Abstract
Objectives:
There are limited data concerning the assessment of renal function in beta-thalassaemia major, with no study of such involvement in Omani patients. The objective of this study was to establish the pattern of renal glomerular and tubular function using traditional and specific laboratory tests in patients with beta-thalassaemia major.
Methods:
This cross-sectional study, from January–July 2008, included 30 patients of the Thalassaemia Clinic at the Royal Hospital, Oman, with transfusion-dependent homozygous beta-thalassaemia major. They included 15 males and 15 females, aged 16–32 years with mean ± standard deviation of 21.23 ± 3.42 years. The medical records were reviewed and renal function states assessed as follows: serum creatinine, estimated glomerular filtration rate (eGFR); urea; phosphate, fractional excretion of filtered sodium (FENa); urine albumin: creatinine index; urine ß2-microglobulin:creatinine index; tubular reabsorption of phosphate (TRP), and tubular maximum phosphate reabsorption (TmP)/GFR.
Results:
All patients had eGFR >90 ml/min/1.73m2; serum creatinine <90 μmol/L; serum urea <6.0 mmol/L, and urine albumin:creatinine <2.5 mg/mmol. Only 2 (6.7%) patients had FENa >1% and 3 (10.0%) patients had urine ß2-microglobulin: creatinine >22 μg/mmol. All patients had TRP >0.85, of whom seven (23.3%) patients had values within the range of 0.85–0.95 and 23 (76.7%) had values >0.95. Also, all patients had TmP/GFR >1.0 mmol/L, of whom only one (3.3%) patient had TmP/GFR of 1.0–1.5, and 29 (96.7%) patients had TmP/GFR >1.5 mmol/L. Finally, 24 (80%) patients had serum phosphate >1.4 mmol/L. Linear regression revealed a highly significant correlation between serum phosphate and TmP/GFR (r = 0.904, P < 0.001).
Conclusion:
Renal function, glomerular and tubular, appears to be well preserved in beta-thalassaemia major. Almost all renal function indicators were within the recommended ranges. Raised TmP/GFR and TRP were noted in the majority of patients, reflecting an up-trend in serum phosphate and therefore increasing renal phosphate reabsorption.
Keywords: Beta-thalassaemia, Glomerular, Tubular, Renal function, Oman
Advances in Knowledge
The cornerstone in the management of patients with beta-thalassaemia major is life-long blood transfusion with frequent iron chelation therapy. Despite this, these patients are prone to long-term organ dysfunction particularly in their cardiovascular, hepato-biliary, endocrine and skeletal systems. The renal system, particularly the kidneys, also undergoes pathophysiological changes and may be affected by the burden of iron and the chelation therapy. This is the first study of the assessment of laboratory-based renal function tests in patients with beta-thalassaemia major in Oman.
Glomerular tests are usually within the reference ranges, while alteration in certain tubular tests (such as raised TRP and TmP/GFR) may reflect an upward trend in serum phosphate and, as a consequence, in increasing renal phosphate reabsorption. Otherwise, renal function appeared to be well preserved with no overt renal disease.
Application to Patient Care
This study helps health care professionals to select and interpret renal function tests in patients with beta-thalassaemia major using glomerular indicators (eGFR, serum creatinine, urine microalbumin) and tubular indicators (FENa, ß2-microglobulinuria, TRP and TmP/GFR).
Haemoglobinopathies are a common medical problem worldwide including in Oman.1 A recent prospective neonatal screening programme in two major cities of Oman, which included consecutive cord blood samples from a total of 7,837 neonates, revealed that the overall incidence of alpha-thalassaemia (alpha-thal) was 48.5%. Beta-globin-related abnormalities accounted for 9.5% of the samples (4.8% sickle cell trait, 2.6% beta-thalassaemia trait, 0.9% haemoglobin (Hb) E trait, 0.8% Hb D trait, 0.08% Hb C trait, 0.3% sickle cell disease and 0.08% homozygous beta-thalassaemia).2 Also, data from an earlier community-based survey of the most common genetic blood disorders among Omani children reported a prevalence rate of 2% for beta-thalassaemia trait, 0.07% for beta-thalassaemia major, 6% for sickle cell trait and 0.2% for sickle cell disease.3 Comparable data has also been reported from Gulf Cooperation Council states and other neighbouring countries.4,5,6,7
Beta-thalassaemia major is an inherited monogenic disorder that was first described in 1925 by Cooley and Lee.8 It is caused by a mutation at the ß-globin gene locus resulting in persistence of α-globin chain that is precipitated within the erythroid precursors in the bone marrow associated with severe dyserythropoeitic anaemia.9 The combination of early diagnosis, improvement in monitoring complications and advances in supportive therapy has enabled patients with thalassaemia major to have improved life expectancy.10 The cornerstone of management is life-long blood transfusion with frequent iron chelation therapy to minimise the deleterious effect of chronic iron deposition and its accumulation in tissues.9 Despite this, these patients are prone to long-term organ dysfunction particularly the cardiovascular, hepato-biliary, endocrine and skeletal systems.11 The renal system, particularly the kidneys, is an important functional unit that is exposed to the pathophysiological changes as well as the burden of iron deposition and chelation therapy in beta-thalassaemia. However, it appears that there is limited data available concerning renal involvement in this disease, and no such data is available for patients with beta-thalassaemia in Oman.
The objective of this study was to evaluate the degree of renal dysfunction, both glomerular and tubular, using traditional and specific laboratory tests of renal function in adult Omani patients with transfusion-dependent homozygous beta-thalassaemia major at the Royal Hospital, Oman.
Methods
This cross-sectional study was conducted during a six months period (1 January to 31 July 2008) and covered adult Omani patients with transfusion-dependent homozygous beta-thalassaemia major registered with the Thalassaemia Clinic at the Royal Hospital, Oman. This is one of a number of thalassaemia clinics in Oman which together deal patients with this disease. The study included 30 patients (15 male, 15 female), aged 16–32 years who were seen at the clinic at 3 monthly intervals. The diagnosis of homozygous thalassaemia was based on the characteristic haematological criteria (peripheral blood evaluation and haemoglobinopathy screening) at presentation or screening from the early years of life.
The study protocol was a naturalistic observation, an integral part of routine clinical procedure through reviewing the medical records of these thalassaemic patients from the hospital computer records including the haematologists’ clinical review as well as results of laboratory investigations. The clinical haematologists are regularly consulted on the management of these patients which includes supervision of blood transfusion and chelation therapy, as well as monitoring of organ dysfunction due to predicted iron deposition in tissues. The patients had been regularly transfused every three weeks since the early years of life with packed red blood cells, and were regularly taking iron chelators such as desferrioxamine (40 mg/kg body weight) as subcutaneous infusions 5 days per week, and deferiprone (75 mg/kg body weight) tablets daily.
For the laboratory investigations, blood samples were collected in the fasting state, and early morning urine specimens were provided from all the patients during their visits to the clinic. In addition to complete blood count and serum ferritin, different biochemical function profiles are regularly done to screen for any possible dysfunction. Evaluation of renal function is usually performed, together with other core function profile tests. The biochemical tests included: serum creatinine, and creatinine-based estimated glomerular filtration rate (eGFR); urea; phosphate as well as fractional excretion of filtered sodium (FENa); urine albumin: creatinine index (for microalbuminuria); urine ß2-microglobulin: creatinine index (for ß2-microglobulinuria); tubular reabsorption of phosphate (TRP), and tubular maximum phosphate reabsorption/GFR (TmP/GFR). All these parameters were measured in the Clinical Biochemistry Laboratory at the Royal Hospital. Creatinine was analysed by kinetic Jaffe reaction; urea and sodium by ion-selective electrodes; phosphate by molybdenum blue, and albumin by the immunoturbidemetric method (all from Synchron LX20, Beckman Coulter, Inc, CA, USA), and ß2-microglobulin by chemiluminescent microparticle immunoassay method on AXSym (Abbott, USA).12 Urinary ratios of microalbumin and ß2-microglobulin in relation to urine creatinine, as well as FENa were calculated.12 The eGFR was calculated by the Modification of Diet in Renal Disease (MDRD) equation13 and TRP as well as TmP/GFR were calculated as recommended by Payne.14 The reference ranges for the concerned biochemical parameters were as follows: serum creatinine (female 40–90 μmol/L; male 50–100 μmol/L); eGFR (>90 ml/min/1.73m2); serum urea (3.0–6.0 mmol/L); serum phosphate (0.7–1.4 mmol/L); FENa (<1%); urine albumin: creatinine ratio (female < 3.5 mg/mmol, male < 2.5 mg/mmol); urine ß2 microglobulin: creatinine ratio (ß22 μg/mmol); TRP (0.85–0.95), and TmP/GFR (1.0–1.5 mmol/L).
The results were analysed and numerical data presented as mean ± standard deviation (SD) and range. ANOVA (analysis of variance) was used to compare the differences in the means of results between the different groups. Pearson’s correlation coefficient was also used to compare between certain parameters where appropriate, which include serum phosphate and TmP/GFR. The statistical significance was assigned at P < 0.05.15
Results
Thirty homozygous ß-thalassaemia patients (15 male, 15 female), aged 16–32 years, mean 21.23 years, SD of ± 3.42, were evaluated in this study. The recommended cut-off for the studied renal function tests include: eGFR >90 ml/min/1.73m2; serum creatinine <90 μmol/L (in females) and <100 μmol (in males); serum urea <6.0 mmol/L; serum phosphate <1.40 mmol/; FENa <1.0 %; urine albumin: creatinine <3.5 mg/mmol (in females) and <2.5 mg/mmol (in males); urine ß2micglobulin: creatinine <22 μg/mmol; TRP 0.85–0.95, and TmP/GFR 1.0–1.5 mmol/L.
The results of the renal function tests, glomerular and tubular, are shown in Table 1. For eGFR, and due to the possible effect of imprecision and analytical bias in creatinine assays at concentrations within the reference range, it has been recommended to report eGFR values higher than 90 ml/min/1.73m2 as >90 ml/min/1.73m2 with no numerical value.16 Hence, statistical data was not applied for eGFR results when calculated eGFR >90 ml/min/1.73m2. All thirty patients had eGFR >90 ml/min/1.73m2; serum creatinine <90 μmol/L (all group median 43, range 23–73 μmol/L); serum urea <6.0 mmol/L (median 3.3, range 1.3–5.6 mmol/L), and urine albumin: creatinine <2.5 mg/mmol (median 0.50, range 0.1–1.7 mg/mmol). Only two (6.7%) patients had FENa >1% (median 0.30, range 0.06–1.35%), and three (10%) patients had urine ß2-microglobulin: creatinine >22 μg/mmol (median 7.1, range 1.14–60.4 μg/mmol).
Table 1:
Indicators of renal glomerular and tubular function tests in 30 patients with homozygous beta-thalassaemia.
| Parameter | Mean ± SD of Results | No. and (%) of Patients | |||
|---|---|---|---|---|---|
| Normal Values | Abnormal Values* | Abnormal Values | Normal Values | ||
| eGFR (ml/min/1.73m2) | > 90 | - | 30 (100%) | 0 (0.0%) | |
| Serum creatinine (μmol/L) | 44.4 ± 10 | - | 30 (100%) | 0 (0.0%) | |
| Serum urea (mmol/L) | 3.4 ± 1.0 | - | 30 (100%) | 0 (0.0%) | |
| Serum phosphate (mmol/L) | 1.33 ± 0.12 | 1.91 ± 0.39 | 6 (20%) | 24 (80) | |
| Urine albumin: creatinine (mg/mmoL) | 0.69 ± 0.5 | - | 30 (100%) | 0 (0.0%) | |
| FENa (%) ** | 0.35 ± 0.22 | 1.24 ± 0.14 | 28 (93.5%) | 2 (6.7%) | |
| Urine ß2.micglobulin: creatinine (μg/mmoL) ** | 7.1 ± 4.9 | 39.3 ± 18.3 | 27 (90.0%) | 3 (10.0%) | |
| TRP *** | 0.96 ± 0.02 | - | 30 (100%) | 0 (0.0 %) | |
| TmP/GFR *** (mmol/L) | 2.28 ± 0.646 | - | 30 (100%) | 0 (0.0 %) | |
Notes
All patients marked as (-) had values within the reference ranges with no patient exceeded the cut-off for the renal function test
Only 2 patients had high results for FENa and 3 patients had high results for urine, B2.microglobulin: creatinine; statistical data are not presented as the number is low
Abnormal values indicate high values for all parameters, except for TRP and TmP/GFR which are indicated by low values
Legend: eGFR = estimated glomerular filtration rate; FENa = fractional excretion of filtered sodium; TRP = tubular reabsorption of phosphate; TmP/GFR = tubular maximum phosphate reabsorption/glomerular filtration rate.
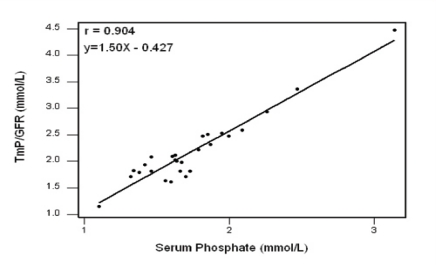
Concerning renal phosphate handling, all patients had TRP >0.85 (all group median 0.97, range 0.91–0.99), and TmP/GFR >1.0 mmol/L (median 2.01, range 1.15–4.48 mmol/L). Further characterisation of the results revealed that seven (23.3%) patients had TRP within the recommended reference range of 0.85–0.95 and 23 (76.7%) patients had values >0.95, with no patient having TRP <0.85. For TmP/GFR, only one (3.3%) patient had TmP/GFR within the reference range of 1.0–1.5 mmol/L, and 29 (96.7%) patients had raised TmP/GFR >1.5 mmol/L; 13 (43.3%) patients had values 1.51–2.00 mmol/L, nine (30%) had values 2.01–2.50 mmol/L, five (16.7%) had values 2.5–3.0 mmol/L and two (6.7%) had values >3.0 mmol/L; no patient had TmP/GFR <1.0 mmol/L. Also, six (20%) and 24 (80%) patients had serum phosphate ≤1.4 and >1.4 mmol/L respectively (median 1.66, range 1.10–3.14 mmol/L). A linear regression analysis revealed a highly significant correlation between serum phosphate and TmP/GFR (r = 0.904, P <0.001), [Figure 1].
Figure 1:
Correlation between serum phosphate and tubular maximum phosphate reabsorption/glomerular filtration rate (TmP/GFR) in 30 homozygous beta-thalassaemia major patients.
In order to compare the parameters of phosphate handling, the results were also assessed in relation to the endocrine complications that were previously reported and reviewed in these patients.17 The patients were grouped as those with ≥ one endocrinopathy, n = 22; hypogonadism alone, n = 12; hypogonadism with diabetes mellitus, n = 7; with primary hypoparathyroidism, n = 1; with hypoparathyroidism and diabetes mellitus, n = 1; and with hypoparathyroidism and hypothyroidism, n = 1, and those with no endocrinopathy, n = 8. A significant difference (P <0.005) was noted in TmP/GFR (2.456 ± 0.69 versus 1.818 ± 0.255 mmol/L) and in serum phosphate (1.928 ± 0.42 versus 1.46 ± 0.18 mmol/L); however, no significant difference was noted in TRP (0.962 ± 0.021 versus 0.960 ± 0.025) in patients with and without endocrinopathy respectively. The three patients with primary hypoparathyroidism had the highest TmP/GFR values (4.48, 3.36, and 2.32 mmol/L). No significant difference was noted in serum ferritin between these two groups (6480 ± 3418.6 versus 5647 ± 3663 μg/L).
Discussion
Patients with beta-thalassaemia major are prone to metabolic complications, including different organ dysfunction which can occur as single or multiple involvements. Although the actual mechanism is not definitive, the most likely explanation is related to anaemia and iron overload, in addition to lipid peroxidation, oxidative stress and free radical release.18
In this study, all 30 beta-thalassaemia major patients had normal glomerular renal function tests as indicated by their normal levels of eGFR >90 ml/min/1.73m2; serum creatinine <90 μmol/L; serum urea <6.0 mmol/L and urine albumin: creatinine <2.5 mg/mmol. Despite the increased iron deposition and the consequence of its possible oxidative stress on the renal parenchyma of these patients, many workers have also reported normal serum creatinine and creatinine clearance in patients with beta-thalassaemia major.19,20 The absence of microalbuminuria in our patients is another reflector of intact renal glomerular function. Microalbuminuria is an early indicator of nephropathy in patients at risk of developing kidney involvement, such as those with underlying chronic diseases including diabetes mellitus, hypertension or cardiovascular disease.21
In this study, almost all patients also had normal tubular renal function tests. This is indicated by the normal levels of FENa and urine ß2-microglobulin: creatinine, with only two (6.7%) patients having slightly raised FENa >1% and three (10%) patients with raised urine ß2-microglobulin: creatinine >22 μg/mmol; serum ß2-microglobulin was not measured in these three patients. Also, no patient had TRP <0.85 or TmP/GFR <1.0 mmol/L, which exclude the possibility of a renal tubular phosphate leak. Hence, all our patients had normal renal indicators for handling microalbumin and phosphate, with the vast majority of them having normal renal indicators for handling sodium and ß2-microglobulin. These are amongst the important tubular functions tests, which have almost excluded a significant impairment in tubular renal function in our patients’ series. Gosling reported an estimate that when there is complete failure of renal tubular absorption, there will be an approximately 1,800-fold normal increase in ß2-microglobulin loss and 20-fold normal increase in urine albumin loss.22 In comparison with other studies, Aldudak et al. reported no significant difference in creatinine clearance and FENa between thalassaemics and healthy controls.23 Kalman et al. also reported no significant difference in many tubular function indicators that include fractional excretion of sodium, magnesium, uric acid as well as calcium, protein, glucose, urine ß-2.microglobulin, N-acetyl-ß-D-glucosaminidase (NAG) levels and TRP in children with beta-thalassaemia major and thalassaemia intermedia.24 However, there are reports by other researchers who observed impairment in renal function when using other sensitive renal tubular function tests. Smolkin et al.25 reported normal creatinine clearance, FENa and TRP; however, urine NAG excretion was significantly raised in beta-thalassaemics, as also reported by Aldudak et al.23 and Sumboonnnanonda et al.26, who observed increase in both urinary NAG and malondialdehyde (MDA) excretion. MDA is an indicator of lipid peroxidation.27 Hence anaemia and increased oxidative stress, possibly iron induced, may play a role in precipitating some degree of tubular dysfunction, which may be mild, and so can not be detected when using other tubular function indicators.
In our study, 96.7% patients had TmP/GFR of >1.5 mmol/L and 76.7% patients had TRP values >0.95, indicating enhanced renal tubular phosphate reabsorption. This may be due to the raised phosphate level, due to the accelerated turnover of erythrocytes from the excess haemolysis in beta-thalassaemia. This is supported by the observation that 80% of our thalassaemics had raised serum phosphate and the significant correlation (r = 0.904) observed between serum phosphate and TmP/GFR levels. This compares with other studies where some researchers revealed no change24,26 while others observed some degree of altered TRP.23 However, no data is available in the literature for comparing TmP/GFR as a renal tubular index in beta-thalassaemic patients, although this test is more representative of renal tubular handling of phosphate than TRP alone, but there is limited awareness about its importance.14
Also, in this study significantly higher mean TmP/GFR and serum phosphate values were observed in beta-thalassaemics with endocrinopathies compared with those with no endocrinopathy. Hypogonadism, the most frequent endocrine disorder that was observed in 22 (73.3%) thalassaemics in our series, appears to be the most likely underlying aetiology.17 The upward trend in TmP/GFR in these patients may be due to the decline in sex hormones (estrogens in women and androgens in men) as a consequence of impaired gonadal function resulting in a decline in the stimulating effect of sex hormones on calcitonin secretion, and accelerated bone resorption through inhibiting the formation of osteoclasts.28 Multiple pathways have been described for the inhibitory effect of estrogens and androgens on osteoclasts with calcitonin receptors and sex hormones have been demonstrated at multiple steps in the osteoclast lineage.29,30 Dundar et al. reported low calcitonin, osteocalcin, gonadal steroids, and pituitary hormones with high phosphate and N. telopeptide in beta-thalassaemic patients.31 In addition, other hormone deficiencies, such as parathyroid, thyroid and insulin, which are known to influence calcitonin secretion and skeletal protection as well as direct iron toxicity on osteoblasts, also contribute to the pathogenesis of altered bone metabolism in beta-thalassaemia.32 The highest TmP/GFR values in our patients were observed in three beta-thalassaemics who had primary hypoparathyroidism in addition to hypogonadism. While the main function of the parathyroid hormone (PTH) is calcium homeostasis, it is also an important determinant of phosphate reabsorption, with TmP/GFR being increased in hypoparathyroidism and decreased in hyperparathyroidism.33 Normal or raised TmP/GFR may offer an advantage, as low values are associated with hypercalciuria, nephrocalcinosis and tubular proteinuria. This is presumably a result of direct tubular dysfunction, although it is controversial and may be difficult to prove.34,35,36 However, regardless of the underlying mechanism, in patients with renal tubular dysfunction whether due to the effect of metabolites, drugs or toxins, the response of low TmP/GFR to therapy may assist in monitoring these patients.
Conclusion
Renal function, glomerular as well as tubular, appears to be well preserved in beta-thalassaemic adult patients. In this study, almost all renal indicators that include eGFR, serum creatinine and urea as well as FENa, urine microalbumin, and ß2-microglobulin were within the recommended reference ranges. Raised TRP and TmP/GFR values were noted in the majority of thalassaemics, studied reflecting an upward trend in serum phosphate and its consequence in increasing renal reabsorption of phosphate. Despite the impairment in certain laboratory-based renal tubular indicators in some patients, especially those reported in the reviewed literature, these beta-thalassaemics had no overt renal disease, and so it remains questionable whether such functional abnormalities would have any long-term effect.
Footnotes
CONFLICT OF INTEREST
The authors reported no conflict of interest.
References
- 1.Rajab AG, Patton MA, Modell B. Study of hemoglobinopathies in Oman through a national register. Saudi Med J. 2000;21:1168–72. [PubMed] [Google Scholar]
- 2.AlKindi S, Al Zadjali S, Al Madhani A, Daar S, Al Haddabi H, Al Abri Q, et al. Forecasting hemoglobinopathy burden through neonatal screening in Omani neonates. Hemoglobin. 2010;34:135–44. doi: 10.3109/03630261003677213. [DOI] [PubMed] [Google Scholar]
- 3.Al-Riyami A, Ebrahim GJ. Genetic blood disorders survey in the Sultanate of Oman. J Trop Pediatr. 2003;49:i1–20. [PubMed] [Google Scholar]
- 4.Al-Siliman A. Prevalence of beta-thalassarmia trait in premarital screening in Al-Hassa, Saudi Arabia. Ann Saudi Med. 2006;26:14–6. doi: 10.5144/0256-4947.2006.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed AM, Al-Hilli F, Nadkarni KV, Bhagwat GP, Bapat JP. Hemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency in hospital births in Bahrain. Ann Saudi Med. 1992;12:536–9. doi: 10.5144/0256-4947.1992.536. [DOI] [PubMed] [Google Scholar]
- 6.Miller CJ, Dunn EV, Berg B, Abdouni SF. A hematological survey of preschool children of the United Arab Emirates. Saudi Med J. 2003;24:609–13. [PubMed] [Google Scholar]
- 7.Karimi M, Jamalian N, Yarmohammadi H, Askarnejad A, Afrasiabi A, Hashemi A. Premarital screening for beta-thalassaemia in Southern Iran: options for improving the programme. J Med Screen. 2007;14:62–6. doi: 10.1258/096914107781261882. [DOI] [PubMed] [Google Scholar]
- 8.Cooley TB, Lee P. A series of cases of splenomegaly in children with anemia and peculiar changes. Trans Am Pediatr Soc. 1925;37:29–30. [Google Scholar]
- 9.Thein SL. Beta-thalassaemia. Baillieres Clin Haematol. 1998;11:91–126. doi: 10.1016/s0950-3536(98)80071-1. [DOI] [PubMed] [Google Scholar]
- 10.Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, et al. Survival in medically treated patients with homozygous beta-thalassaemia. N Engl J Med. 1994;331:574–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 11.Britton RS, Ramm GA, Olynyk J, Singh R, O’Neill R, Bacon BR. Pathophysiology of iron toxicity. Adv Exp Med Biol. 1994;356:239–53. doi: 10.1007/978-1-4615-2554-7_26. [DOI] [PubMed] [Google Scholar]
- 12.Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 14th ed. St. Louis, Missouri: Elsevier Saunders; 2006. [Google Scholar]
- 13.Levey AS, Greene T, Kusek JW, Beck GL. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 14.Payne RB. Renal tubular reabsorption (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35:201–6. doi: 10.1177/000456329803500203. [DOI] [PubMed] [Google Scholar]
- 15.Hill AB, Hill ID. Bradford Hill’s Principles of Medical Statistics. 12th ed. London: Hodder Arnold; 1991. [Google Scholar]
- 16.Lamb EJ, Tomson CRV, Roderick PJ. Estimating kidney function in adults using formulae. Ann Clin Biochem. 2005;42:321–45. doi: 10.1258/0004563054889936. [DOI] [PubMed] [Google Scholar]
- 17.Mula-Abed WA, Al-Hashmi HS, AlMuslahi MN, AlMuslahi HN, AlLamki MA. Prevalence of endocrinopathies in patients with beta-thalassaemia major: A cross-sectional study in Oman. Oman Med J. 2008;23:257–62. [PMC free article] [PubMed] [Google Scholar]
- 18.Walter PB, Macklin EA, Porter J, Evans P, Kwiatkowski JL, Neufeld EJ, et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators desferasirox (ICL670) or desferoxamine: an ancillary study of the Novartis CICL670A0107 trial. Haematologica. 2008;93:817–25. doi: 10.3324/haematol.11755. [DOI] [PubMed] [Google Scholar]
- 19.Ong-ajyooth L, Malasit P, Ong-ajyooth S, Fucharoen S, Pootrakul P, Vasuvattkul S, et al. Renal function in adult beta-thalassemia/Hb E disease. Nephron. 1998;78:156–61. doi: 10.1159/000044904. [DOI] [PubMed] [Google Scholar]
- 20.Kotopodis KP, Elisaf MS, Pappas HA, Theodorou JC, Milionis HJ, Bourantas KL, et al. Renal abnormalities in patients with sickle cell beta-thalassemia. J Nephrol. 1997;10:163–7. [PubMed] [Google Scholar]
- 21.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 22.Gosling P. Proteinuria. In: Marshall WJ, Bangert SK, editors. Clinical Biochemistry: Metabolic and Clinical Aspects. 2nd ed. New York: Elsevier; 2008. pp. 156–73. [Google Scholar]
- 23.Aldudak B, Karabay Bayazit A, Noyan A, Ozel A, Anarat A, Sasmaz I, et al. Renal function in pediatric patients with beta-thalassemia major. Pediatr Nephrol. 2000;15:109–12. doi: 10.1007/s004670000434. [DOI] [PubMed] [Google Scholar]
- 24.Kalman S, Atay AA, Sakallioglu O, Ozgurtas T, Gok F, Kurt I, et al. Renal tubular function in children with beta-thalassemia minor. Nephrology. 2005;10:427–9. doi: 10.1111/j.1440-1797.2005.00484.x. [DOI] [PubMed] [Google Scholar]
- 25.Smolkin V, Halevy R, Levin C, Mines M, Sakran W, Ilia K, et al. Renal function in children with beta-thalassemia major and thalassemia intermedia. Pediatr Nephrol. 2008;23:1847–51. doi: 10.1007/s00467-008-0897-8. [DOI] [PubMed] [Google Scholar]
- 26.Sumboonnanonda A, Malasit P, Tanphaichitr VS, Ong-ajyooth S, Sunthornchart S, Pattanakakitsakul S, et al. Renal tubular function in beta-thalassemia. Pediatr Nephrol. 1998;12:280–3. doi: 10.1007/s004670050453. [DOI] [PubMed] [Google Scholar]
- 27.Del Rio D, Stewart AJ, Pellergini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Mundy GR. Factors regulating bone resorbing and bone forming cells. In: Mundy GR, editor. Bone remodeling and its disorders. 2nd ed. London: Martin Dunitz; 1999. pp. 45–82. [Google Scholar]
- 29.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogens stimulate gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–70. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 30.Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of androgen receptors in human bone. J Clin Endocrinol Metab. 1997;82:3493–7. doi: 10.1210/jcem.82.10.4319. [DOI] [PubMed] [Google Scholar]
- 31.Dundar U, Kupesiz A, Ozdern S, Gilgil E, Tuncer T, Yesilipek A, et al. Bone metabolism and mineral density in patients with beta-thalassemia major. Saudi Med J. 2007;28:1525–9. [PubMed] [Google Scholar]
- 32.Voskaridou E, Terpos E. Pathogenesis and management of osteoporosis in thalassemia. Pediatr Endocrinol Rev. 2008;6:86–93. [PubMed] [Google Scholar]
- 33.Shaw NJ, Wheeldon J, Brocklebank JT. Indices of intact serum parathyroid hormone and renal excretion of calcium, phosphate, and magnesium. Arch Dis Child. 1990;65:1208–11. doi: 10.1136/adc.65.11.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wikstrom B, Backman U, Danielson BG, Fellstrom B, Johansson G, Ljunghall S, et al. Phosphate metabolism in renal stone formers. (II): Relation to renal tubular functions and calcium metabolism. Scand J Urol Nephrol. 1981;61(II):1–26. [PubMed] [Google Scholar]
- 35.Jaeger P, Portmann L, Ginalski JM, Jacquet AF, Temler E, Burckhardt P. Tubulopathy in nephrolithiasis: conseque rather than cause. Kidney Int. 1986;29:563–71. doi: 10.1038/ki.1986.35. [DOI] [PubMed] [Google Scholar]
- 36.Alpern RJ, Sakhaee K. Does hyperphosphaturia underlie hypercalciuria? Lancet. 1997;349:518–9. doi: 10.1016/s0140-6736(97)80080-3. [DOI] [PubMed] [Google Scholar]