Abstract
Objectives
The objective of this study was to evaluate the influence of acute pulmonary tuberculosis and the effect of drug therapy on markers of oxidative stress (malondialdehyde [MDA] and total antioxidant status [TAS]), C-reactive protein (CRP) and iron body status indices.
Methods
Forty patients with active pulmonary tuberculosis from the Advisory Clinic for Chest and Respiratory Diseases in Mosul City, Iraq, were included in this study, with fifty healthy age and sex matched subjects as controls. Assessment of serum concentrations of MDA, TAS, CRP, serum iron, total iron binding capacity, transferring saturation percent and ferritin were done for both patients and controls. After two months of therapy with a daily dose of isoniazid 75 mg, rifampicin 150mg, pyraziamide 400 mg, and ethambutol 275 mg, the same parameters were reassessed for the patients.
Results
After two months of therapy, there was a significant reduction in the levels of MDA, CRP, and ferritin, with a significant increase in the TAS, serum iron, and transferring saturation percentage with an insignificant effect on the total iron binding capacity in comparison with the patients’ pre-therapy values.
Conclusion
Active pulmonary tuberculosis is associated with oxidative stress; the increase in the levels of CRP indicated that pulmonary tuberculosis is associated with an inflammatory response. The initial two months therapy led to significant improvement in oxidative stress and suppression of inflammatory responses. Newly diagnosed cases of pulmonary tuberculosis often had chronic anaemia of inflammation, but this therapy resulted in a significant correction of such anaemia.
Keywords: Pulmonary tuberculosis, Oxidant/antioxidant status, C-reactive protein, Iron status
Advances in Knowledge
Newly diagnosed cases of pulmonary tuberculosis are associated with oxidative stress as indicated by enhanced lipid peroxidation (reflected in the malondialehyde level) and reduced total antioxidant status.
They are also associated with an intense inflammatory response as indicated by increase levels of acute phase protein C-reactive protein.
Newly diagnosed cases of pulmonary tuberculosis often had chronic anaemia of inflammation, although the possibility of iron deficiency anaemia cannot be ruled out.
Application to Patient Care
Initial therapy with antituberculosis drugs is very important to suppress oxidative stress, acute inflammation and chronic anaemia of inflammation in patients with active pulmonary tuberculosis.
Mycobacteria are capable of inducing reactive oxygen species production by activating both mononuclear and polymorphonuclear phagocytes that may possess antimicrobial activity. The enhanced level of free radical production, although designed to combat the invader, has the potential to damage the host; however, host tissue damage is limited by the concurrent enhancement of the antioxidant defences of the host.1 In tuberculosis (TB) patients, there are also some reports of poor antioxidants defence that may expose to oxidative host tissue damage.2,3
C-reactive protein (CRP), an acute phase protein, is synthesised by hepatocytes in response to proinflammatory cytokines in particular IL-6.4 CRP has been reported to be significantly elevated in patients with active tuberculosis (TB), normalising over time on therapy, thereby correlating with clinical response.5,6 The physiological roles of CRP are numerous, one of the critical functions being its importance in host defense. In the presence of calcium, CRP specifically binds to polysaccharides such as phosphocholine moieties present on the cell surface of many pathogenic microbes.7 CRP binding activates the classical complement pathway and initiates opsonization, phagocytosis, and lyses of invading cells. CRP can also augment cell mediated cytotoxicity i.e. amplify the immune response.7
Iron has a crucial role in TB infection. On the one hand, it influences both the innate and acquired immune response, and it is required for bacterial replication.8 Indeed, iron levels can be a determining factor during infection because overload in human and mice results in exacerbation of the disease.9 The concentration in serum of iron and the iron-binding protein, transferrin, varies markedly in different pathological and physiological conditions. Both concentrations (with transferrin being expressed as total iron-binding capacity) with or without serum ferritin are used in identifying various types of anaemia.10 Both iron deficiency and anaemia of chronic inflammation may coexist in patients with pulmonary tuberculosis. Chronic anaemia of inflammation has several features in common with iron deficiency anaemia, thus confusing the aetiological diagnosis. Low serum iron and transferrin saturation percentages, characteristic laboratory findings of iron-deficiency anaemia, are also seen in chronic inflammatory anaemia.11 It is, therefore, difficult to establish the exact mechanism of associated anaemia in pulmonary tuberculosis patients by the routine investigations for diagnosis of anaemia.
The aims of this study were to assess the influence of an initial two months of treatment on serum levels of lipid peroxidation products: malondialdehyde (MDA), total antioxidant status (TAS), CRP, and indices of iron body status (serum iron, total iron binding capacity (TIBC), transferrin saturation percentage, and serum ferritin) in patients with active pulmonary TB in comparison to controls.
Methods
Patients included in this study, which was undertaken from December 2007 to April 2008, were obtained from Advisory Clinic for Chest and Respiratory Diseases in Mosul City, Iraq. The analytical work was performed in the Department of Pharmacology, College of Medicine, at the University of Mosul. Approval was obtained from Ethical Committees of the main Health Centre in Ninevah, Mosul-City, and the College of Medicine of the University of Mosul, Iraq.
Eligibility for entry into the study included typical symptoms of pulmonary TB: fibrocavitary lung infiltrate on chest radiograph and at least one sputum specimen staining positive with Ziehl-Neelsen for acid-fast bacilli. All patients included in the study were non-smokers and had no history of drug usage (including vitamins, iron, or antibiotics) at the time of initial assessment. They also had no history of blood transfusion in the previous 6 months and no recent history of blood loss. Additional criteria for females included neither being pregnant nor lactating, nor menstruating at the time of blood collection. Patients with a history of previous treatment for tuberculosis were not included in order to exclude those with previous lung fibrosis or infection with multidrug-resistant tuberculosis. Also, patients were excluded who had co-existing lung pathology, defined as a history of previous respiratory disease or clinical or radiological evidence of lung pathology other than tuberculosis, cardiac disease or metabolic problems including chronic liver disease, renal failure or diabetes mellitus. Out of 48 patients interviewed and examined, only forty patients with active TB (29 males, 11 females, age range from 19–70 years), with a mean age of 39.95 ± 13.46 years consented to participate in this study. They were followed up for two months after receiving the initial course of anti-tuberculosis drugs described below.
Fifty apparently healthy volunteers (36 males and 14 females, age range from 18–70 years), with a mean age of 43.88 ± 14.26 years, and with no previous history of tuberculosis, were recruited as controls to establish the normal values for MDA, TAS, CRP, serum iron, total iron binding capacity, transferrin saturation percentage, and serum ferritin.
After diagnosis, patients with pulmonary tuberculosis were treated according to the standard protocol at the Advisory Clinic. Patients were given 4 tablets of Rimstar®, a fixed-dose tablet containing 4 anti-tuberculosis drugs (isoniazid (INH) 75 mg, rifampicin 150 mg, pyraziamide 400 mg, ethambutol 275 mg) to be swallowed daily before breakfast for the initial two months. Approximately 10 ml of venous blood was drawn using disposable plastic syringes from tuberculosis patients prior to initiation of anti-tuberculosis treatment, and again at the end of the two month intensive phase of treatment, and transferred immediately into disposable plain tubes. The serum was separated after centrifugation of the blood and kept frozen at −20 °C to be analysed. Blood samples from the healthy control subjects were collected and processed in the same way.
Serum MDA levels were estimated using a thiobarbituric acid (TBA) assay,12 and TAS according to the method described by Miller et al.,13 using a Randox TAS kit (Randox Laboratories Ltd., Antrim, UK). CRP was determined semi-quantitatively by slide agglutination using a CRP Latex kit (Chemelex, Spain). Serum iron was measured colorimetrically (Direct method)14 using a Ferene assay kit (Biolabo Reagents, France). TIBC was measured using a REF 92308 assay kit (Biolabo Reagents, France). Transferrin saturation percentage was calculated according to the following formula15:
Finally, serum ferritin was measured, using the ELFA technique (enzyme linked fluorescent assay), with VIDAS instrument, and a Ferritin kit (both from Bio Merieux, France).
The data of the study, subjected to statistical analysis, were expressed as mean ± standard deviation (SD). Statistical comparisons were performed using the Student’s t-test. The Fisher-Freeman-Halton test was used to compare the results of CRP of tuberculosis patients with the controls. The paired Wilcoxon Z-test was used to compare the results of CRP in tuberculosis patients before and after therapy. Linear regression analysis and Pearson correlation coefficients(r), were performed to determine the relationships between different parameters. Analysis of variance was performed for determining the relationships between CRP and other parameters. A P-value of <0.05 was considered to be statistically significant.
Results
The serum levels of MDA, TAS, iron, total iron binding capacity, transferrin saturation percent, ferritin and CRP from healthy subjects and patients with pulmonary TB before and after two months of therapy are shown in Tables 1 and 2.
Table 1.
Concentrations of malondialdehyde, total antioxidant status and iron body status indices in healthy controls and patients with pulmonary tuberculosis before and after therapy
| Parameters | Control (n = 50) | TB cases (n = 40) | |
|---|---|---|---|
| Before therapy | After therapy | ||
| MDA (μmol/l) | 1.24 ± 0.17 | 3.18 ± 0.58*** | 2.82 ± 0.60***§§§ |
| TAS (mmol/l) | 1.86 ± 0.30 | 1.06 ± 0.12*** | 1.27 ± 0.11***§§§ |
| Iron (μg/dl) | 99.12 ± 27.5 | 55.73 ± 16.78*** | 76.95 ± 28.29***§§§ |
| TIBC (μg/dl) | 397.1 ± 68.5 | 394.8 ± 148.7 | 376.7 ± 136.0 |
| Transferrin saturation % | 25.58 ± 8.4 | 15.89 ± 7.0*** | 21.37 ± 8.7*§§§ |
| Ferritin (ng/ ml) | 183.1 ± 65.6 | 441.76 ± 319.4*** | 185.64 ± 129.1 §§§ |
Note: Results are expressed as mean ± SD
Legend: MDA = malondialdehyde;
Significant difference from control at P <0.001; TAS = total antioxidant status;
Significant difference from before at P <0.001; TIBC = serum iron, total iron binding capacity;
Significant difference from control at P <0.05.
Table 2.
Comparison of C-reactive protein (CRP) concentrations between healthy controls and patients with pulmonary tuberculosis before and after therapy
| CRP mg/l | Control (n = 50) | Before drug (n = 40) | After drug (n = 40) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| <6 | 39 | 78.0 | 0 | 0.0 | 12 | 30.0 |
| 6 | 9 | 18.0 | 6 | 15.0 | 19 | 47.5 |
| 12 | 2 | 4.0 | 11 | 27.5 | 7 | 17.5 |
| 24 | 0 | 0.0 | 10 | 25.0 | 2 | 5.0 |
| 48 | 0 | 0.0 | 4 | 10.0 | 0 | 0.0 |
| 96 | 0 | 0.0 | 9 | 22.5 | 0 | 0.0 |
| P-value versus control | <0.001 | <0.001 | ||||
The serum levels of MDA were significantly higher (P <0.001) and TAS were significantly lower (P <0.001) in patients with pulmonary TB before starting therapy in comparison with the control group. After two months of therapy, there was a highly significant reduction (P <0.001) in the serum MDA with a highly significant increase (P <0.001) in the serum TAS from initial pre-treatment levels.
Both serum iron and transferrin saturation percentages were significantly lower (P <0.001), and serum ferritin concentration was significantly higher (P <0.001) in patients with pulmonary TB before starting therapy in comparison with the control subjects. However, TIBC showed insignificant differences compared to the control values. After two months therapy, there was highly significant elevation in the levels of serum iron (P <0.001) and transferrin saturation percentage (P <0.001) and a highly significant reduction in ferritin levels (P <0.001); however, TIBC showed insignificant differences after therapy.
As shown in Table 2, the serum levels of CRP were significantly higher (P <0.001) in patients with pulmonary TB before starting therapy in comparison with the control group and, after two months therapy, there was a highly significant reduction (P <0.001) in CRP levels.
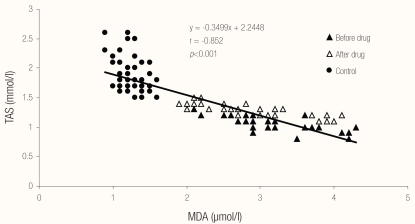
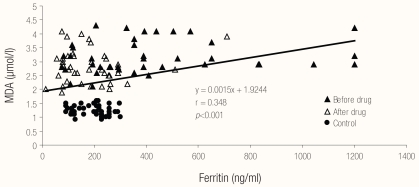
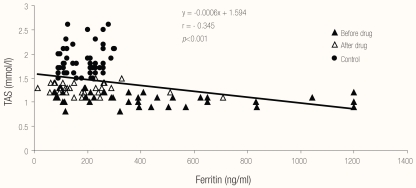
Regarding correlations between different biochemical parameters, Figures 1, 2 and 3 show the relationships between MDA, TAS and serum ferritin in patients with pulmonary TB (before and after therapy) and in the control group. There was a highly significant negative correlation (r = −0.852; P <0.001) between serum MDA and TAS, a highly significant positive correlation (r = 0.348; P <0.001) between serum ferritin and MDA and a highly significant negative correlation (r = −0.345; P <0.001) between serum ferritin and TAS in patients with pulmonary TB (before and after therapy) and in the control group.
Figure 1.
Relationship between MDA and TAS in the studied cases and control.
Legend: MDA = malondialdehyde; TAS = total antioxidant status
Figure 2.
Relationship between ferritin and MDA in the studied cases and control.
Legend: MDA = malondialdehyde
Figure 3.
Relationship between ferritin and total antioxidant status in the studied cases and control
Legend: TAS = total antioxidant status
Tables 3, 4 and 5 show the relationships between CRP, MDA, TAS and serum ferritin in patients with pulmonary TB (before and after therapy) and in the control group. There was a highly significant correlation between CRP and MDA, between CRP and TAS and between CRP and serum ferritin in patients with pulmonary TB (before and after therapy) and in the control group.
Table 3.
Relationship between C-reactive protein (CRP) and malondialdehyde (MDA) in the studied cases and control group
| CRP | n | MDA, Mean ±SD | P value |
|---|---|---|---|
| <6 | 51 | 1.56 ±0.70 | |
| 6 | 34 | 2.51 ±0.88 | |
| 12 | 20 | 2.72 ±0.78 | <0.001 |
| 24 | 12 | 3.30 ±0.46 | |
| 48 | 4 | 3.00 ±0.12 | |
| 96 | 9 | 3.44 ±0.65 |
Table 4.
Relationship between C-reactive protein (CRP) and total antioxidant status (TAS) in the studied cases and control.
| CRP | n | TAS, Mean ±SD | P value |
|---|---|---|---|
| <6 | 51 | 1.74 ±0.37 | |
| 6 | 34 | 1.36 ±0.33 | |
| 12 | 20 | 1.25 ±0.27 | <0.001 |
| 24 | 12 | 1.10 ±0.10 | |
| 48 | 4 | 1.00 ±0.08 | |
| 96 | 9 | 1.01 ±0.11 |
Table 5.
Relationship between C-reactive protein (CRP) and ferritin in the studied cases and control group
| CRP | N | Ferritin, Mean ±SD | P value |
|---|---|---|---|
| <6 | 51 | 142.18 ±88.00 | |
| 6 | 34 | 94.37 ±97.43 | |
| 12 | 20 | 203.24 ±189.68 | <0.001 |
| 24 | 12 | 419.09 ±425.85 | |
| 48 | 4 | 561.07 ±323.15 | |
| 96 | 9 | 653.39 ±281.07 |
Discussion
In the present study, it was observed that the serum MDA levels were significantly high and the antioxidant status was significantly low in newly diagnosed patients with pulmonary TB as compared to healthy controls. It was also observed that the administration of antimicrobial agents to such patients for a period of 2 months was associated with a significant reduction in MDA concentrations and a significant increase in total antioxidant status. Although considerable improvement was noted in these parameters, still there were significant differences from the control values. Our finding of enhanced lipid peroxidation product and reduced TAS provides evidence for the presence of oxidative stress in newly diagnosed patients with active pulmonary tuberculosis. Furthermore, the decrease in levels of MDA, with a concomitant increase in TAS after two month of therapy seen in our study, may indicate a good response to therapy.
Plit et al. found that even after 6 months of successful chemotherapy, pulmonary TB is still associated with increased levels of circulating lipid peroxides and low plasma concentrations of vitamin E.3 These findings are in line with our results of significant differences in MDA concentrations and TAS between patients after two months of therapy and the control group which indicate that oxidative stress were ongoing even after two months of chemotherapy.
In an attempt to kill Mycobacteria, host cells (namely macrophages, neutrophils and monocytes) generate huge amounts of reactive oxygen species (ROS).16 One of the manifestations of these reactive species is lipid peroxidation. These high levels of reactive species are often cytotoxic and may cause host tissue damage such as lung fibrosis and lung dysfunction in patients with pulmonary TB if antioxidant defences of the host are deficient.17 Malnutrition is more common in patients with pulmonary TB than in the healthy subjects, and could possibly explain the low levels of antioxidant potential. Furthermore, patients with tuberculosis are unable to produce a sufficient amount of antioxidant to cope with their increased oxidative stress.18
In the present study, TAS was measured instead of determining individual antioxidant enzymes and molecules. Low levels of TAS were observed in pulmonary TB. This might be due to malnutrition or exhaustion in an attempt to neutralise the heavy load of free radical in these patients. This study showed a highly significant negative correlation between serum MDA levels and TAS in patients with pulmonary TB (before and after therapy) and in the control group. This suggests increased utilisation by reactive oxygen species as an important contributing factor to the lower concentrations of antioxidants in tuberculosis patients.18,19 In fact, the combination of malnutrition (leading to decreased “supplementation” of antioxidants) and enhanced ROS generation (leading to increased utilisation of these compounds) may represent a pathogenic loop that results in markedly enhanced oxidative stress during tuberculosis infection.18,19
This study also showed that the CRP concentrations in newly diagnosed patients were significantly higher than those of healthy controls and that the administration of anti-tuberculosis drug therapy for an initial two months was associated with a significant reduction in serum levels. CRP has been reported to be significantly elevated in patients with active TB, normalising over weeks on therapy, thereby correlating with clinical response.5,6 Higher CRP concentrations have also been associated with more severe tuberculosis TB and poor prognosis.20 This observation is supported by reports that patients with more severe lung dysfunction, as evidenced by lung cavitation, had significantly higher CRP levels than patients without cavitation.21 Mycobacterium tuberculosis and its components have been shown to stimulate mononuclear phagocytes in vitro to release IL-6, which are regarded as inflammatory mediators. IL-6 is known to induce hepatic acute phase reactants (including CRP). Several clinical and laboratory findings support the significant relation between IL-6 and CRP.22 Bekker et al. suggested that the decrease in CRP concentration in patients treated for pulmonary tuberculosis is paralleled by a similar reduction in plasma cytokines such as IL-6, the principal cytokine that induces CRP synthesis, and that the fall in serum CRP concentration during treatment of pulmonary tuberculosis might be correlated with the resolution of the systemic inflammatory process.23
In the present study, the serum iron levels and transferrin saturation percentage were observed to be significantly lower in patients with active pulmonary tuberculosis before starting therapy in comparison with the controls. This study also demonstrated that after therapy the serum levels of both parameters showed significant elevation. Although considerable improvements were reported in these parameters, still there were highly significant differences in serum iron levels and mildly significant differences in transferrin saturation percentages after two months of therapy in comparison to control values. Kassu et al. found that the concentrations of iron were significantly lower in the serum of patients with pulmonary TB in comparison with the control group, with no significant differences at the end of the intensive phase of chemotherapy.24 Although these are in agreement with our findings of lower pre-treatment serum iron levels in patients in comparison to the healthy controls, they disagree with our finding of a significant increase in serum iron levels after therapy.
The hypoferraemia in TB patients may be induced by the shift of iron from a transferrin-bound available state to a ferritin-incorporated storage state.24 The condition may have evolved as a cytokine-mediated defence against microbial pathogens, effectively withholding iron from microbes, which incidentally also deprives erythroid precursors of their iron supply.25 Gobin and Horwitz suggested that Mycobacteria acquire their iron from the host’s own transferrin/ferritin iron binding proteins, and they achieve this by releasing a type of iron-capturing siderophore called an exochelin. This, in turn, transfers and donates the iron back to the mycobactins which exist in the cell walls of the Mycobacteria.26 Baynes et al. suggested that patients with pulmonary tuberculosis are often anaemic due to restriction of available iron by the host.5 This restriction occurs as a major component of the host natural immunity and is characterised by the induction of an acute phase response that limits the amount of circulating iron, primarily accomplished by iron sequestration. The sequestration of iron by the host also limits iron-induced cellular damage to the host itself through binding by serum proteins such as transferrin, ferritin and lactoferrin. Overall, an attempt is made by the host to induce an iron deficiency upon the pathogen.27 The low serum iron levels seen in this study may also be attributed to low levels of dietary iron intake, low socioeconomic status and blood loss by haemoptysis.
Values of TIBC are usually high in iron deficiency anaemia and low in inflammatory anaemia.28 The TIBC reflects transferrin, the protein to which virtually all iron in the blood is bound.29 In this study, we assessed TIBC as an indirect measurement of serum transferrin concentrations, and found no significant differences in TIBC between patients with pulmonary TB before and at the end of intensive phase of therapy and control subjects. Al-Omar and Oluboyede reported low initial (pre-treatment) TIBC levels in patients with pulmonary tuberculosis.30 Also, Devi et al. reported low TIBC concentrations in patients with pulmonary tuberculosis, and they attributed their findings to an acute phase response since transferrin is a negative acute phase protein and serum levels may be decreased in inflammatory conditions.11
Our results also showed a significantly higher serum ferritin concentration in tuberculosis patients before starting therapy in comparison with healthy controls. There was also a significant reduction in the serum levels at the end of the intensive phase of chemotherapy. Ferritin concentration in blood is considered to be a specific indicator of body iron stores;31 however, the concentration can rise following an inflammatory response, irrespective of iron status. Some reports indicated that ferritin synthesis is stimulated in pulmonary tuberculosis as a consequence of the inflammatory process.32 Al-Omar and Oluboyede attributed the rise in serum ferritin levels in their study to two factors: first, increased production by monocytes and macrophages, monocytosis having been observed in pulmonary TB; second, serum ferritin is an acute phase protein that increases in inflammatory conditions.30 In favour of this conclusion, we found a positive correlation between serum ferritin and CRP. The raised ferritin levels seen in our patients point more towards the possibility of anaemia of inflammation, although an association with iron deficiency anaemia cannot be completely ruled out. In patients with anaemia of chronic inflammation, there appear to be a defect in the freeing of iron from macrophages, the loading of iron onto plasma transferrin, or both. Reticuloendothelial iron is plentiful in bone marrow macrophages, but this iron is not available to erythroid precursors.33
This study showed that after two months of therapy there were significant corrections in serum iron levels, transferrin saturation percent and serum ferritin despite the fact that our patients received no iron supplementation. These observations give rise to the possibility that the anaemia in our patients is of chronic inflammation rather than iron deficiency anaemia; however, the possibility of iron deficiency anaemia cannot be ruled out, since the only effective treatment for anaemia of chronic inflammation is correction of the underlying disorder.
Both CRP and ferritin have been reported to reflect the extent of oxidative stress and inflammation in individual patients and may be useful markers of disease activity and mortality risk.34 In the present study, we have investigated the relationship between markers of oxidative stress and markers of inflammation in TB patients and in healthy controls. Subjects with higher serum ferritin and CRP concentrations, indicative of greater inflammatory response in such individuals, also had higher serum MDA levels and lower TAS than those with lower serum ferritin and CRP concentrations. This picture was observed in the figures which showed that both serum CRP and ferritin levels were positively correlated with lipid peroxidation product and negatively correlated with TAS.
Nguyen-Khoa et al. conducted a study to investigate the role of inflammation in exacerbating oxidative stress in haemodialysis patients, and they found that plasma CRP levels were correlated positively with plasma levels of lipid peroxidation products, and negatively with plasma α-tocopherol and albumin levels.35 They suggested that inflammatory processes induce an increase production of ROS which lead to the consumption of fat-soluble antioxidants and subsequently to the generation of lipid peroxidation products. They also found that the increase in plasma ferritin levels observed in haemodialysis patients was not associated with an increase in the level of lipid and protein oxidative stress markers. Ferritin expression is modulated by a variety of conditions associated with oxidative stress that act either directly on gene expression or indirectly via the modification of iron responsive elements activity. Superoxide anions produced during oxidative stress can mobilise iron from ferritin and this may augment the intensity of oxidative stress by catalysing the production of highly toxic hydroxyl radicals by Fenton and Haber-Weiss reactions.36
Conclusion
This study concluded that pulmonary tuberculosis is associated with oxidative stress as indicated by enhanced lipid peroxidation products (MDA) and reduced total antioxidant status and that the administration of anti-tuberculosis treatments for the initial two months (intensive phase) reduces the serum MDA level, elevates TAS and decreases oxidative stress. The increased levels of acute phase proteins (CRP and serum ferritin) indicate that pulmonary TB is associated with an inflammatory response and that the administration of treatment for the initial two months brings about suppression of the inflammatory response with significant reduction in these proteins. This study also concluded that newly diagnosed patients with pulmonary TB often had anaemia of chronic inflammation as shown by disturbances in iron body status indices, although the possibility of iron deficiency anaemia cannot be ruled out, and that therapy resulted in a significant correction of such anaemia.
Footnotes
CONFLICT OF INTEREST
The authors reported no conflict of interest.
References
- 1.Madebo T. Thesis. Centre for International Health, University of Bergen; Norway: 2003. Clinical and operational challenges in the control of tuberculosis in south Ethiopia. [Google Scholar]
- 2.Jack CL, Jackson MJ, Hind CR. Circulating markers of free radical activity in patients with pulmonary tuberculosis. Tuber Lung Dis. 1994;75:132–7. doi: 10.1016/0962-8479(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 3.Plit ML, Theron AJ, Fickl H, Van Rensburg CE, Pendel S, Anderson R. Influence of antimicrobial chemotherapy and smoking status on the plasma concentrations of vitamin C, vitamin E, beta carotene, acute phase reactants, iron and lipid peroxides in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 1998;2:590–6. [PubMed] [Google Scholar]
- 4.Ribeiro MA. Levels of C-reactive protein in serum samples from healthy children and adults in São Paulo, Brazil. Braz J Med Biol Res. 1997;30:1055–9. doi: 10.1590/s0100-879x1997000900002. [DOI] [PubMed] [Google Scholar]
- 5.Baynes RD, Flex H, Bothwell TH, Bezwoda WR, Macphail AP, Alkinson P, et al. Haematological and iron related measurements in active pulmonary tuberculosis. Scand J Haematol. 1986;36:280–7. doi: 10.1111/j.1600-0609.1986.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 6.Peresi E. Cytokines and acute-phase serum proteins as markers of inflammatory regression during pulmonary tuberculosis treatment. J Venom Anim Toxins Incl Trop Dis. 2008;14:190. doi: 10.1590/s1806-37132008001100009. [DOI] [PubMed] [Google Scholar]
- 7.Reeves G. Abnormal laboratory results C-reactive protein. Aust Prescr. 2007;30:74–6. [Google Scholar]
- 8.Rodriguez GM. Control of iron metabolism in Mycobacterium tuberculosis. TRENDS Microbiol. 2006;14:320–7. doi: 10.1016/j.tim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Gangaidzo IT, Moyo VM, Mvundura E, Aggrey G, Murphree NL, Khumalo H. Association of pulmonary tuberculosis with increased dietary iron. J Infect Dis. 2001;184:936–9. doi: 10.1086/323203. [DOI] [PubMed] [Google Scholar]
- 10.Peter F, Wang S. Serum iron and total iron-binding capacity compared with serum ferritin in assessment of iron deficiency. Clin Chem. 1981;27:276–9. [PubMed] [Google Scholar]
- 11.Devi U, Rao CM, Srivastava VK, Rath PK, Das BS. Effect of iron supplementation on mild to moderate anemia in pulmonary tuberculosis. Brit J Nutr. 2003;90:541–50. doi: 10.1079/bjn2003936. [DOI] [PubMed] [Google Scholar]
- 12.Buege JA, Aust SD. Thiobarbuturic acid assay. Methods Enzymol. 1978;52:306–7. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 13.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 14.Douglas JH, Reid GR, Smith FE, Thompson SL. Ferene: a new spectrophotometric reagent for iron. Can J Chem. 1984;62:721–4. [Google Scholar]
- 15.Al-Buhairan AM, Oluboyede OA. Determination of serum iron, total iron binding capacity and serum ferritin in healthy Saudi adults. Ann Saudi Med. 2001;21:100–3. doi: 10.5144/0256-4947.2001.100. [DOI] [PubMed] [Google Scholar]
- 16.Kaur K, Kishan J, Bedi GK, Ahi RS. Oxidants stress and antioxidants in pulmonary tuberculosis. Chest. 2005;128:397s. [Google Scholar]
- 17.Kwiatkowska S, Piasecka G, Zieba M, Piotrowski W, Nowak D. Increased serum concentrations of conjugated dienes and malondialdehyde in patients with pulmonary tuberculosis. Respir Med. 1999;93:272–6. doi: 10.1016/s0954-6111(99)90024-0. [DOI] [PubMed] [Google Scholar]
- 18.Reddy YN, Murthy SV, Krishna DR, Prabhakar MC. Role of free radicals and antioxidants in tuberculosis patients. Indian J Tuberc. 2004;51:213–8. [Google Scholar]
- 19.Madebo T, Lindtj⊘rn B, Aukrust P, Berge RK. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. Am J Clin Nutr. 2003;78:117–22. doi: 10.1093/ajcn/78.1.117. [DOI] [PubMed] [Google Scholar]
- 20.Scott GM, Murphy PG, Gemidjioglu ME. Predicting deterioration of treated tuberculosis by corticosteroid reserve and C-reactive protein. J Infect. 1990;21:61–9. doi: 10.1016/0163-4453(90)90669-y. [DOI] [PubMed] [Google Scholar]
- 21.De Beer FC, Nel AE, Gie RP, Donald PR, Strachan AF. Serum amyloid A protein and C-reactive protein levels in pulmonary tuberculosis: relationship to amyloidosis. Thorax. 1984;39:196–200. doi: 10.1136/thx.39.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ünsal E, Aksaray S, Köksal D, Sipit T. Potential role of interleukin-6 in reactive thrombocytosis and acute phase response in pulmonary tuberculosis. Postgrad Med J. 2005;81:604–7. doi: 10.1136/pgmj.2004.030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekker LG, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178:580–4. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]
- 24.Kassu A, Yabutani T, Mahmud ZH, Mohammad A, Nguyen N, Huong BT, et al. Alteration in serum levels of trace elements in tuberculosis and HIV infections. Europ J Clin Nutr. 2006;60:580–6. doi: 10.1038/sj.ejcn.1602352. [DOI] [PubMed] [Google Scholar]
- 25.Jurado RI. Iron, infection, and anemia of inflammation. Clin Infect Dis. 1997;25:888–95. doi: 10.1086/515549. [DOI] [PubMed] [Google Scholar]
- 26.Gobin J, Horwitz MA. Exochelins of Mycobacterium tuberculosis remove iron from human iron binding proteins and donate iron to mycobactrins in the M. tuberculosis cell wall. J Exp Med. 1996;183:1527–32. doi: 10.1084/jem.183.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermid JM, Prentice AM. Iron and infection: effects of host iron status and the iron-regulatory genes haptoglobin and NRAMPI (SLCIIAI) on hostpathogen interactions in tuberculosis and HIV. Clin Sci. 2006;110:503–24. doi: 10.1042/CS20050273. [DOI] [PubMed] [Google Scholar]
- 28.Punnonen K, Irjala K, Rajamaki A. Iron deficiency anemia is associated with high concentrations of transferrin receptors in serum. Clin Chem. 1994;40:774–6. [PubMed] [Google Scholar]
- 29.Wish JB. Assessing iron status: Beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1:S4–8. doi: 10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 30.Al-Omar IA, Oluboyede AO. Serum ferritin and other iron parameters in patients with pulmonary tuberculosis. Saudi Med J. 2002;23:244–6. [PubMed] [Google Scholar]
- 31.Lammi-Keefe CJ, Lickteig ES, Ahluwalia N, Haley NR. Day-to-day variation in iron status indexes is similar for most measures in elderly women with and without rheumatoid arthritis. J Am Diet Assoc. 1996;96:247–51. doi: 10.1016/S0002-8223(96)00075-2. [DOI] [PubMed] [Google Scholar]
- 32.Wessels G, Schaaf HS, Beyers N, Gie RP, Nel E, Donald PR. Haematological abnormalities in children with tuberculosis. J Trop Pediatr. 1999;45:307–10. doi: 10.1093/tropej/45.5.307. [DOI] [PubMed] [Google Scholar]
- 33.Andrews NC. Disorders of iron metabolism. N Engl J Med. 2000;34:364. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 34.Huang HH, Yan HC, Han CL, Yu FC, Kao WY, Chen WT. Association of in vitro oxidative stress, serum ferritin concentration and C-reactive protein in febrile emergency room patients. Clin Invest Med. 2005;28:48–54. [PubMed] [Google Scholar]
- 35.Nguyen-Khoa T, Massy ZA, De Bandt JP. Oxidative stress and haemodialysis: role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant. 2001;16:335–40. doi: 10.1093/ndt/16.2.335. [DOI] [PubMed] [Google Scholar]
- 36.Rehma A. Thesis. Department of Biochemistry, University of Tartu; Estonia: 2004. Assessment of nonhaem ferrous iron and glutathione redox ratio as markers of pathogenecity of oxidative stress in different clinical groups. [Google Scholar]