surgical resection followed by fractionated external radiation. Exclusion criteria were recurrent tumour, prior brain radiotherapy, and multifocal disease. The primary clinical endpoint was overall survival. Median survival in the intent-to-treat (ITT) population was 13.9 months for the Gliadel®-treated group, and 11.6 months for the placebo-treated group, with a 29% reduction in the risk of death in the treatment group. Time to decline in KPS and clinical parameters (e.g. vital signs, level of consciousness, neurological status), were also significantly improved in the treatment group compared to the placebo group. The adverse events (AE) were comparable for the two groups, except for cerebrospinal fluid (CSF) leak (5% in the Gliadel® group versus 0.8% in the placebo group) and intracranial hypertension (9.1% in the Gliadel® group versus 1.7% in the placebo group). This study is the largest clinical investigation of Gliadel® activity to date.25
In a later study, the authors reported the longterm follow-up results of the above patient group regarding survival benefits at 2 and 3 years after Gliadel® implantation.26 Of the 59 patients available for long-term follow-up, 11 were alive at 56 months; 9 having received Gliadel® and 2 placebo wafers. The median survival of patients treated with Gliadel® was 13.8 months versus 11.6 months in placebo-treated patients (P = 0.017), with a hazard ratio of 0.73 (P = 0.018), representing a significant risk reduction of 27%. This survival advantage was maintained at 1, 2, and 3 years and was statistically significant (P = 0.01) at 3 years. Two of the 207 GBM patients remained alive at the end of the follow-up period, both in the Gliadel® group. The authors concluded that malignant glioma patients treated with Gliadel® at the time of initial surgery in combination with radiation therapy demonstrated a continuous and significant survival advantage at 2 and 3 years follow-up compared with placebo patients.26
Additional studies were aimed at increasing the clinical effects of Gliadel® treatment using different strategies. Olivi et al. treated 44 adult patients with surgery and implantation of BCNU wafers.31 Six patients per dose level were studied using wafers with 6.5%, 10%, 14.5%, 20%, and 28% BCNU (weight/weight). Toxicity was assessed one month after wafer implantation. No dose-limiting toxicity was identified at the 6.5%, 10%, or 14.5% dose levels, although delayed wound healing, seizures, and brain oedema were noted. At the 20% dose, these side effects seemed more prominent, and 6 additional patients were treated at this dose and tolerated treatment well. Three of 4 patients receiving 28% BCNU wafers developed severe brain oedema and seizures. Nine additional patients received 20% wafers, confirming this as the maximum tolerated dose (MTD). Maximum BCNU plasma concentrations with the 20% wafers were 27 ng/ml. Overall median survival of the patients was 12 months. The authors concluded that 20% BCNU wafers are relatively well tolerated and result in minimal systemic BCNU exposure.31 Additional studies are however needed to establish the efficacy of high-dose BCNU wafers and a possible significant extension of survival due to the increased concentration of BCNU.
Beginning in 2004, concomitant temozolomide (TMZ) during fractionated brain irradiation (Stupp protocol) became the standard of care at most neurooncology centres in Europe and the USA.18 Patients with newly diagnosed GBM and Gliadel® implantation received therefore concomitant TMZ and irradiation radiochemotherapy); however, it remained unstudied whether combining Gliadel® and radiochemotherapy is safe or further improves survival in such patients. In 2010, McGirt et al. reviewed retrospectively the initial experience with Gliadel® and radiochemotherapy in GBM patients.32 Thirty-three patients were treated with the combined modalities and the median survival in the group was 20.7 months, with a 2-year survival rate of 36%. Six-month morbidity included surgical site infection in 1 case (3%), perioperative seizures in 2 cases (6%), deep-vein thrombus in 1 (3%), pulmonary embolism in 3 (9%), and cerebral oedema requiring intravenous dexamethasone administration in 1 case (3%). Myelosuppression required premature termination of TMZ in 7 patients (21%): (thrombocytopenia in 5, neutropenia in 2 cases). In patients ≤ 70 years of age, Gliadel® and radiochemotherapy were independently associated with improved median survival (21.3 versus 12.4 months, P = 0.005). Moreover, the combined modalities were not associated with an increase in postoperative morbidity in comparison with Gliadel® or radiation only. The authors concluded that radiochemotherapy can be safely administered to patients receiving Gliadel® wafers after resection of newly diagnosed GBM.32,33
Conclusions and Future Developments–Gliadel® Therapy
Despite the convenience and relative simplicity of use of intraoperatively placed Gliadel® wafers, there are some factors limiting their widespread clinical application. Most importantly, there is only a modest short-term clinical benefit in most patients, while long-term survival is prolonged in a small subset of malignant glioma patients only. The side effects of Gliadel® are not negligible, and the high cost of treatment is an important issue, at least in most European Union (EU) countries. In the future, local release wafers will need to be optimised for increased anti-tumour activity, either by increasing the concentration and improving the release kinetics of well-known agents such as BCNU, or by employing novel agents with a more potent anti-tumour effect, e.g. mitoxantrone or IL-2.34 Combinations of local release wafers with standard adjuvant therapies such as radiochemotherapy or single-drug oral chemotherapy (e.g. with TMZ) may become a standard of care if more reliable clinical evidence is presented for increased survival and unchanged morbidity with these combinations.33
Targeted Toxins (Immunotoxins)
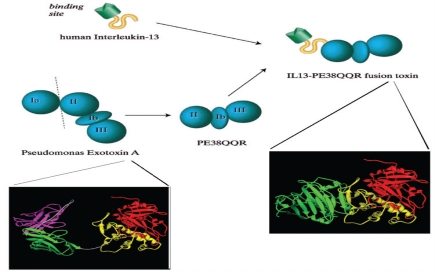
Targeted toxins represent a new class of anticancer agents providing high specificity for tumour cells selectively overexpressing some surface proteins.35,36 Currently used toxins are recombinant polypeptide molecules consisting of a tumour-selective ligand coupled to a highly potent peptide toxin, which is truncated to abolish native toxicity.37 The most frequently used and best researched ligands bind to tumour-associated molecules with receptor signalling properties, such as epidermal growth factor receptor (EGFR), transferrin receptor (TfR), interleukin-13 receptor (IL-13R), or interleukin-4 receptor (IL-4R).38 The toxin part of the molecule in all clinically used toxins is a polypeptide derived from bacteria (Corynebacterium diphtheriae, Pseudomonas aeruginosa), which has been modified by deleting the native targeting and internalisation chains of the polypeptide and replacing them with one of the above ligands [Figure 2].39,40
Figure 2:
Schematic and 3D-ribbon representations of the structure of the recombinant fusion toxin IL13-PE38 (IL13-PE38QQR). A derivative of the native Pseudomonas exotoxin A (PE), PE38QQR, in which domain Ia (amino acids 1 to 252) has been completely deleted and domain Ib (amino acids 365 to 380) has been partially deleted; lysine residues at positions 590 and 606 have been replaced by glutamine, and at position 613 by arginine, and it has been fused to the carboxy terminus of the human IL-13 to produce the fusion toxin IL13-PE38QQR (IL13-PE38).
The mechanism of action of targeted toxins may have important advantages over that of radiation and classic chemotherapeutic agents. Toxins are effective against radiation-resistant hypoxic tumour cells and far more potent than any chemotherapy drug. One single molecule of toxin is sufficient to cause tumour cell death independent of any malignancy-associated genetic alterations and/or mutations [Figure 3].37 Multidrug resistance and apoptosis resistance are therefore not an issue with toxins; after receptor binding and internalisation, no tumour cell is able to survive the toxin part of the molecule.38,41,42 A few targeted toxins have advanced from the laboratory to the stage of phase I and II clinical studies, with two of these toxins reaching phase 3 trials.43
Figure 3:
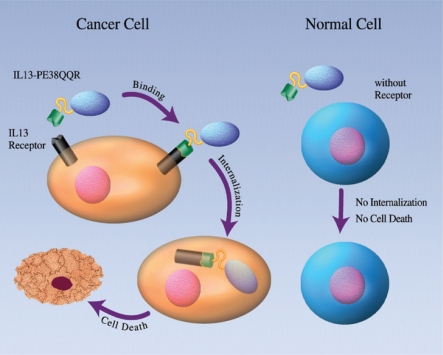
Schematic representation of the mode of action of the recombinant fusion toxin IL13-PE38 (IL13-PE38QQR). IL-13 receptor (IL-13R-ß2 subunit) is a tumour-specific protein in human malignant glioma cells. Immune cells, endothelial cells and normal glia and neurons express none or very low amounts of IL-13R. IL13-PE38 is highly selective and potent in killing human glioblastoma multiforme cells by binding to IL-13R, but does not kill normal cells, which are IL-13R-negative.
IL4-PE (NBI-3001)
This chimeric recombinant fusion protein (also known as NBI-3001, Neurocrine Inc., S. Diego, CA) is composed of circularly permuted interleukin-4 (IL-4) and a truncated form of P. aeruginosa exotoxin (PE) A.44 PE is a 66 kD protein with three domains: Ia/Ib, II, and III. When domain Ia is removed, the resulting molecule (termed PE-40) retains its translocation function and elongation factor 2 (EF-2) inhibition properties, but is unable to bind to and kill human cells.35 Domain II is the site of proteolytic cleavage and is responsible for catalysing translocation of the toxin into the cytosol. Domain III, located at the C-terminus, possesses ADP-ribosylation activity which in turn leads to inactivation of EF-2 and to cell death. In the genetically engineered PE molecule PE38KDEL, amino acids (AA) 253–364 were linked to AA 381–608, which in turn were fused to KDEL (an endoplasmic reticulum retaining sequence) at position 609–612. To improve the binding of IL-4 toxin to the IL-4 receptor (IL4-R), a circularly permuted form of IL-4 was fused to the toxin. This new agent was termed IL-4(38–37)-PE38KDEL, or IL4-PE.45,46 IL4-PE was found to have a 16-fold higher affinity for binding to GBM cell lines than the native PE toxin, and was 3–30-fold more toxic to GBM cells.42,47
IL-4 is a pleiotropic cytokine composed of 4 α-helices which is primarily produced by Th2-type T lymphocytes. Whereas normal CNS cells such as endothelial cells, microglia, or astrocytes express low levels of IL4-R, malignant glioma cells express high levels of this receptor on their surface.44,48 In one series, 16 of 21 surgical samples were determined to express high numbers of IL4-R.36 In another series, all of the 25 tested surgical specimens of high-grade gliomas expressed IL4-R. In contrast, no detectable IL-4R was expressed in a normal brain as determined by immunohistochemistry.49
Cell culture studies and animal models have confirmed that IL4-PE is highly cytotoxic to glioma cells.50 IL4-PE has a 16-fold higher affinity for binding to GBM cell lines than the native PE toxin and is 3–30-fold more toxic to GBM cells.47,48 Whereas normal CNS cells such as endothelial cells, microglia, or astrocytes express low levels of IL-4R, malignant glioma cells do express high levels of this receptor on their surface.42 A recent study demonstrated that 15 of 18 GBM and 12 other brain tumour samples were moderately to intensely positive for IL-4R. In contrast, no detectable IL-4R was expressed in a normal brain as determined by immunohistochemistry.44
Weber et al. carried out an open-label, doseescalation trial of intratumoural administration of IL4-PE in patients with recurrent malignant glioma.51 A total of 31 adult patients were enrolled, 25 with GBM and 6 with anaplastic astrocytoma. Patients were assigned to one of 4 groups receiving different doses of toxin in different volumes of infusate. The toxin was administered by CED via an external pump connected to stereotactically placed catheters. The overall median survival for the whole group of WHO grade 3 and 4 tumours was 8.2 months, with a median survival of 5.8 months for GBM patients. Six months survival was 52% and 48%, respectively. Drug-related grade 3 or 4 CNS toxicity was seen in all groups in a total of 39% of all patients, and in 22% of patients at the MTD of 6 μg/ml in 40 ml. Treatment-related AE were limited to the CNS. No deaths were attributable to treatment. No drug-related systemic toxicity was apparent in any patient. Gadolinium-enhanced MRI of the brain in some of the treated patients showed areas of decreased signal intensity within the tumour consistent with toxin-mediated tumour necrosis.51
A multicentre, randomised, open-label phase 2 study with IL4-PE was carried out in patients with recurrent GBM to investigate continuous intratumoural infusion of the toxin followed by surgical resection of the tumour. The study was designed to evaluate the efficacy of IL4-PE, with a secondary objective to evaluate the safety and tolerability of the toxin.52 In the toxin group, patients received an intratumoural infusion of toxin at total doses of up to 90 μg and underwent surgical resection of the tumour between 2 and 7 days after the end of toxin infusion. Patients in the control group underwent tumour resection without prior toxin treatment. A total of 30 adult patients with unilateral, unifocal tumours with a volume <100 ml and a KPS ≤60 were enrolled. Recruitment was completed in 2003, but no final published results of the study are available yet.52 There are currently no phase 3 protocols using IL4-PE.
TP-38
TP-38 (Teva Pharmaceuticals, formerly IVAX Inc., Miami, FL, USA) is a recombinant chimeric protein composed of transforming growth factor α (TGF-α), an epidermal growth factor receptor (EGFR) ligand, and the genetically engineered form of PE described above (see IL4-PE).
The 170–180 kD transmembrane glycoprotein, epidermal growth factor (EGF) receptor, is one of four members of the erbB family of receptor tyrosine kinases, which consist of an extracellular domain that can bind ligands, a transmembrane domain and an intracellular tyrosine kinase domain.53 Binding of a ligand (EGF or TGF-α) to EGFR causes receptor dimerisation leading to tyrosine kinase activation.54 The resultant receptor autophosphorylation initiates signal-transduction cascades involved in cell proliferation and survival.53 After ligand binding, the whole receptor-ligand complex undergoes endocytosis and is translocated to the lysosomes, where the ligand dissociates from the receptor.55
Human malignant gliomas and many other malignant tumours that metastasise to the brain express EGFR, and this is commonly associated with amplification and/or mutation of the EGFR gene during neoplastic transformation.54 Amplification and high expression of EGFR in gliomas may drive tumour growth and proliferation to a significant degree.55 By contrast, EGFR is expressed at very low levels, or is undetectable on normal human glial cells and neurons thus suggesting a potential therapeutic window. The ratio of EGFR expression in glioma versus normal control brain specimens has been shown to be as high as 300-fold.56
Animal studies with TP-38 have been carried out in rodent and primate models. In tumour bearing mice, a total dose of 0.1 μg TP-38 at a concentration of 5 μg/ml was found to be safe and efficacious in prolonging survival. The MTD of intracerebral TP-38 in normal rats was 0.666 μg at a toxin concentration of 33.3 μg/ml. In non-human primates, it was demonstrated that an intracerebral bolus dose of 2 μg TP-38 at a concentration of 10 μg/ml is safe.50
Sampson et al. investigated TP-38 in a phase 1 clinical trial.57 The primary objective of the study was to define MTD and dose limiting toxicity of TP-38 delivered by CED in patients with recurrent malignant glioma. A secondary objective was to detect the efficacy of the toxin. Twenty patients were enrolled in the study and doses were escalated from 25 to 100 ng/ml. TP-38 was infused by two stereotactically placed catheters at a flow rate of 0.4 ml/h for each catheter. A total volume of 40 ml was infused. TP-38 was tolerated well, and an MTD was not established. Non-specific toxicity was not found at any of the dose levels. The toxicity encountered was solely neurologic and mostly related either to infusion volume, recurrent tumour, or stereotactic catheter placement, but not directly to TP-38. Fifteen of the patients in this study died from progressive disease. When the study closed, 4 further patients had not had a recurrence of tumour and were 55, 56, 69, and 116 weeks from the time of TP-38 therapy. Overall median survival after TP-38 for all patients was 23 weeks, whereas for those without radiographic evidence of residual disease at the time of therapy, the median survival was 31.9 weeks. Overall, 3 of 15 patients with residual disease at the time of therapy have demonstrated radiographic responses.57
A multicentre randomised open-label phase 2 study was conducted in adult patients with recurrent GBM.58 Patients were randomised to two dose levels of TP-38, 50 ng/ml or 100 ng/ml, and toxin was administered by CED via stereotactically placed catheters. Patients did not have to undergo tumour resection prior to treatment. The end points of the study were time to progression, progression-free survival, and overall survival. Three catheters were stereotactically placed in investigator-determined locations within the enhancing tumour area. The infusion rate was 200 μl/h per catheter. Each catheter delivered 13.4 ml over 67 h. The total volume infused was approximately 40 ml, and the total dose of TP-38 infused was 2 μg (50 ng/ml) or 4 μg (100 ng/ml). One patient had a complete response 48 weeks after infusion, and another showed a partial response stable over 60 weeks. Twenty-four patients remained stable. Post-infusion MRI scans in most patients showed unspecific treatment-related changes such as halo contrast enhancement around the infusion sites, which made assessment of response rather difficult. These changes usually resolved by 20 weeks post-treatment. There were no grade 3 and 4 toxicities related to TP-38, and all adverse effects of the treatment were reversible.58 A phase 3 clinical protocol employing TP-38 seems to be in development, but no further details are currently known.
IL 13-PE 38
IL13-PE38 (IL13-PE38QQR, also known as cintredekin besudotox, (NeoPharm Inc., Lake Forest, IL, USA) is a recombinant chimeric toxin consisting of human IL-13 fused to the mutated form of PE mentioned above [Figure 2]. The T-helper cell 2-derived immunoregulatory cytokine interleukin-13 (IL-13) inhibits the production of inflammatory cytokines in monocytes. The cell surface receptor for IL-13 (IL-13R) is expressed on many human cancer cells, including glioblastoma, AIDS-associated Kaposi’s sarcoma, ovarian carcinoma, renal cell carcinoma, but also fibroblast cell lines.59 The IL-13R structure varies according to cell type, existing in three different forms known as type I, type II and type III.60
Subunit structure studies of the IL-13R complex in primary brain tumour cells established the IL-13Rα2 subunit as a tumour-specific protein.59 Immune cells, endothelial cells and normal glia and neurons generally express none or very low amounts of IL-13R.39
IL13-PE38 was found to be highly selective and potent in killing human glioma cells in culture. The activity of IL13-PE38 is mediated via IL-13R, as its cytoxicity is blocked by exposing cell lines to a 10– to 100-fold excess of human IL-13.61 Furthermore, IL-13R-negative cell lines or cell lines expressing low numbers of IL-13R, including human bone marrow-derived cells and PHA-activated T-cells, are not susceptible to IL13-PE38 mediated cytotoxicity [Figure 3].62
The significant antitumour activity of IL13-PE38 was observed in animal models of human glioma, with intratumoural injection proving more efficacious than intravenous (i.v.) or intraperitoneal (i.p.) administration. In nude mice with intracerebral glioma, the MTD of IL13-PE38 was 4 μg when delivered by bolus injection, and 10 μg when delivered by CED.39
Several phase 1 and 2 studies have been initiated to investigate IL13-PE38 as an antitumour agent for the treatment of patients with recurrent malignant glioma. In a phase 1/2 study, IL13-PE38 was infused intratumourally in adult patients with recurrent malignant glioma (including anaplastic oligoastrocytoma) to determine the MTD of the drug.63 Patients received two IL13-PE38 infusions at 8-week intervals via two stereotactically placed intratumoural catheters at a constant infusion rate. One aim of the study was to determine MTD and toxicity of IL13-PE38 administered by CED via intratumoural catheters at 200 μl/h/catheter for 96 h (total 38.4 ml), in two courses 8 weeks apart. Escalation was planned through 9 levels ranging from 0.125 to 12.0 μg/ml (total dose 4.8 to 460.8 μg) in cohorts of 3 patients per level. Cohorts of 3 patients received 0.5, 1.0, 2.0 and 4.0 μg/ml. Concentrations of up to 2.0 μg/ml were safe and well tolerated. The AE reported across all dose ranges were mild and mainly neurological. The most frequent drug-related AE were headache (24%), hemiparesis (24%), brain oedema (14%), aphasia (10%), and ataxia (10%). In the phase 1 portion of the study, two histopathological and two radiographic responses were observed, with progression-free survival ranging from 3 to 88 weeks and overall survival of 147 weeks. No results have been published yet for the phase 2 portion of this study.63
Another phase 1 study investigated IL13-PE38 in adult supratentorial malignant glioma. In this four-stage study, the primary objectives were to determine effective dose of IL13-PE38 either prior to or post-resection of the tumour.64,65 In stage one, after biopsy and intratumoural catheter placement on day 1, IL13-PE38 was administered for 48 h at 400 μl/h on days 2 to 4 at escalating doses ranging from 0.25 to 2 μg/ml. The tumour was resected on day 8 and tissue was evaluated for necrosis adjacent to the catheter. Following resection, 2 or 3 catheters were placed into the brain adjacent to the tumour resection cavity and on days 10 to 14, IL13-PE38 was given at 750 μl/h for 96 h at a fixed dose of 0.25 μg/ml (total dose 18 μg). In stage 2, intratumoural infusion was omitted and patients underwent resection of the tumour followed by a 96-h peritumoural infusion of IL13-PE38 at 0.5 μg/ml and 1.0 μg/ml. In stage 3, the duration of peritumoural infusion was increased from 5 to 7 days at a fixed dose of 0.5 μg/ml. Tumour necrosis up to 2.5 cm from the catheter tip was demonstrated radiologically in at least 5 patients with preoperative intratumoural toxin infusion. Tumour specimens from at least two patients after intratumoural injection of 0.5 μg/ml IL13-PE38 toxin revealed regional necrosis in an ovoid zone extending 1 to 2 cm from the catheter tip. Peritumoural post-resection MTD was defined as 0.5 μg/ml. Similar AEs occurred across all cohorts, most being neurological. The most frequent AE were headache (53%), hemiparesis (27%), sensory disturbance (20%), fatigue (17%), aphasia (13%), facial paresis (13%), abnormal gait (13%), convulsions (10%), hypaesthesia (10%) and lymphopenia (10%). Prolonged individual patient survival has been observed after peritumoural therapy at concentrations of 0.25 μg/ml (total dose 18 μg) and above.39,66,67
A further multicentre phase 1/2 study investigated IL13-PE38 in adult recurrent supratentorial malignant glioma with confirmed radiographic evidence of recurrent or progressive tumour. The primary objective of the phase 1 portion of the study was to determine the optimum duration of infusion and drug concentration of IL13-PE38 delivered by CED via 1 or 2 intratumoural catheters prior to surgical resection of the tumour.64,67 The phase 2 objective was to determine the proportion of patients surviving at 6 months. In phase 1, cohorts of 3–6 patients received a fixed drug concentration (0.5 μg/ml) with escalating duration of infusion (4–7 days) to determine MTD based on duration. After determining maximum duration, the drug concentration was escalated from 1.0 to 4.0 μg/ml in cohorts of 3–6 patients to determine a MTD based on concentration. Tumour necrosis was assessed 6 to 13 days after the end of infusion, at the time of tumour resection. There were no severe drug-related AE in the 10 treated patients. Five patients experienced AE grade 2–3. The most frequent AE were headache (70%), hemiparesis (40%), seizures (40%), oedema (40%) and aphasia (30%). Progression-free survival ranged from 6 to 30 weeks and overall survival from 10 to 30 weeks.39,64
In all of the above studies, intratumoural infusion of IL13-PE38, with or without tumour resection in patients with recurrent or progressing malignant glioma, seemed to be well tolerated and did not result in any grade 3 and 4 AE, or in any AE in peripheral organs such as the liver, kidney and lungs. Neurological AE during and after toxin infusion were, however, encountered in a significant proportion of the treated patients. These included brain oedema, meningitis, seizures, headache, and symptoms of increased intracranial pressure. All these AE were temporary and mostly controllable by administration of steroids. In general, IL13-PE38 doses (0.5–2 μg/ml) showing biological activity (e.g. necrosis on MRI scans) in tumours were below the threshold of widespread neurotoxicity (4–12 μg/ml). Selecting of patients without incipient mass effect in the brain (due to tumour size and/or peritumoural oedema) seemed a particularly important factor for avoiding serious unwanted side effects. Some prolonged survival was observed in this selected population of patients.39,66,67
A multicentre phase 3 randomised, open label, active control, parallel assignment efficacy study (PRECISE) was carried out in order to determine whether overall survival duration, safety, and quality of life were improved for patients treated with IL13-PE38 compared to patients treated with Gliadel® wafers following surgical tumour removal in the treatment of first recurrence of GBM after initial surgery and external beam radiation therapy.43,68 Patients were randomised 2:1 to receive IL13-PE38 or Gliadel®. The IL13-PE38 toxin (0.5 μg/ml, total flow rate 0.75 ml/h) was administered over 96 hours via 2–4 intraparenchymal catheters placed after tumour resection. The primary endpoint was overall survival from the time of randomisation. Secondary and tertiary endpoints were safety and health-related quality-of-life assessments. From March 2004 to December 2005, 296 patients were enrolled at 52 centres. Median survival was 36.4 weeks (9.1 months) for the toxin group and 35.3 weeks (8.8 months) for the Gliadel® group (P = 0.476). For the efficacy evaluable population, the median survival was 45.3 weeks (11.3 months) for toxin and 39.8 weeks (10 months) for Gliadel® (P = 0.310). The AE profile was similar in both arms, except that pulmonary embolism was higher in the toxin arm (8% versus 1%, P = 0.014). This was the first randomised phase 3 evaluation of an agent administered via CED and the first with an active control group in GBM patients. There was no survival difference between IL13-PE38 toxin administered via CED and Gliadel® wafers placed at the time of surgery. Drug distribution was not assessed, but may be crucial for evaluating future CED-based therapeutics.43,68
TransMID-107 (Tf-CRM107)
TransMID™ (known as TransMID-107, Tf-CRM107, or KSB-311; KS Biomedix Holdings plc, UK, now Xenova Group plc, UK, and Celtic Pharmaceutical Holdings PL, Bermuda) is a thioether conjugate of human transferrin (Tf) with a truncated natural mutant form of diphtheria toxin (DT) known as CRM-107, which lacks receptor binding.69
Native DT is produced by the bacterium C. diphtheriae and is composed of two disulphidelinked subunits. The A subunit catalyses ADP-ribosylation of EF-2, thereby stopping protein synthesis and killing the cell. The B subunit has two functions: binding to cell-surface receptors and translocation of the A subunit into the cytosol.70 In the natural mutant form of DT, designated CRM-107, two point mutations (phenylalanine at positions 390 and 525 of the DT sequence) reduce the binding 8,000-fold and its toxicity 10,000-fold, compared to native DT.71 However, conjugation of CRM-107 by thioether linkage to various new binding moieties was able to reconstitute the full toxicity of the mutant.72 By linking CRM-107 with Tf, a receptor-ligand complex was created that is rapidly internalised to an environment that facilitates toxin translocation.70
The Tf receptor (TfR) is a transmembrane glycoprotein that mediates cellular uptake of iron. TfR are overexpressed on rapidly dividing cells, such as hematopoietic and neoplastic cells, including glioma cells.72 TfR in normal brains are sparse and their expression is largely restricted to the luminal surface of brain capillaries.73 It is this relative difference in the density of TfR that TransMID™ uses to generate differential toxicity to highly TfR expressing neoplastic cells, while sparing low expressing normal brain cells.74 Low-density background expression of the respective target receptor in normal tissue remains, however, a major concern with TransMID™ (and with all other targeted toxin based therapies).
TransMID™ has been thoroughly evaluated in cell culture75 and in vivo. The efficacy of locally administered TransMID™ against human glioma in nude mice was demonstrated by Laske et al. in 1994.76 These authors studied TransMID™ compared to 454A12-rRA, and administered toxins intratumourally in a subcutaneous model. Repeated intratumoural injections of 10 μg TransMID™, or 10 μg 454A12-rRA, were compared to equimolar doses of the untargeted native toxin CRM-107, 454A12 antibody, rRA, and phosphate-buffered saline. TransMID™ administration resulted in almost complete tumour regression (>95%), without evidence of recurrence by day 30. Unconjugated toxin components (CRM-107, 454A12, or rRA) caused significant, but less potent tumour growth inhibition than the conjugated toxin.76 In a more recent study in 2002, Engebraaten et al. compared the efficacy of TransMID™ with that of PE conjugated with the 425.3 antibody directed against EGFR.77 Mice with subcutaneous gliomas or rats with intracranial gliomas received different doses (1–10 μg) of TransMID™, or 425.3-PE, injected intratumourally in established tumours. Both toxins showed significant antitumour effects in subcutaneous tumours, but only 425.3-PE was effective in intracranial gliomas in rats. Intracerebral TransMID™ was toxic in doses above 10 ng, while intracerebral 425.3-PE was tolerated up to a dose of 4 μg per animal. The authors concluded that both toxins have promising efficacy in brain tumour models, but that 425.3-PE is better tolerated and has a more specific activity at higher doses.77
In 1997, Laske et al. treated 18 patients with recurrent malignant glioma with intratumoural high flow interstitial microinfusion of TransMID™ in a dose-escalating single arm phase 1 clinical trial.78 The drug was infused at a maximum flow rate of 4–10 μl/min at a toxin concentration of 0.1 μg/ml. Nine of the 15 evaluable patients responded to treatment by at least 50% reduction in tumour volume on MRI, including 2 complete responses. Reduction in tumour volume occurred no earlier than 1 month after completion of the first toxin infusion. In 4 patients, the response was not maximal until 6–14 months after the first treatment. Tumour response appeared to be concentration and dose dependent. Median survival after treatment in the responder group was 74 weeks, with 3 of the responders still alive at 102–142 weeks after the first treatment. Non-responders survived a median of 36 weeks. Intratumoural infusions of TransMID™ with total volumes of 5–180 ml were well tolerated. There were no treatment related deaths or lifethreatening or irreversible toxicity. In this first clinical trial, the relationship between dose, drug concentration, and sustained neurotoxicity established a MTD of 26.8 μg (40 ml at 0.67μg/ml). The trial results indicated that therapy with TransMID™ can reduce the size of malignant brain tumours refractory to conventional therapy without producing severe neurologic or systemic toxicity.78
An open-label, single-arm, multicentre phase 2 study investigated intratumoural CED infusion of TransMID in recurrent or progressive malignant glioma in adults.69 The primary study objective was evaluation of efficacy and safety of TransMID™, and the primary endpoint was a 50% reduction in tumour volume (measured by MRI) within 12 months after the second treatment. Patients received TransMID™ (0.67 μg/ml) at an escalating rate up to 200 μl/h per catheter for 4–5 days, until a total volume of 40 ml was delivered. Forty-four patients were enrolled in total and all received at least one TransMID™ infusion. Of the 34 patients evaluable for analysis, there were a total of 5 complete responders and 7 partial responders (total of 35% response), which was a statistically significant result. Median survival time for all 44 patients was 37 weeks. Infusions of TransMID™ within this clinical protocol resulted in symptomatic progressive brain oedema in 8 of 44 patients (14%). The results of this phase 2 clinical trial have confirmed the safety and tumour response data of the phase 1 trial.69
A multicentre randomised, open label, active control, parallel assignment, safety and efficacy phase 3 study (KSB311R/CIII/001 trial) was launched in 2004 in order to compare TransMID™ with the best standard treatment available for patients with progressive and/or recurrent non-resectable GBM.79,80 Best standard treatment involved a chemotherapeutic regimen considered to be the best standard of care at the respective institution, and consisting of either nitrosoureas, platinum compounds, temozolomide, procarbazine, or PCV (procarbazine, CCNU [lomustine], vincristine). The primary endpoint of the study was overall survival time. Adult patients eligible for enrolment into this trial had to be diagnosed with histologically confirmed GBM and had to have undergone conventional treatment, including surgery (biopsy or debulking) and/or radiation therapy and/or chemotherapy. Patients were required to have a recurrent and/or progressive tumour ≥1.0 cm and ≤4.0 cm in diameter.79,80 The trial was however prematurely terminated in 2007 after an interim conditional power analysis showed that it was extremely unlikely that TransMID™ would meet the trial criteria for efficacy.80 No data from this clinical trial has yet been published.
Conclusions and Future Developments–Immunotoxins
Targeted toxins have shown considerable promise in phase 1 and 2 clinical trials with recurrent malignant gliomas. There are however some major obstacles that need to be overcome before targeted toxins may enter the mainstream of brain tumour therapies. The most important of them are the heterogeneity of target receptor expression in malignant glioma cells, the non-uniform and poorly predictable distribution of the large molecules of immunotoxin in the microenvironment of the tumour and/or brain, as well as non-specific associated toxicity to glial and neuronal cells.19 With the introduction of MRI-based functional imaging and 3D volumetric techniques in routine clinical practice, it will be possible to determine quantitatively brain tumour volumes and the volume of infusate in the tumour and normal brain.81,82 Diffusion tensor imaging (DTI) is a new MRI imaging technique sensitive to directional movements of water molecules induced by tissue barriers.83 CED makes use of the extracellular space of the brain as a natural pathway for the widespread distribution of agents infused in an aqueous solution, and therefore DTI could be used for imaging of CED delivery without the need for contrast agents. Further key developments in MR imaging and MR-related computer software as well as infusion catheter design and computerised simulation and delivery modelling within the brain may provide much needed new resources to be combined with the biologically targeted toxins.19 Further improvements in the molecular diagnostics of malignant glioma with genetic profiling of individual patients and tumours should allow for selection of subgroups of cases with high expression of the target receptor, who are most likely to benefit from the administration of a targeted immunotoxin.
Clinical protocols have explored several points of presumed significance, such as the use of different numbers of catheters, positioning of catheters, surgical resection of the tumour before or after toxin infusion, and single versus repeated infusion, but there is no clear answer to any of these questions. It remains unknown whether there are benefits from combining targeted toxins with chemotherapy and/or with fractionated external radiation. Protocols have investigated patients with recurrent or progressing GBM, and the role of targeted toxin infusion in newly diagnosed malignant glioma remains to be determined. Evidence of prolonged survival and late local recurrences in treated glioma patients has raised the question of periodically repeated application of toxin for extended control of local recurrence and progression. The randomised clinical trials were able to provide some answers as to the efficacy of the studied toxins and clinical protocols; however, more clinical research is needed to address the above mentioned global issues.
GENETICALLY MODIFIED VIRUSES
Wild type viruses have been long known for their capability to destroy malignant tumour cells upon infection and intracellular replication. Genetic engineering of some of these viruses was, however, only recently done in an attempt to improve their utility as targeted gene transfer anti-cancer agents.84 Recombinant and usually non-replicating viruses transferring to tumour cells genes with toxicity-inducing intracellular effects are known as virus vectors, while replicating viruses with a lytic life cycle selectively destroying tumour cells are known as oncolytic viruses.85 Most of the viruses employed in clinical trials are derived from a retrovirus (RV), adenovirus (AV), or herpes simplex virus type 1 (HSV1) [Figure 4a and b]. Other viruses, such as the Newcastle disease virus (NDV) or reovirus, have been also employed in clinical trials, but to a much lesser extent.19,20,86–88
Figure 4a:
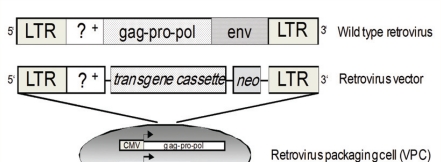
Schematic diagrams of virus vectors. Schematic representation of a wild type retrovirus (RV) and a RV vector with the corresponding packaging cell line (VPC). Wild type RV has a double-stranded RNA genome which is converted to DNA by viral reverse transcriptase and then integrated into the host genome at non-specific sites. The genetically modified RV vector genome contains the cis-acting elements required for replication (long terminal repeats - LTR, packaging sequence - Ψ), but lacks the viral genes (capsid core proteins - gag, reverse transcriptase, integrase and protease - pol, and the envelope antigens - env), which are replaced by up to 8 kb of foreign coding sequences (transgene cassette, e.g. cDNA encoding HSV-tk). A selectable marker (e.g. neomycin resistance gene - neo) allows for antibiotic selection of cells expressing the RV vector. In order that RV vectors replicate, it is necessary to provide the missing viral genes in trans, e.g. expressed within a genetically engineered RV VPC. VPC are most frequently of murine fibroblast origin. Separation of the packaging function from the genetic material to be transferred ensures that a replication incompetent RV is generated and makes RV vectors biologically safe.
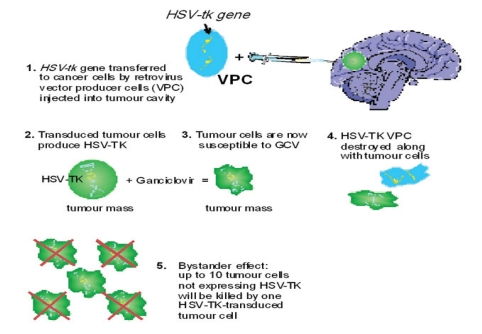
The effects of virus-mediated local therapy of malignant gliomas have been investigated only since the 1990s. The initially favoured and best explored gene therapy approach included the use of a replication-disabled, genetically modified virus vector capable of insertion of a toxicity-inducing gene into tumour cells.89–91 Most frequently, the inserted gene (transgene) rendered the infected tumour cells and their clonal progeny differentially sensitive to treatment with a pro-drug.91,92 The gene/vector system most widely utilised in initial clinical trials was the HSV thymidine kinase (HSV-tk) gene transferred by a replication-incompetent RV vector which was released in situ by fibroblast-derived virus-producing cells (VPC) [Figure 5].93 The most severe limitation of clinical gene therapy with non-replicating viruses was, however, their inability to achieve sufficiently high levels of gene expression in sufficiently large numbers of target tumour cells to result in a clinical benefit.19,94,95 A modified approach was therefore introduced in order to improve anti-tumour toxicity and virus distribution within the tumour mass and the surrounding normal brain. It employed conditionally replicating oncolytic viruses with a lytic life cycle instead of their non-replicating counterparts.96–100
Figure 5:
Schematic representation of the mode of action of recombinant retrovirus (RV) vector-mediated suicide gene therapy in patients with malignant glioma. Up to 20 ml of a suspension of viable RV-producing cells (VPC) carrying the transgene coding for herpes simplex virus (HSV) thymidine kinase (HSV-tk) are manually injected into the walls of the tumour resection cavity at the end of the surgical removal of a human glioblastoma multiforme (GBM). RV vectors, which have been genetically engineered to become replication-deficient, are produced in high titres by the implanted VPC. GBM cells are highly mitotic and, after infection by RV, are able to produce most RV proteins, including HSV-tk. This enzyme converts the low-toxicity prodrug ganciclovir (GCV) into highly toxic metabolites, which block DNA replication during mitosis and render the tumour cells apoptotic. After repeated i.v. application of GCV to patients, all cells expressing HSV-tk are killed, including VPC. Cells not expressing HSV-tk may also be killed by the so-called “bystander effect” - transfer of toxic GCV metabolites from HSV-tk-expressing to HSV-tk-negative cells by direct cellular contact. Up to 10 non-expressing cells may be killed by one HSV-tk-expressing cells in cell culture.
NON-REPLICATING RETROVIRUS
Starting in the late 1990s, several clinical studies were published which investigated the use of RV-mediated HSV-tk gene transfer delivered by intratumoural injections of VPC and followed by systemic administration of ganciclovir (GCV).89,90,91,101 The earliest phase 1/2 clinical study was published by Ram et al. in 1997.93 These authors investigated, in 15 patients with recurrent malignant glioma, the tolerability and efficacy of RV-producing VPC (RV-VPC) delivered by a single stereotactic intratumoural injection. Treatment resulted in anti-tumour activity in 4 patients with relatively small tumours. One patient with GBM had a complete response with a recurrence-free survival of 50 months.93 The limited efficiency of histologically demonstrated RV-mediated gene transfer suggested the presence of a bystander effect conferring toxicity to non-transduced tumour cells.102,103 In 1998, Klatzmann et al. treated 12 patients with recurrent GBM in a phase 1/2 study by local injections of RV-VPC into the margins of the tumour cavity after tumour debulking.104 Using the same method and RV-VPC preparation, Shand et al. treated 48 patients with recurrent GBM in a phase 1/2 study.105 The authors of both reports independently concluded that RV-VPC injections were safe. Gene therapy in both trials resulted in a prolonged delay of recurrence in individual cases.
Izquierdo et al. conducted phase 1 studies in 7 patients with recurrent GBM. Five patients received a single intratumoural RV-VPC injection and 2 patients intraoperative RV-VPC injections, later followed by repeated RV-VPC applications into the tumour resection cavity.106,107 In 2003, Prados et al. treated 30 patients in a phase 1/2 study with recurrent GBM by repeated direct injection of RV-VPC, initially immediately after tumour resection in the walls of the resection cavity, and a week later via an intracavitary catheter.108 Fifty per cent of the patients experienced serious side effects possibly related to RV-VPC therapy. The median survival of all patients in the treatment group was 8.4 months. Puumalainen et al. showed low tumour transduction efficiency with a RV-VPC construct carrying the marker gene lacZ.109 The same study also evaluated AV-mediated marker gene transfer and found much higher transduction rates in some areas of the tumours. Harsh et al. performed a gene-marking and neuropathological study in 5 patients with recurrent GBM who received multiple stereotactically guided intratumoural injections of RV-VPC during a single surgical session.94 This study showed an extremely low gene transduction efficiency of the RV-VPC used.
Finally, a large prospective randomised multicentre phase 3 clinical trial was carried out to allow definitive evaluation of RV-VPC treatment in patients with newly diagnosed GBM.101 A total of 248 patients with previously untreated GBM were randomised to two groups and treated using either standard surgical resection and radiotherapy (n = 124) or standard therapy plus adjuvant RV-VPC injected in the walls of the resection cavity at tumour surgery (n = 124). Systemic administration of GCV (5 mg/kg i.v. twice daily) was started two weeks after surgery and continued for another 2 weeks. Somewhat surprisingly, this study found no significant difference in the progression-free, median, or 12-month survival of both the standard and the gene-therapy groups, although the approach was proven exceptionally safe. Based on the results of this trial, local therapy of malignant glioma with RV-VPC expressing HSV-TK has been largely abandoned.101
NON-REPLICATING ADENOVIRUS
Studies with AV vectors were conducted and published, mostly in the late 1990s. Trask et al. conducted a phase 1 study of AV-HSV-tk in 12 patients with recurrent malignant glioma.110 A single stereotactic intratumoural injection was used to deliver AV doses of 2×109–2×1012 pfu. Adequate safety was reported with all doses except the highest, which caused significant CNS toxicity. Median survival in this study was only 4 months, however 3 patients showed long-term survival of 2 years or longer, which was interpreted as evidence for some local tumour control. Judy and Eck treated 13 patients with primary or recurrent malignant gliomas in a modified phase 1 study.111 Twelve patients received stereotactic intratumoural AV-HSV-tk injections and a week later underwent tumour mass resection with additional AV injections in the walls of the resection cavity. Total virus doses of 2×108–2×1011 pfu were used. Transient side effects, such as increased intracranial pressure (ICP), were noted only at the highest dose level. The median survival of all patients was 10 months, while 5 patients were alive at 1 year and 1 patient at 3 years after therapy.111 Germano et al. carried out a dose-escalating phase 1 study in 11 patients with recurrent GBM.112
AV-HSV-tk doses of 2.5×1011–9×1011 virus particles were administered intraoperatively to the walls of the resection cavity immediately after tumour resection. The median survival of treated patients was 59 weeks. No major toxicities occurred, and the few serious AEs were not related to AV administration.112 Smitt et al. conducted another phase 1 dose escalation study in 14 patients with recurrent malignant glioma.113 Patients received 4.6×108–4.6×1011 virus particles injected manually in the walls of the tumour resection cavity at the end of tumour removal. Median overall survival was 4 months, however 4 patients survived for longer than 1 year following therapy. The treatment was safe and well tolerated.113
Some groups were able to report significantly better clinical outcomes using the AV-HSV-tk approach. Sandmair et al. enrolled 21 patients with primary or recurrent malignant glioma in a comparative phase 1/2 study employing HSV-tk gene transfer by RV-VPC or by AV and comparing these with a third group receiving AV carrying only a marker gene, lacZ.114 The mean survival in the AV-HSV-tk group was significantly longer (15 months) than that of the other groups with mean survival of 7.4 months for RV-VPC and 8.3 months for AV-LacZ. No serious AE were reported in any of the groups.
Building on these promising results, Immonen et al. conducted a randomised prospective phase 2 study to evaluate further the efficacy and safety of AV-HSV-tk in patients with primary or recurrent malignant glioma.115 Seventeen of the 36 patients received intraoperative injections of AV-HSV-tk (3×1010 pfu) into the walls of the surgically created resection cavity followed by GCV (5 mg/kg twice daily for 14 days), and 19 patients had tumour surgery without AV injections. Standard radiotherapy was carried out in all patients with previously untreated tumours. The mean survival of patients in the AV-HSV-tk group was significantly longer (70.6 weeks) than in the control group (39.0 weeks) (P = 0.0095). An additional post hoc subgroup analysis excluding patients with anaplastic astrocytoma showed that there still was a significant survival benefit in GBM patients (55.3 versus 37.0 weeks, P = 0.0214). The treatment was well tolerated and showed no major side effects.115
A phase 3 randomised and standard carecontrolled multicentre pivotal trial (also known as study 904) using the above AV-HSV-tk vector115 designated as sitimagene ceradenovec (Cerepro®, Arc Therapeutics Ltd., London, UK) has been carried out in patients with newly diagnosed malignant glioma.116 Patients were randomised to either standard care plus Cerepro® or standard care alone. Standard care was surgery and radiotherapy or surgery and radiotherapy followed by temozolomide, resulting in 4 treatment groups. This allowed comparison of the efficacy of Cerepro® and temozolomide in the same trial without denying patients the best possible standard care. The primary endpoint was survival, defined as time to death or re-intervention.116
The overall primary endpoint analysis in the ITT population (n = 236) compared Cerepro® with and without TMZ against controls with and without TMZ. It showed a 42 day improvement in median survival (310 days versus 268 days) in the two groups receiving Cerepro®. The improvement over standard care reached statistical significance (P < 0.032). At the primary endpoint, the group with Cerepro® and TMZ showed an improvement of 68% in median survival time compared with standard care (surgery and radiotherapy) controls (350 days versus 208 days). Against the same controls, treatment with Cerepro® alone showed an improved median survival trend approaching 50%, similar to those given treatment with TMZ alone after standard care (300 days and 307 days, respectively versus 208 days with standard care). Improvements in the combined Cerepro® and TMZ treatment group (n = 58) and TMZ alone group (n = 76) were significant (P <0.05). In the Cerepro® alone treatment group (n = 61), the effect was not statistically significant (P < 0.065). Of the total 53 patients still to report an event, only 7 are in the surgery and radiotherapy control group and thus confidence intervals and statistical significance levels in all treatment groups might be expected to improve with time.
Whilst increases were observed in hemiparesis, aphasia and pyrexia following therapy, the serious AE reports for Cerepro® were in line with those in the previous studies, indicating that the virus has an acceptable safety profile.116 The results of the study have not been published yet, and final data still need to be provided. Preliminary information in the public space however states that the trial may be statistically underpowered and that Cerepro® has failed to show sufficient primary endpoint efficacy.117
ONCOLYTIC VIRUSES
Genetically modified and conditionally replicating AV and HSV have been the most frequently used viruses in early clinical studies with oncolytic viruses.87,89,97,99 Four phase 1 trials in recurrent malignant glioma have used intracerebrally inoculated G207 - a conditionally replicating HSV with defects in both ICP6 and ICP34.5 genes, which has demonstrated anti-tumour efficacy in preclinical studies in glioma.89,118,119 Markert et al. in 2000 carried out a dose escalation study treating 21 patients with recurrent malignant glioma using stereotactic intratumoural injections of G207 (up to 3×109 pfu total dose).89 MRI studies and neuropathological analysis suggested some anti-tumour activity of the treatment. The best responding 4 patients survived for a mean of 12.8 months, while the rest of the group had a mean survival of 6.2 months after therapy. Most importantly, this early trial showed that inoculation of a genetically engineered oncolytic HSV virus in the human brain is safe, despite the concerns about possible encephalitis well known from its wild type counterpart.89
Rampling et al. (2000) conducted a first phase 1 dose escalation study using another selectively replicating HSV mutant with disrupted ICP34.5 genes known as HSV1716.120 Nine patients with recurrent malignant glioma received stereotactic intratumoural injections of HSV1716 up to a dose of 1×105 pfu. Four patients were alive for 14–24 months after virus injection. No signs of encephalitis or major neurological side effects were noted.120 Papanastassiou et al. (2002) used intratumoural injections of HSV1716 (1×105 pfu) in another phase 1 study in 12 patients with malignant glioma.87 The authors were able to demonstrate replication of virus in tumours resected 4–9 days after the HSV1716 injection. No toxicity was apparent. Additional data from the above studies were published separately and suggested intratumoural persistence and replication of HSV1716, allowing the virus to kill tumour cells over extended periods of time. Analysis of tumour explants showed viral replication for up to 9 days after initial injection, and the amount of recovered virus exceeded the input dose in some tumour samples. In addition, 1 patient was free of tumour progression at nearly 3 years, and 2 patients were alive and stable after almost 4 years.121 Harrow et al. (2004) followed 12 patients with newly diagnosed or recurrent malignant glioma treated in a previous study.122 Three long-time survivors with GBM were clinically stable at 15–22 months following surgery and virus injection. No long-term toxicity was reported.122
ONYX-015, a naturally occurring AV mutant with deletion in the viral E1B gene, has been previously used in clinical trials for head and neck cancer and gastro-intestinal tract malignancies.97,123,124 The first multicentre phase 1 study using ONYX-015 in patients with recurrent malignant glioma was published by Chiocca et al. in 2004.124 Twenty-four patients received intraoperative doses of ONYX-015 (1×107–1×1010 pfu) injected manually in the walls of the surgical cavity immediately after tumour resection. The median survival time for all patients was 6.2 months. No definitive anti-tumour effect could be demonstrated, however none of the patients, at any virus dose, experienced serious AE and there was no dose-limiting neurological toxicity.124
Conclusions and Future Developments–Genetically Modified Viruses
The clinical efficacy of local therapy for malignant glioma with non-replicating viruses has been so far rather disappointing, possibly with the exception of the studies with AV-HSV-tk.115,116 No clinical studies using non-replicating virus vectors are currently enrolling patients.
Encouraging anti-tumour activity has been demonstrated in the some of the studies in malignant glioma treated with local injections of oncolytic AV and HSV.97–99,122 Systemic chemotherapy has been found to potentiate the anti-tumour effect of virus mediated oncolysis in other cancer types.123,125 Factors likely to be an issue with any type of oncolytic virus are the physical limitations of manual injection of virus into tumours or tumour-invaded surrounding brain, as well as intrinsic barriers to intra- and peritumoural spread in a glioma-harbouring brain, such as cysts, fibrotic membranes, and tumour necrosis.84,97
Therapy with oncolytic viruses seems to hold more promise in the few early clinical trials than the therapy with non-replicating virus vectors, although both approaches were already proven to be safe and lacking major side effects.96–99, 119,122,126 However, further major advancements in virus designs, application modalities, and understanding of the interactions of the host immune system with the virus are clearly needed before oncolytic virus therapy of malignant brain tumours may enter routine clinical use.
Final Conclusions and Future Developments of Drug Delivery Modalities to Brain Tumours
The therapeutic success of any tumour-targeting and locally delivered agent will depend not only on its ability to kill tumour cells selectively, but to a high degree also on the delivery mode and distribution throughout a tumour and the surrounding normal brain tissue.19,84 Diffusion of particles (e.g. viruses) or large molecules (e.g. recombinant toxins) in tissue is a rather inefficient way of distribution and will depend not only on the concentration of the compound, but also on its size, molecular weight, polarity, and its avidity for the target receptor.40,92,127 To circumvent this limitation, distribution of agents by CED, a much more efficient and fast mode for interstitial delivery by high-flow infusion, has been studied in several animal models.100,127 Local delivery of targeted toxins by CED seems to be the best approach to circumvent the limitations of the blood-brain barrier (BBB) and to increase therapeutic efficacy by high local concentrations of drug. All clinical trials with targeted toxins have adopted CED as the delivery mode of choice.19
Virus particles can also be delivered by CED, although this modality has been studied mainly in animal experiments,20,84,127,128 while all human clinical trials carried out so far have employed bolus injections of virus or carrier suspensions.91,94,101,124 Intratumoural stereotactically guided injections can provide adequate virus delivery only to spatially limited areas of tumour, since the number of injection sites is limited for practical reasons by tumour size, length of surgery and increasing risk of haemorrhage.94 Direct intratumoural injections into the walls of the tumour resection cavity, although they can be performed under direct visual control and with multiple virus depots close to each other, have the same basic limitations as stereotactic procedures.93,101 Moreover, the depth of injection is limited to 10–15 mm from the visible resection border, which seems to be insufficient to reach tumour cells migrating away from the main mass.101
CED has been shown to improve significantly virus distribution in and around experimental brain tumours.127 Virus particles may be efficiently delivered by CED over large areas of tumour and surrounding brain and achieve widespread distribution and much larger coverage than with bolus injections.92,127,128 Since a large amount of clinical experience has become available in the clinical trials employing CED of targeted toxins, it seems a logical step that clinical protocols with CED of viruses should be implemented in future. Such trials may be able to combine the biological advantages of oncolytic viruses with the spatial distribution affordable by CED.
Figure 4b:
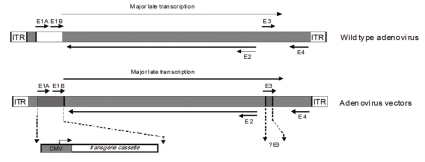
Schematic representation of the genome of a wild type adenovirus (AV) and recombinant AV vectors. The wild type AV genome is flanked by the inverted terminal repeats (ITR) and divided into E1A, E1B, E2, E3, and E4 regions, whose genes are expressed in a defined temporal sequence. It contains 36 kb of double-stranded DNA, of which several regions can be deleted to accommodate up to 10 kb of foreign DNA (e.g. insertion of a therapeutic transgene cassette in the E1 region, deletion of E3 region). Replication-deficient AV vectors are generated by placing the AV genome in a plasmid and replacing E1 with a transgene (e.g. HSV-tk), then transfecting the plasmid into a packaging cell line (VPC) that provides E1 functions in trans.
Footnotes
CONFLICT OF INTEREST
NGR has received research grants from Neurocrine Inc. (San Diego, CA) and IVAX Inc. (Miami, FL). The authors have no financial interests in any of the biotechnology companies mentioned in the review.
References
- 1.Kleihues P, Cavenee WK. Pathology and genetics of tumours of the nervous system. Lyon: IARC Press; 2000. [Google Scholar]
- 2.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–89. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 3.Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on surveillance, epidemiology, and end results (SEER) data, 1973–1991. J Neurosurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 4.Davis FG, McCarthy BJ, Berger MS. Centralized databases available for describing primary brain tumor incidence, survival, and treatment: Central brain tumor registry of the United States; surveillance, epidemiology, and end results; and National cancer data base. Neurooncol. 1999;1:205–11. doi: 10.1093/neuonc/1.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson KR, Alvord EC, Jr, Murray JD. Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Br J Cancer. 2002;86:14–8. doi: 10.1038/sj.bjc.6600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton EC, Prados MD. Malignant gliomas. Curr Treat Options Oncol. 2000;1:459–68. doi: 10.1007/s11864-000-0073-2. [DOI] [PubMed] [Google Scholar]
- 7.Janus TJ, Kyritsis AP, Forman AD, Levin VA. Biology and treatment of gliomas. Ann Oncol. 1992;3:423–33. doi: 10.1093/oxfordjournals.annonc.a058228. [DOI] [PubMed] [Google Scholar]
- 8.Salcman M. Malignant glioma management. Neurosurg Clin N Am. 1990;1:49–63. [PubMed] [Google Scholar]
- 9.Schiffer D, Cavalla P, Dutto A, Borsotti L. Cell proliferation and invasion in malignant gliomas. Anticancer Res. 1997;17:61–9. [PubMed] [Google Scholar]
- 10.Guha A, Mukherjee J. Advances in the biology of astrocytomas. Curr Opin Neurol. 2004;17:655–62. doi: 10.1097/00019052-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell P, Ellison DW, Mendelow AD. Surgery for malignant gliomas: mechanistic reasoning and slippery statistics. Lancet Neurol. 2005;4:413–22. doi: 10.1016/S1474-4422(05)70118-6. [DOI] [PubMed] [Google Scholar]
- 12.Whittle IR. Surgery for gliomas. Curr Opin Neurol. 2002;5:663–9. doi: 10.1097/01.wco.0000044761.39452.38. [DOI] [PubMed] [Google Scholar]
- 13.Giese A, Westphal M. Treatment of malignant glioma: a problem beyond the margins of resection. J Cancer Res Clin Oncol. 2001;127:217–25. doi: 10.1007/s004320000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess CF, Schaaf JC, Kortmann RD, Schabet M, Bamberg M. Malignant glioma: patterns of failure following individually tailored limited volume irradiation. Radiother Oncol. 1994;30:146–9. doi: 10.1016/0167-8140(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 15.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–36. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 16.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 17.Schiff D, Shaffrey ME. Role of resection for newly diagnosed malignant gliomas. Expert Rev Anticancer Ther. 2003;3:621–30. doi: 10.1586/14737140.3.5.621. [DOI] [PubMed] [Google Scholar]
- 18.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 19.Chiocca EA, Broaddus WC, Gillies GT, Visted T, Lamfers ML. Neurosurgical delivery of chemotherapeutics, targeted toxins, genetic and viral therapies in neuro-oncology. J Neurooncol. 2004;69:101–17. doi: 10.1023/b:neon.0000041874.02554.b3. [DOI] [PubMed] [Google Scholar]
- 20.Rainov NG, Kramm CM. Vector delivery methods and targeting strategies for gene therapy of brain tumors. Curr Gene Ther. 2001;1:367–83. doi: 10.2174/1566523013348445. [DOI] [PubMed] [Google Scholar]
- 21.Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. J Control Release. 2001;74:63–7. doi: 10.1016/s0168-3659(01)00311-x. [DOI] [PubMed] [Google Scholar]
- 22.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3:721–8. doi: 10.6004/jnccn.2005.0042. [DOI] [PubMed] [Google Scholar]
- 23.Brem H, Mahaley MS, Jr, Vick NA, Black KL, Schold SC, Jr, Burger PC, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74:441–6. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 24.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer Brain Tumor Treatment Group. Lancet. 1995;345:1008–12. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 25.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neurooncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006;148:269–75. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 27.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41:403–19. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 28.Brem H, Ewend MG, Piantadosi S, Greenhoot J, Burger PC, Sisti M. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neurooncol. 1995;26:111–23. doi: 10.1007/BF01060217. [DOI] [PubMed] [Google Scholar]
- 29.Kleinberg LR, Weingart J, Burger P, Carson K, Grossman SA, Li K, et al. Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: implications for patient management. Cancer Invest. 2004;22:1–9. doi: 10.1081/cnv-120027575. [DOI] [PubMed] [Google Scholar]
- 30.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44–8. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Olivi A, Grossman SA, Tatter S, Barker F, Judy K, Olsen J, et al. Dose escalation of carmustine in surgically implanted polymers in patients with recurrent malignant glioma: a new approaches to brain tumor therapy CNS consortium trial. J Clin Oncol. 2003;21:1845–9. doi: 10.1200/JCO.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 32.McGirt MJ, Brem H. Carmustine wafers (Gliadel) plus concomitant temozolomide therapy after resection of malignant astrocytoma: growing evidence for safety and efficacy. Ann Surg Oncol. 2010;17:1729–31. doi: 10.1245/s10434-010-1092-2. [DOI] [PubMed] [Google Scholar]
- 33.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583–8. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhines LD, Sampath P, DiMeco F, Lawson HC, Tyler BM, Hanes J, et al. Local immunotherapy with interleukin-2 delivered from biodegradable polymer microspheres combined with interstitial chemotherapy: a novel treatment for experimental malignant glioma. Neurosurgery. 2003;52:872–80. doi: 10.1227/01.neu.0000053211.39087.d1. [DOI] [PubMed] [Google Scholar]
- 35.Pastan I, Chaudhary VK, Fitzgerald DJ. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–54. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- 36.Pastan I, Fitzgerald D. Recombinant toxins for cancer treatment. Science. 1992;254:1173–7. doi: 10.1126/science.1683495. [DOI] [PubMed] [Google Scholar]
- 37.Hall WA, Fodstad O. Immunotoxins and central nervous system neoplasia. J Neurosurg. 1992;76:1–12. doi: 10.3171/jns.1992.76.1.0001. [DOI] [PubMed] [Google Scholar]
- 38.Reiter Y. Recombinant immunotoxins in targeted cancer cell therapy. Adv Cancer Res. 2001;81:93–124. doi: 10.1016/s0065-230x(01)81003-4. [DOI] [PubMed] [Google Scholar]
- 39.Husain SR, Puri RK. Interleukin-13 receptor-directed cytotoxin for malignant glioma therapy: from bench to bedside. J Neurooncol. 2003;65:37–48. doi: 10.1023/a:1026242432647. [DOI] [PubMed] [Google Scholar]
- 40.Rustamzadeh E, Low WC, Vallera DA, Hall WA. Immunotoxin therapy for CNS tumor. J Neurooncol. 2003;64:101–16. doi: 10.1007/BF02700025. [DOI] [PubMed] [Google Scholar]
- 41.Gottstein C, Winkler U, Bohlen H, Diehl V, Engert A. Immunotoxins: is there a clinical value? Ann Oncol 5 Suppl. 1994;1:97–103. doi: 10.1093/annonc/5.suppl_1.s97. [DOI] [PubMed] [Google Scholar]
- 42.Puri RK, Siegel JP. Interleukin-4 and cancer therapy. Cancer Invest. 1993;11:473–86. doi: 10.3109/07357909309018879. [DOI] [PubMed] [Google Scholar]
- 43.The PRECISE Trial Study of IL13-PE38QQR compared to GLIADEL wafer in patients with recurrent glioblastoma multiforme. From: http://clinicaltrials.gov, study identifier NCT00076986. Accessed: July 2010.
- 44.Puri RK, Leland P, Kreitman RJ, Pastan I. Human neurological cancer cells express interleukin-4 (IL-4) receptors which are targets for the toxic effects of IL4-Pseudomonas exotoxin chimeric protein. Int J Cancer. 1994;58:574–81. doi: 10.1002/ijc.2910580421. [DOI] [PubMed] [Google Scholar]
- 45.Kreitman RJ, Puri PK, Pastan I. A circularly permuted recombinant interleukin 4 toxin with increased activity. Proc Natl Acad Sci USA. 1994;91:6889–93. doi: 10.1073/pnas.91.15.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreitman RJ, Puri RK, Pastan I. Increased antitumor activity of a circularly permuted IL-4-toxin in mice with IL-4 receptor bearing human carcinoma. Cancer Res. 1995;55:3357–63. [PubMed] [Google Scholar]
- 47.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–34. [PubMed] [Google Scholar]
- 48.Puri RK, Hoon DS, Leland P, Snoy P, Rand RW, Pastan I, et al. Preclinical development of a recombinant toxin containing circularly permuted interleukin 4 and truncated Pseudomonas exotoxin for therapy of malignant astrocytoma. Cancer Res. 1996;56:5631–7. [PubMed] [Google Scholar]
- 49.Joshi BH, Leland P, Asher A, Prayson RA, Varricchio F, Puri RK. In situ expression of interleukin-4 receptors in human brain tumors and cytotoxicity of a recombinant interleukin-4 (IL-4) cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001;61:8058–61. [PubMed] [Google Scholar]
- 50.Husain SR, Behari N, Kreitman RJ, Pastan I, Puri RK. Complete regression of established human glioblastoma tumor xenograft by interleukin-4 toxin therapy. Cancer Res. 1998;58:3649–53. [PubMed] [Google Scholar]
- 51.Weber F, Asher A, Bucholz R, Berger M, Prados M, Chang S, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64:125–37. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 52.NBI-3001 followed by surgery in treating patients with recurrent glioblastoma multiforme. From: http://clinicaltrials.gov, study identifier NCT00014677. Accessed: July 2010.
- 53.Hunter T. The epidermal growth factor receptor gene and its product. Nature. 1984;311:414–6. doi: 10.1038/311414a0. [DOI] [PubMed] [Google Scholar]
- 54.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84:6899–903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humphrey PA, Wong AJ, Vogelstein B, Friedman HS, Werner MH, Bigner DD, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenographs. Cancer Res. 1988;48:2231–8. [PubMed] [Google Scholar]
- 56.Liberman TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, et al. Amplification, enhanced expression, and possible rearrangement of EGF receptor gene in primary human brain tumors of glial origin. Nature. 1985;313:144–7. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 57.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. Progress report of a phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 58.Weber F. Preliminary results of a phase II study of TP-38 immunotoxin delivered via convection-enhanced delivery to patients with recurrent glioblastoma multiforme: report of the TP-38 study group. Ann Meeting German Soc Neurosurg. From: http://www.egms.de/en/meetings/dgnc2005/05dgnc0094.htm. Accessed: July 2010.
- 59.Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–72. [PubMed] [Google Scholar]
- 60.Joshi BH, Kawakami K, Leland P, Puri RK. Heterogeneity in interleukin-13 receptor expression and subunit structure in squamous cell carcinoma of head and neck: differential sensitivity to chimeric fusion proteins comprised of interleukin-13 and a mutated form of Pseudomonas exotoxin. Clin Cancer Res. 2002;8:1948–56. [PubMed] [Google Scholar]
- 61.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–8. [PubMed] [Google Scholar]
- 62.Puri RK, Leland P, Obiri NI, Husain SR, Kreitman RJ, Haas GP, et al. Targeting of interleukin-13 receptor on human renal cell carcinoma cells by a recombinant chimeric protein composed of interleukin-13 and a truncated form of Pseudomonas exotoxin A (PE38QQR) Blood. 1996;87:4333–9. [PubMed] [Google Scholar]
- 63.Weingart J, Strauss LC, Grossman SA. Phase I/II study: intratumoral infusion of IL13-PE38QQR cytotoxin for recurrent supratentorial malignant glioma. Neurooncol. 2002;4:379. [Google Scholar]
- 64.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–11. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 65.Kunwar S, Prados M, Chang S, Berger MS, Lang FF, Piepmeier JM, et al. Peritumoral convection-enhanced delivery of IL13-PE38QQR in patients with recurrent malignant glioma - phase I interim results. Neurooncol. 2003;5:4. [Google Scholar]
- 66.Chang SM. Neuroradiographic changes following convection-enhanced delivery (CED) of the conjugated toxin IL13-PE38QQR following resection of recurrent malignant glioma. Neurooncol. 2003;5:4. [Google Scholar]
- 67.Prados MD, Lang FF, Stauss LC, Fleming CK, Aldape K, Kunwar S, et al. Intratumoral and intracerebral microinfusion of IL13-PE38QQR cytotoxin: phase I/II study of pre-and post-resection infusions in recurrent resectable maliganant glioma. Proc Am Soc Clin Oncol. 2002;21:2087. [Google Scholar]
- 68.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. Phase III randomized trial of CED of IL13-PE38QQR vs. Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871–81. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65:3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 70.Johnson VG, Wilson D, Greenfield L, Youle RJ. The role of the diphtheria toxin receptor in cytosol translocation. J Biol Chem. 1988;263:1295–300. [PubMed] [Google Scholar]
- 71.Greenfield L, Johnson VG, Youle RJ. Mutations in diphtheria toxin separate binding from entry and amplify immunotoxin selectivity. Science. 1987;238:536–9. doi: 10.1126/science.3498987. [DOI] [PubMed] [Google Scholar]
- 72.Recht L, Torres CO, Smith TW, Raso V, Griffin TW. Transferrin receptor in normal and neoplastic brain tissue: implications for brain tumor immunotherapy. J Neurosurg. 1990;72:941–5. doi: 10.3171/jns.1990.72.6.0941. [DOI] [PubMed] [Google Scholar]
- 73.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–3. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 74.Johnson VG, Wrobel C, Wilson D, Zovickian J, Greenfield L, Oldfield EH, et al. Improved tumor-specific immunotoxins in the treatment of CNS and leptomeningeal neoplasia. J Neurosurg. 1989;70:240–8. doi: 10.3171/jns.1989.70.2.0240. [DOI] [PubMed] [Google Scholar]
- 75.Hall WA, Godal A, Juell S, Fodstad O. In vitro efficacy of transferrin-toxin conjugates against glioblastoma multiforme. J Neurosurg. 1992;76:838–44. doi: 10.3171/jns.1992.76.5.0838. [DOI] [PubMed] [Google Scholar]
- 76.Laske DW, Ilercil O, Akbasak A, Youle RJ, Oldfield EH. Efficacy of direct intratumoral therapy with targeted protein toxins for solid human gliomas in nude mice. J Neurosurg. 1994;80:520–6. doi: 10.3171/jns.1994.80.3.0520. [DOI] [PubMed] [Google Scholar]
- 77.Engebraaten O, Hjortland GO, Juell S, Hirschberg H, Fodstad O. Intratumoral immunotoxin treatment of human malignant brain tumors in immunodeficient animals. Int J Cancer. 2002;97:846–52. doi: 10.1002/ijc.10137. [DOI] [PubMed] [Google Scholar]
- 78.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–8. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 79.Study of therapy with TransMID™ compared to best standard of care in patients with glioblastoma multiforme. From: http://clinicaltrials.gov, study identifier NCT00083447, see also NCT00088400 and NCT00087230. Accessed: July 2010.
- 80.Celtic Pharma Terminates Transmid Trial KSB311R/CIII/001. From: http://www.drugs.com/news/celtic-pharma-terminates-transmid-trial-ksb311r-ciii-001-5246.html. Accessed: July 2010.
- 81.Briellmann RS, Pell GS, Wellard RM, Mitchell LA, Abbott DF, Jackson GD. MR imaging of epilepsy: state of the art at 1.5 T and potential of 3 T. Epileptic Disord. 2003;5:3–20. [PubMed] [Google Scholar]
- 82.Tummala RP, Chu RM, Liu H, Truwit CL, Hall WA. Application of diffusion tensor imaging to magnetic-resonance-guided brain tumor resection. Ped Neurosurg. 2003;39:39–43. doi: 10.1159/000070879. [DOI] [PubMed] [Google Scholar]
- 83.Cruz Junior LC, Sorensen AG. Diffusion tensor magnetic resonance imaging of brain tumors. Neurosurg Clin N Am. 2005;16:115–34. doi: 10.1016/j.nec.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Zlokovic BV, Apuzzo ML. Cellular and molecular neurosurgery: pathways from concept to reality - part II: vector systems and delivery methodologies for gene therapy of the central nervous system. Neurosurgery. 1997;40:805–13. doi: 10.1097/00006123-199704000-00028. [DOI] [PubMed] [Google Scholar]
- 85.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–98. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 86.Coffey MC, Strong JE, Forsyth PA, Lee PW. Retrovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–4. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 87.Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5-) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 88.Rainov NG, Kramm CM, Aboody-Guterman K, Chase M, Ueki K, Louis DN, et al. Retrovirus-mediated gene therapy of experimental brain neoplasms using the herpes simplex virus-thymidine kinase/ganciclovir paradigm. Cancer Gene Ther. 1996;3:99–106. [PubMed] [Google Scholar]
- 89.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 90.Oldfield EH, Ram Z, Culver KW, Blaese RM, DeVroom HL, Anderson WF, et al. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993;4:39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- 91.Rainov NG, Ren H. Gene therapy for human malignant brain tumors. Cancer J. 2003;9:180–8. doi: 10.1097/00130404-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Muldoon LL, Kroll RA, Pagel MA, Roman-Goldstein S, Fiamengo SA, Neuwelt EA. Delivery of therapeutic genes to brain and intracerebral tumors. In: Chiocca EA, Breakefield XO, editors. Gene therapy for neurological disorders and brain tumors. Boston: Humana Press; 1997. pp. 128–39. [Google Scholar]
- 93.Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–61. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 94.Harsh GR, Deisboeck TS, Louis DN, Hilton J, Colvin M, Silver JS, et al. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: a gene-marking and neuropathological study. Neurosurgery. 2000;92:804–11. doi: 10.3171/jns.2000.92.5.0804. [DOI] [PubMed] [Google Scholar]
- 95.Ram Z, Barnett G, Vogelbaum M, Constantini S, Lillehei K, Sherman J, et al. Pre-operative infusion of IL13-PE38QQR cytotoxin by convection-enhanced delivery (CED) in recurrent malignant glioma: A phase I/II study. Proc Am Soc Clin Oncol. 2003;22:403. [Google Scholar]
- 96.Andreansky S, Soroceanu L, Flotte ER, Chou J, Markert JM, Gillespie GY, et al. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–9. [PubMed] [Google Scholar]
- 97.Chiocca EA. Oncolytic viruses. Nat Cancer Rev. 2002;2:938–51. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 98.Dirven C, Van Beusechem V, Lamfers M, Grill J, Gerritsen WR, Vandertop WP. Oncolytic adenoviruses for treatment of brain tumours. Expert Opin Biol Ther. 2002;2:943–52. doi: 10.1517/14712598.2.8.943. [DOI] [PubMed] [Google Scholar]
- 99.Heise C, Kirn DH. Replication-selective adenoviruses as oncolytic agents. J Clin Invest. 2000;105:847–51. doi: 10.1172/JCI9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors I. Role of interstitial pressure and convection. Microvasc Res. 1989;37:77–102. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 101.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 102.Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–83. [PubMed] [Google Scholar]
- 103.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–81. [PubMed] [Google Scholar]
- 104.Klatzmann D, Valery CA, Bensimon G, Marro B, Boyer O, Mokhtari K, et al. A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Hum Gene Ther. 1998;9:2595–604. doi: 10.1089/hum.1998.9.17-2595. [DOI] [PubMed] [Google Scholar]
- 105.Shand N, Weber F, Mariani L, Bernstein M, Gianella-Borradori A, Long Z, et al. A phase 1–2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. Hum Gene Ther. 1999;10:2325–35. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- 106.Izquierdo M, Cortes ML, Martin V, de Felipe P, Izquierdo JM, Pérez-Higueras A, et al. Gene therapy in brain tumours: implications of the size of glioblastoma on its curability. Acta Neurochir Suppl. 68:111–7. doi: 10.1007/978-3-7091-6513-3_21. 19097. [DOI] [PubMed] [Google Scholar]
- 107.Izquierdo M, Martin V, de Felipe P, Izquierdo JM, Pérez-Higueras A, Cortés ML, et al. Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Ther. 1996;3:491–5. [PubMed] [Google Scholar]
- 108.Prados MD, McDermott M, Chang SM, Wilson CB, Fick J, Culver KW, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol. 2003;65:269–78. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 109.Puumalainen A, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, et al. ß-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–74. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 110.Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 111.Judy KD, Eck SL. The use of suicide gene therapy for the treatment of malignancies of the brain. In: Lattime EC, Stanton LC, editors. Gene therapy of cancer. San Diego, CA: Academic Press; 2002. pp. 505–12. [Google Scholar]
- 112.Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex–thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–89. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 113.Smitt PS, Driesse M, Wolbers J, Kros M, Avezaat C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003;7:851–8. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 114.Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 115.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–72. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Preliminary Cerepro® Phase III results meet primary endpoint - Secondary endpoints yet to be established with 45% of patients still alive. From: http://www.dggt.de/docs/CereproPhase.pdf. Accessed: July 2010.
- 117.Mitchell P. Ark’s gene therapy stumbles at the finish line. Nat Biotech. 2010;28:183–4. doi: 10.1038/nbt0310-183. [DOI] [PubMed] [Google Scholar]
- 118.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–43. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 119.Markert JM, Parker JN, Gillespie GY, Whitley RJ. Genetically engineered human herpes simplex virus in the treatment of brain tumours. Herpes. 2001;8:17–22. [PubMed] [Google Scholar]
- 120.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–66. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 121.Harland J, Papanastassiou V, Brown SM. HSV1716 persistence in primary human glioma cells in vitro. Gene Ther. 2002;9:1194–8. doi: 10.1038/sj.gt.3301782. [DOI] [PubMed] [Google Scholar]
- 122.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–58. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 123.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 124.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–66. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 125.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 126.Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–7. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 128.Nilaver G, Muldoon LL, Kroll RA, Pagel MA, Breakefield XO, Davidson BL, et al. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood-brain barrier disruption. Proc Natl Acad Sci USA. 1995;92:9829–33. doi: 10.1073/pnas.92.21.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]