Abstract
Incidental renal tumours are becoming an important clinical problem that many physicians will need to deal with. A good knowledge of the nature of these tumours and how to manage them is therefore needed. The aim of this paper is to review the literature about incidental renal tumours in adults. Many incidentally discovered small renal tumours (<4 cm) are benign and of low stage, grade and progression potential. The preferred management in young fit patients is open or laparoscopic nephron-sparing surgery. Treatment alternatives include needle-ablative therapies and surveillance in elderly unfit patients. Tumour renal biopsy is encouraged prior to needle-ablative therapy and surveillance. Awareness about incidental renal masses and their management is essential for treating doctors.
Keywords: Incidental, Tumors, renal, Renal cell carcinoma
Cancer of the kidney accounts for approximately 3.5% of all malignancies and is the third most common cancer of the urinary tract.1 With the increased frequency and sophistication of radiological imaging, the rate of incidental findings of renal cortical tumours has steadily increased. The greatest incidence of asymptomatic small renal masses occurs in elderly patients with multiple comorbidities.2 Incidentally discovered small renal masses are being diagnosed with greater frequency3 and they now account for 48% to 66% of renal cell carcinoma (RCC) diagnosis.4 The incidence of renal cell carcinoma (RCC) at autopsy has been reported to be approximately 2%.5 The incidence of RCC has been increasing in all age groups, with the greatest increase occurring in patients with localised tumours, suggesting a migration toward earlier stages as a result of earlier detection.6
Considering the improved prognosis of incidental RCC, the difficulty in diagnosing benign tumours preoperatively, the excellent results associated with partial nephrectomy, and the possibility that these lesions themselves may have a less aggressive biology, it becomes apparent that not all solid renal masses are optimally treated by radical nephrectomy.7 Consequently, changes in the approach to the management of these masses have occurred. Traditionally, renal parenchymal-sparing procedures were reserved for patients with a functional or an anatomic impairment of the contralateral kidney. Currently, appropriately located lesions 4 cm or less in size are treated, with excellent long-term survival, by nephron-sparing surgery even in patients with a normal contralateral kidney.8 The aim of this paper is to review the literature about incidental renal tumours in adults. A literature search was performed using the PubMed database and selecting papers from 1950 to the present using the term ‘incidental renal tumours’.
Differential Diagnosis
The differential diagnosis of an incidentally detected renal mass includes both benign and malignant processes. Malignant renal masses include RCC, sarcoma, lymphoma, metastatic disease (especially lung, breast, prostate, colon, testes), and urothelial-based tumours of the pelvis and collecting system. Of these, RCCs account for most such masses. Lesions classified as benign include simple cysts, oncocytoma, adenoma, angiomyolipoma, fibroma, and lipoma, [Table 1].8
Table 1.
Differential diagnosis of renal masses in adults
| Cysts | Tumours | Inflammatory lesions |
|---|---|---|
| Simple | Malignant masses | Infection |
| Complex | Renal cell carcinoma | Infarction |
| Multiple | Lymphoma | Trauma (hematoma) |
| Sarcomas | ||
| Metastases | ||
| Benign masses | ||
| Renal adenomas | ||
| Angiomyolipomas | ||
| Oncocytomas | ||
| Others |
Tumour Growth Rate
Studies on the natural history of small renal tumours have shown that their growth was, in general, slow or undetectable. Approximately one-quarter to one-third of small renal tumours did not show radiographic growth, and the overall mean growth rate has been reported to be 0.28 cm per year (range: 0.09–0.86).4,9 A meta-analysis by Chawla et al. found that, over a mean follow-up of 34 months, a majority of 234 untreated renal lesions had a slow growth rate (approximately 0.28 cm per year), and metastases were rare (1%).9 Kouba et al.2 published the results of a retrospective study including 43 patients with 46 renal masses (24% of tumours >4 cm) who underwent active surveillance of enhancing solid or cystic, Bosniak IV (i.e. thick wall, thick septations, course calcifications, density more than 20, enhancement) renal masses. A subset of 13 patients who ultimately underwent surgical intervention was also examined. The mean delay to intervention was 12 months. At a mean follow-up of 36 months, renal masses grew in 74% of patients with a mean (median) growth rate of 0.70 (0.35) cm per year, no patient died of RCC, and none had evidence of metastatic disease. Initial tumour size (3.1 versus 2.6 cm, P = 0.450) was similar in the intervention and non-intervention group, and the growth rate did not correlate with initial tumour size. The authors suggested that active surveillance for renal masses is an appropriate option for selected patients, especially those with competing comorbidities. Delayed intervention after an initial period of surveillance did not appear to impact pathological outcomes adversely.
In a large retrospective study by Frank et al.,10 2,935 solid renal tumours were treated in a 30-year period. Of these tumours, 12.8% were benign and 87.2% were malignant. Of the tumours <1 cm in diameter, 46.3% were benign while 98% of malignant tumours were low grade. Of 250 tumours <2 cm in diameter, 30% were benign and only 9.2% were high grade malignancies. As the tumour size increases, there is a significantly greater probability that the tumour is malignant versus benign, clear cell versus papillary RCC, and high-grade versus low-grade RCC.
Initial tumour size is not a predictor of the subsequent growth rate. In the study conducted by Kouba et al.,2 tumours with an initial size > 4 cm had growth rates similar to those of smaller tumours. The majority of small renal masses grow and the majority is cancerous. One cannot safely assume that a lack of growth on serial computed tomography (CT) scan confirms the absence of malignancy. No clinical or radiological predictors of growth rate have been identified.11 Tumour growth rate determination alone probably will not give clinicians enough information to predict accurately the behaviour of all small renal masses. Cytogenetic, immunohistochemical, or other investigations done on needle biopsies and the study of new radiologic parameters using computed tomography (CT) scanning and magnetic resonance imaging (MRI) may be helpful in identifying patients who have renal tumours with good prognostic factors and a long natural history and who therefore may primarily be followed conservatively.12
Role of Renal Biopsy
The use of renal mass biopsy to guide treatment decisions is still controversial. The current indications for renal mass biopsy are: a) to differentiate benign from malignant small renal tumours;13 b) the use of molecular markers obtained from tumour biopsies for separating indolent from aggressive tumours;14 c) prior to or during ablative therapies and during follow up after ablative therapies, especially radiofrequency ablation, for defining treatment success or failure,15 and d) to rule out non-renal cell primary tumours (metastasis and lymphoma) or benign conditions (abscess), which may not require surgery. Biopsy has also been used to confirm the diagnosis and the histological subtype of renal primary lesion in patients with disseminated metastases or unresectable retroperitoneal masses.16 Urothelial carcinoma carries a higher risk of seeding than RCC and a percutaneous biopsy in the presence of radiological suspicion of renal pelvic tumour or positive urinary cytology should only be performed after careful consideration of the risks and benefits.16
The high proportion of benign lesions (19% to 26%)17 in tumours ≤ 4cm justifies preoperative histological diagnosis to decrease the rate of unnecessary renal surgery which has been reported to be 34%.18 The safety and accuracy of renal core biopsy has been questioned, in particular its accuracy for small lesions.19 In incidental renal masses of <5 cm, it has been reported that renal core biopsy had 100% accuracy for distinguishing benign from malignant lesions and 98% accuracy for determining histological tumour type,18 indicating that this technique can be used successfully for small lesions, including those that are asymptomatic and detected incidentally. However, there are limitations and complications with renal core biopsy of small lesions. The limitation is the considerable proportion of biopsy specimens that have insufficient tissue for analysis or contain only necrotic tissue, normal tissue or blood. This is referred to as the nondiagnostic rate or failure rate which has been reported to range from 0% to 22%.18 This high failure rate in small tumours is partly due to more difficult visualisation and targeting, and the biopsy needle displacing small masses rather than penetrating them.16 The potential complications of renal core biopsy include tumour seeding along the needle track, bleeding, arteriovenous fistula, infection and pneumothorax.16
Literature Overview
Renal cell carcinoma, which represents 2% of all adult cancers, is the most lethal of common urologic cancers, with approximately 35% of patients dying from the disease at the 5-year mark.20 The incidence of RCC has risen steadily each year during the last three decades in most of the world, with an average increase of 2% to 3% per year.21 Tobacco use and obesity are the most consistently identified risk factors for RCC, accounting for about 20% and 30% of cases, respectively.20 Other risk factors for RCC include hypertension and a family history of the disease.
Incidental detection of clinical stage 1 (<7.0 cm), solid, enhancing renal masses accounts for more than 50% of RCC cases, and these tumours are more likely to be organ confined and associated with an improved prognosis.22 The biology of these tumours is heterogeneous, and there are multiple management options available, ranging from observation to radical nephrectomy. Approximately 20% of clinical stage T1 renal masses are benign, and only 20% to 30% of malignant tumours in this size range demonstrate potentially aggressive features, with substantial variance based on patient age, gender and tumour size.23 The differential diagnosis of a renal mass includes: RCC, renal adenoma, oncocytoma, angiomyolipoma, urothelial carcinoma, metastatic tumour, abscess, infarct, vascular malformation or pseudotumour. With the exception of fat-containing angiomyolipoma, no current scanning methods can distinguish between benign and malignant solid tumours or between indolent and aggressive tumour biology. Tumours less than 3 cm may be more likely to be benign24 and the aggressive potential of RCC increases dramatically beyond this size.25
In recent years, the potential role of biopsy for localised renal tumours has been revisited, in part driven by the recognition that 20% clinical stage T1 renal masses may represent benign disease and could be considered for less aggressive management.25 In addition, the accuracy and safety of renal mass biopsy has improved substantially due to further refinements in CT- and MRI-guided techniques. A review of studies since 2001, demonstrates that the false-negative rate with renal mass biopsy is now only 1%, and the incidence of symptomatic complications is relatively low, with only a very small percentage (<2 %) requiring any form of intervention.26 Needle-tract seeding also appears to be exceedingly rare, assuming appropriate patient selection. While another 10–15% of renal mass biopsies are indeterminate, this is much less concerning than a false negative, which would lead to observation of a malignancy. Given the significant heterogeneity in the biological aggressiveness of clinical stage T1 renal masses and the wide range of treatment options now available, renal mass biopsy is now being used increasingly for patient counselling and clinical decision making. This approach is appropriate for patients in whom a wide range of management options are under consideration, ranging from surgery to observation. Renal mass biopsy is not indicated, however, for healthy patients who are unwilling to accept the uncertainty associated with this procedure or for older patients who will only consider conservative management options regardless of biopsy results. Incorporation of molecular analysis has shown great promise to further improve accuracy of renal mass biopsy/aspiration and remains a research priority.16 The most prominent molecular markers are the vhl gene, altered expression of carbonic anhydrase IX (CAIX) or the B7H1 molecule, cell cycle regulators such as p27, cyclin D1, pRb and p53 and markers of cellular proliferation such as Ki-67.
Histopathological evaluation of surgically managed suspicious renal masses in published reports indicate that the frequency of benign pathologic findings is higher for tumours less than 4 cm in size compared to tumours more than 4 cm. This information is important for physicians when counselling patients on treatment options. For tumours 4 cm or less in size, the percentage of benign masses reported were 20% (18 of 90),27 19.8% (23 of 116),28 and 23% (65 of 280).29 For tumours > 4 cm, the percentage of benign masses reported was 8% (8 of 96).27 The data by Luciani et al.,22 in a study of 1,092 patients, confirm a rapid and dramatic change in the epidemiologic and clinical characteristics of renal cancer, with an increasing number of incidentally found tumours: 13% in 1982 compared to 59.2% in 1997. The incidentally discovered tumours presented with a lower stage (74.3% versus 49.1%), grade (75.5% versus 56.9%), and percentage of metastases at presentation (10.4% versus 19.6%) compared to the symptomatic neoplasms. In a large study of 482 patients evaluating the histologic type of renal tumours according to size, Schachter et al.17 found smaller lesions (≤4 cm) that proved to be malignant were less likely to be of clear-cell histology (50% versus 72.8%) and more likely to be papillary (15.8% versus 9.4%) than were larger (>4 cm) lesions. Given the differences in the biological behaviour of the various histological subtypes of these tumours, these data are important in patient counselling about the treated and untreated natural history of small renal masses. The impact of histological subtype on prognosis is important.30 Amin and colleagues found that chromophobe and papillary subtypes have the best prognosis and conventional clear-cell RCCs had a worse prognosis as judged by 5-year disease specific survival.31 In the experience of Cheville and associates, both papillary and chromophobe lesions had a better prognosis than clear-cell carcinoma.30
Summary of Current Treatment
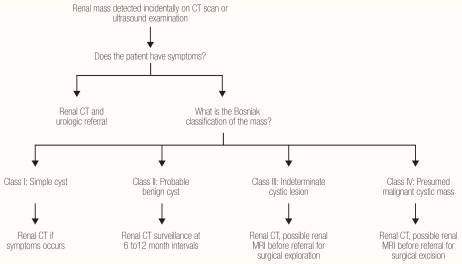
Renal masses are a common clinical problem, often detected in asymptomatic patients. The frequency of masses found incidentally by radiographic studies is increasing as studies with ultrasound (US), CT, and MRI are becoming more routine. The renal lesions can be classified as solid or cystic, malignant or benign, acquired or congenital, unilateral or bilateral, single or multiple, and primary or metastatic. The essential steps in the management of renal masses are: full history and physical examination; radiological evaluation by US or intravenous urogram (IVU) or CT, and determining the nature of the mass whether cystic or solid. If it is a simple cyst no further action is needed. However, if it is a complex cyst then further evaluation by CT or MRI for cyst classification is indicated [Figure 1].32 For solid renal masses, the recommended treatment guidelines for patients with clinical T1 renal masses are as follows.33 In a healthy patient with a clinical T1a (≤4.0 cm) enhancing renal mass, the standard is surgical excision by partial nephrectomy and radical nephrectomy as an alternative standard. In T1a, if the patient has major comorbidities or increased surgical risk, surgical excision by partial nephrectomy or radical nephrectomy should be discussed as a standard of care with an increased risk of surgical complications and chronic kidney disease. Other options include: thermal ablation as a less-invasive treatment, which may be advantageous in a high surgical risk patient, acknowledging the increased risk of local tumour recurrence compared to surgical excision; or active surveillance which can be offered as an acceptable approach to delay or avoid the need for intervention in high-risk patients. The standard treatment in a healthy patient with a clinical T1b (>4.0 cm to <7.0 cm) enhancing renal mass is radical nephrectomy for patients with a normal contralateral kidney, or partial nephrectomy as an alternative, particularly when there is a need to preserve renal function. In T1b patients with major comorbidities or increased surgical risk, the standard is radical nephrectomy for patients with a normal contralateral kidney or partial nephrectomy as a recommended modality when there is a need to preserve renal function. However, active surveillance should be discussed with patients who want to avoid surgery or who are considered high risk for surgical therapy; and thermal ablation may be discussed as treatment option which is less effective due to an increased risk of local recurrence.
Figure 1.
Management of incidental renal mass in adults
Conclusion
With the increased frequency and sophistication of radiological imaging, the rate of incidental findings of renal cortical tumours has steadily increased. Awareness about incidental renal masses and their management is essential as they are becoming an important clinical problem that many physicians will face. The main issue is whether to be aggressive or conservative with small renal masses, knowing that a significant number of these small tumours are benign and most of them grew slowly. In recent years, the potential role of biopsy for localised renal tumours has been revisited, in part driven by the recognition that 20% clinical stage T1 renal masses may represent benign disease and could be considered for less aggressive management. In addition, the accuracy and safety of renal mass biopsy has improved substantially due to further refinements in CT- and MRI-guided techniques. Incorporation of molecular analysis has shown great promise to improve further the accuracy of renal mass biopsy/aspiration and remains a research priority. Currently, small renal masses 4 cm or less are treated, with excellent long-term survival, by nephron-sparing surgery even in patients with a normal contralateral kidney.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Kouba E, Smith A, McRackan D, Wallen EM, Pruthi RS. Watchful waiting for solid renal masses: insight into the natural history and results of delayed intervention. J Urol. 2007;177:466–70. doi: 10.1016/j.juro.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 3.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203–5. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 4.Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer. 2004;100:738–45. doi: 10.1002/cncr.20025. [DOI] [PubMed] [Google Scholar]
- 5.Hellsten S, Johnsen J, Berge T, Linell F. Clinically unrecognized renal cell carcinoma. Diagnostic and pathological aspects. Eur Urol. 1990;18(S2):2–3. doi: 10.1159/000463946. [DOI] [PubMed] [Google Scholar]
- 6.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 7.Morgan WR, Zincke H. Progression and survival after renal-conserving surgery for renal cell carcinoma: experience in 104 patients and extended followup. J Urol. 1990;144:852–7. doi: 10.1016/s0022-5347(17)39608-8. [DOI] [PubMed] [Google Scholar]
- 8.Pantuck AJ, Zisman A, Rauch MK, Belldegrun A. Incidental renal tumors. Urology. 2000;56:190–6. doi: 10.1016/s0090-4295(00)00655-5. [DOI] [PubMed] [Google Scholar]
- 9.Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175:425–31. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 10.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217–20. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 11.Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol. 2007;177:849–53. doi: 10.1016/j.juro.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 12.Israel GM, Bosniak MA. Renal imaging for diagnosis and staging of renal cell carcinoma. Urol Clin North Am. 2003;30:499–514. doi: 10.1016/s0094-0143(03)00019-3. [DOI] [PubMed] [Google Scholar]
- 13.Silverman SG, Gan YU, Mortele KJ, Tuncali K, Cibas ES. Renal masses in the adult patient: the role of percutaneous biopsy. Radiology. 2006;240:6–22. doi: 10.1148/radiol.2401050061. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Cuilleron M, Cottier M, Gentil-Perret A, Lambert C, Genin C, et al. The use of MN/CA9 gene expression in identifying malignant solid renal tumors. Eur Urol. 2006;49:401–5. doi: 10.1016/j.eururo.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Weight CJ, Kaouk JH, Hegarty NJ, Remer EM, O'Malley CM, Lane BR, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008;179:1277–81. doi: 10.1016/j.juro.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 16.Volpe A, Kachura JR, Geddie WR, Evans AJ, Gharajeh A, Saravanan A, et al. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol. 2007;178:379–86. doi: 10.1016/j.juro.2007.03.131. [DOI] [PubMed] [Google Scholar]
- 17.Schachter LR, Cookson MS, Chang SS, Smith JA, Jr, Dietrich MS, Jayaram G, et al. Second prize: frequency of benign renal cortical tumors and histologic subtypes based on size in a contemporary series: what to tell our patients. J Endourol. 2007;21:819–23. doi: 10.1089/end.2006.9937. [DOI] [PubMed] [Google Scholar]
- 18.Shannon BA, Cohen RJ, de BH, Davies RJ. The value of preoperative needle core biopsy for diagnosing benign lesions among small, incidentally detected renal masses. J Urol. 2008;180:1257–61. doi: 10.1016/j.juro.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Rybicki FJ, Shu KM, Cibas ES, Fielding JR, van Sonnenberg E, Silverman SG. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281–7. doi: 10.2214/ajr.180.5.1801281. [DOI] [PubMed] [Google Scholar]
- 20.Lipworth L, Tarone RE, Mclaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:235. doi: 10.1016/j.juro.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 21.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973–1992. Eur J Cancer Prev. 2002;11:171–8. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982–1997) Urology. 2000;56:58–62. doi: 10.1016/s0090-4295(00)00534-3. [DOI] [PubMed] [Google Scholar]
- 23.Lane RB, Babineau D, Kattan MW, Novick AC. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178:42. doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 24.Pahernik S, Ziegler S, Roos F, Melchior SW, Thuroff JW. Small renal tumors: correlation of clinical and pathological features with tumor size. J Urol. 2007;178:414–7. doi: 10.1016/j.juro.2007.03.129. [DOI] [PubMed] [Google Scholar]
- 25.Remzi M, Ozsoy M, Klingler HC, Susani M, Waldert M, Seitz C, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol. 2006;176:896–9. doi: 10.1016/j.juro.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 26.Schmidbauer J, Remzi M, Memarsadeghi M, Haitel A, Klingler HC, Katzenbeisser D, et al. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol. 2008;53:1003–11. doi: 10.1016/j.eururo.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 27.Duchene DA, Lotan Y, Cadeddu JA, Sagalowsky AI, Koeneman KS. Histopathology of surgically managed renal tumors: analysis of a contemporary series. Urology. 2003;62:827–30. doi: 10.1016/s0090-4295(03)00658-7. [DOI] [PubMed] [Google Scholar]
- 28.Filipas D, Fichtner J, Spix C, Black P, Carus W, Hohenfellner R, et al. Nephron-sparing surgery of renal cell carcinoma with a normal opposite kidney: long-term outcome in 180 patients. Urology. 2000;56:387–92. doi: 10.1016/s0090-4295(00)00656-7. [DOI] [PubMed] [Google Scholar]
- 29.McKiernan J, Yossepowitch O, Kattan MW, Simmons R, Motzer RJ, Reuter VE, et al. Partial nephrectomy for renal cortical tumors: pathologic findings and impact on outcome. Urology. 2002;60:1003–9. doi: 10.1016/s0090-4295(02)01967-2. [DOI] [PubMed] [Google Scholar]
- 30.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Amin MB, Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–91. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JC, Fitzgerald JM. Evaluation of incidental renal and adrenal masses. Am Fam Physician. 2001;63:288–94. [PubMed] [Google Scholar]
- 33.Novick AC. Guideline for Management of the Clinical Stage 1 Renal Mass. Washington DC: American Urological Association; 2009. [Google Scholar]