Abstract
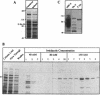
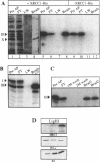
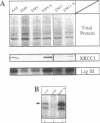
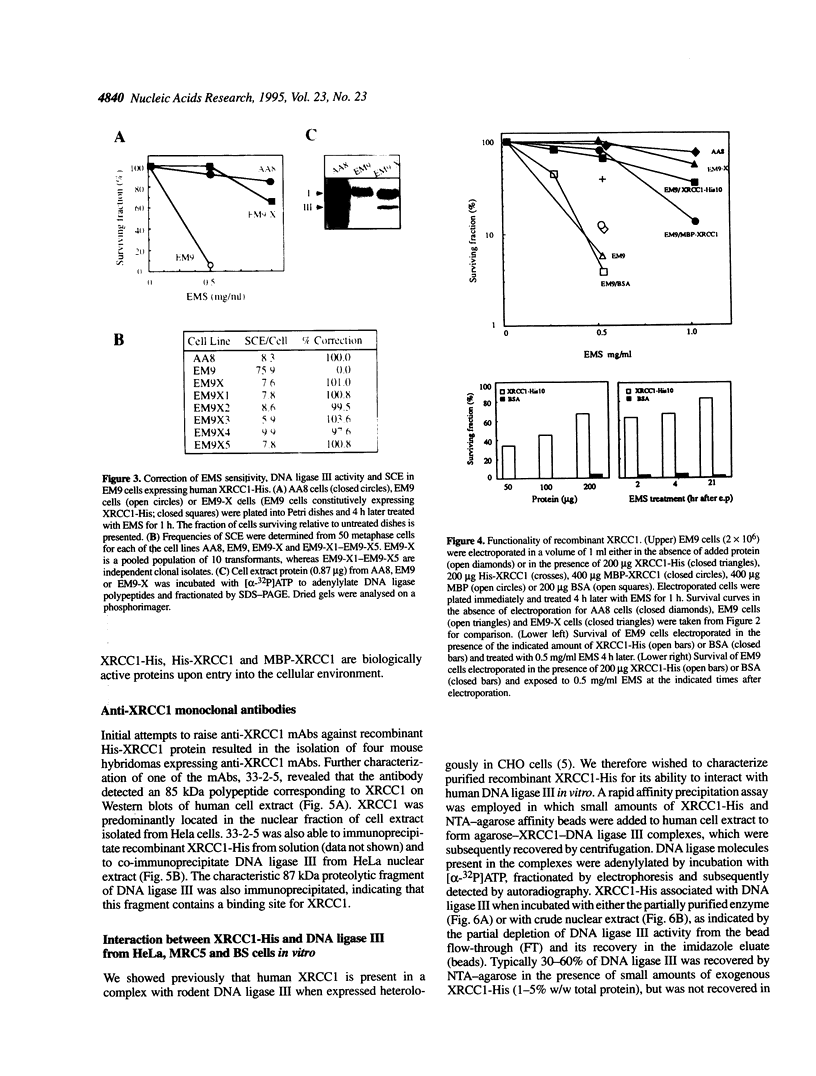
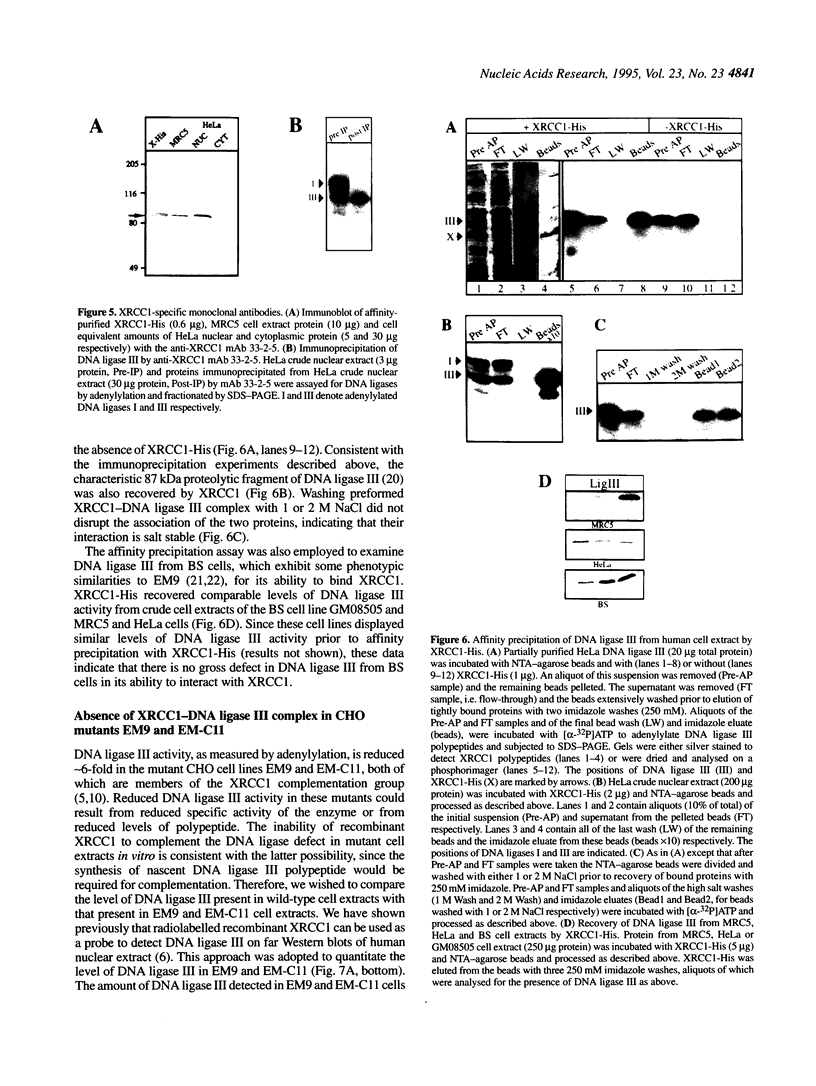
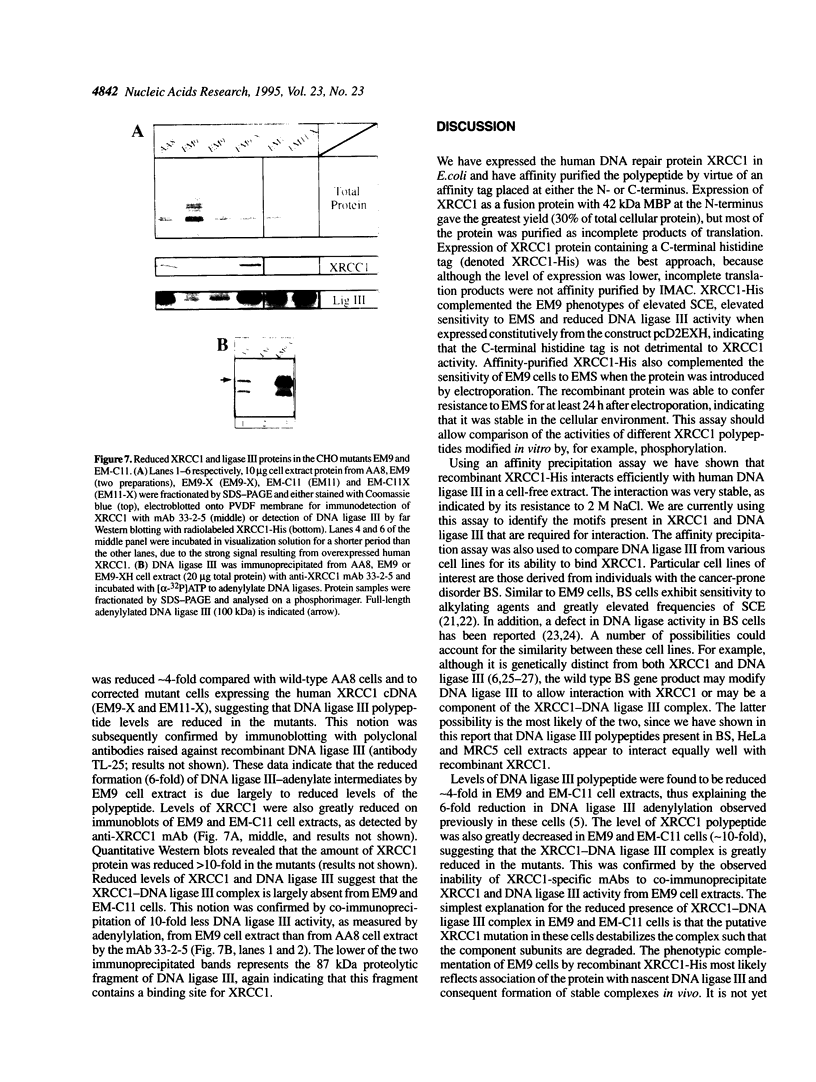
The human DNA repair protein XRCC1 was overexpressed as a histidine-tagged polypeptide (denoted XRCC1-His) in Escherichia coli and purified in milligram quantities by affinity chromatography. XRCC1-His complemented the mutant Chinese hamster ovary cell line EM9 when constitutively expressed from a plasmid or when introduced by electroporation. XRCC1-His directly interacted with human DNA ligase III in vitro to form a complex that was resistant to 2 M NaCl. XRCC1-His interacted equally well with DNA ligase III from Bloom syndrome, HeLa and MRC5 cells, indicating that Bloom syndrome DNA ligase III is normal in this respect. Detection of DNA ligase III on far Western blots by radiolabelled XRCC1-His indicated that the level of the DNA ligase polypeptide was reduced approximately 4-fold in the mutant EM9 and also in EM-C11, a second member of the XRCC1 complementation group. Decreased levels of polypeptide thus account for most of the approximately 6-fold reduced DNA ligase III activity observed previously in EM9. Immunodetection of XRCC1 on Western blots revealed that the level of this polypeptide was also decreased in EM9 and EM-C11 (> 10-fold), indicating that the XRCC1-DNA ligase III complex is much reduced in the two CHO mutants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggerstaff M., Szymkowski D. E., Wood R. D. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. EMBO J. 1993 Sep;12(9):3685–3692. doi: 10.1002/j.1460-2075.1993.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caldecott K. W., McKeown C. K., Tucker J. D., Ljungquist S., Thompson L. H. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994 Jan;14(1):68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott K. W., Tucker J. D., Thompson L. H. Construction of human XRCC1 minigenes that fully correct the CHO DNA repair mutant EM9. Nucleic Acids Res. 1992 Sep 11;20(17):4575–4579. doi: 10.1093/nar/20.17.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F., German J., Ray J. H. Altered DNA ligase I activity in Bloom's syndrome cells. Nature. 1987 Jan 22;325(6102):357–359. doi: 10.1038/325357a0. [DOI] [PubMed] [Google Scholar]
- Drummond J. T., Li G. M., Longley M. J., Modrich P. Isolation of an hMSH2-p160 heterodimer that restores DNA mismatch repair to tumor cells. Science. 1995 Jun 30;268(5219):1909–1912. doi: 10.1126/science.7604264. [DOI] [PubMed] [Google Scholar]
- Ikejima M., Noguchi S., Yamashita R., Ogura T., Sugimura T., Gill D. M., Miwa M. The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J Biol Chem. 1990 Dec 15;265(35):21907–21913. [PubMed] [Google Scholar]
- Krepinsky A. B., Heddle J. A., German J. Sensitivity of Bloom's syndrome lymphocytes to ethyl methanesulfonate. Hum Genet. 1979;50(2):151–156. doi: 10.1007/BF00390236. [DOI] [PubMed] [Google Scholar]
- Ljungquist S., Kenne K., Olsson L., Sandström M. Altered DNA ligase III activity in the CHO EM9 mutant. Mutat Res. 1994 Mar;314(2):177–186. doi: 10.1016/0921-8777(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Masutani C., Sugasawa K., Yanagisawa J., Sonoyama T., Ui M., Enomoto T., Takio K., Tanaka K., van der Spek P. J., Bootsma D. Purification and cloning of a nucleotide excision repair complex involving the xeroderma pigmentosum group C protein and a human homologue of yeast RAD23. EMBO J. 1994 Apr 15;13(8):1831–1843. doi: 10.1002/j.1460-2075.1994.tb06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel L. D., Schultz R. A. Elevated sister chromatid exchange phenotype of Bloom syndrome cells is complemented by human chromosome 15. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7968–7972. doi: 10.1073/pnas.89.17.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinete M., Vermeulen W., Bürkle A., Ménissier-de Murcia J., Küpper J. H., Hoeijmakers J. H., de Murcia G. Overproduction of the poly(ADP-ribose) polymerase DNA-binding domain blocks alkylation-induced DNA repair synthesis in mammalian cells. EMBO J. 1993 May;12(5):2109–2117. doi: 10.1002/j.1460-2075.1993.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J. H., Louie E., German J. Different mutations are responsible for the elevated sister-chromatid exchange frequencies characteristic of Bloom's syndrome and hamster EM9 cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2368–2371. doi: 10.1073/pnas.84.8.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano M. J., Carrano A. V., Thompson L. H. Assignment of a human DNA-repair gene associated with sister-chromatid exchange to chromosome 19. Mutat Res. 1986 Aug;174(4):303–308. doi: 10.1016/0165-7992(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Smider V., Rathmell W. K., Lieber M. R., Chu G. Restoration of X-ray resistance and V(D)J recombination in mutant cells by Ku cDNA. Science. 1994 Oct 14;266(5183):288–291. doi: 10.1126/science.7939667. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Gottlieb T. M., Blunt T., Priestley A., Demengeot J., Mizuta R., Lehmann A. R., Alt F. W., Jackson S. P., Jeggo P. A. Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science. 1994 Sep 2;265(5177):1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Carrano A. V., Mazrimas J. A., Mooney C. L., Minkler J. L. A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat Res. 1982 Aug;95(2-3):427–440. doi: 10.1016/0027-5107(82)90276-7. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Jones N. J., Allen S. A., Carrano A. V. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol Cell Biol. 1990 Dec;10(12):6160–6171. doi: 10.1128/mcb.10.12.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Minkler J. L., Fuscoe J. C., Henning K. A., Carrano A. V. DNA-mediated transfer of a human DNA repair gene that controls sister chromatid exchange. Mol Cell Biol. 1985 Apr;5(4):881–884. doi: 10.1128/mcb.5.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson A. E., Roberts E., Daly G., Totty N. F., Lindahl T. Three distinct DNA ligases in mammalian cells. J Biol Chem. 1991 Nov 15;266(32):21728–21735. [PubMed] [Google Scholar]
- Wei Y. F., Robins P., Carter K., Caldecott K., Pappin D. J., Yu G. L., Wang R. P., Shell B. K., Nash R. A., Schär P. Molecular cloning and expression of human cDNAs encoding a novel DNA ligase IV and DNA ligase III, an enzyme active in DNA repair and recombination. Mol Cell Biol. 1995 Jun;15(6):3206–3216. doi: 10.1128/mcb.15.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A. E., Lindahl T. DNA ligase I deficiency in Bloom's syndrome. Nature. 1987 Jan 22;325(6102):355–357. doi: 10.1038/325355a0. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van der Schans G. P., Natarajan A. T., Thompson L. H., Neuteboom I., Simons J. W. A Chinese hamster ovary cell mutant (EM-C11) with sensitivity to simple alkylating agents and a very high level of sister chromatid exchanges. Mutagenesis. 1992 Jul;7(4):265–269. doi: 10.1093/mutage/7.4.265. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Ménissier de Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994 Apr;19(4):172–176. doi: 10.1016/0968-0004(94)90280-1. [DOI] [PubMed] [Google Scholar]
- van Vuuren A. J., Appeldoorn E., Odijk H., Yasui A., Jaspers N. G., Bootsma D., Hoeijmakers J. H. Evidence for a repair enzyme complex involving ERCC1 and complementing activities of ERCC4, ERCC11 and xeroderma pigmentosum group F. EMBO J. 1993 Sep;12(9):3693–3701. doi: 10.1002/j.1460-2075.1993.tb06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]