Abstract
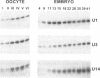
U14 is a member of the rapidly growing family of intronic small nucleolar RNAs (snoRNAs) that are involved in pre-rRNA processing and ribosome biogenesis. These snoRNA species are encoded within introns of eukaryotic protein coding genes and are synthesized via an intron processing pathway. Characterization of Xenopus laevis U14 snoRNA genes has revealed that in addition to the anticipated location of U14 within introns of the amphibian hsc70 gene (introns 4, 5 and 7), additional intronic U14 snoRNAs are also found in the ribosomal protein S13 gene (introns 3 and 4). U14 is thus far a unique intronic snoRNA in that it is encoded within two different parent genes of a single organism. Northern blot analysis revealed that U14 snoRNAs accumulate during early oocyte development and are rapidly expressed after the mid-blastula transition of developing embryos. Microinjection of hsc70 pre-mRNAs into developing oocytes demonstrated that oocytes as early as stages II and III are capable of processing U14 snoRNA from the pre-mRNA precursor. The ability of immature oocytes to process intronic snoRNAs is consistent with the observed accumulation of U14 during oocyte maturation and the developmentally regulated synthesis of rRNA during oogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Ahuja R., Venner T. J., Gupta R. S. Identification of a protein altered in mutants resistant to microtubule inhibitors as a member of the major heat shock protein (hsp70) family. Mol Cell Biol. 1990 Oct;10(10):5160–5165. doi: 10.1128/mcb.10.10.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN D. D., LITTNA E. RNA SYNTHESIS DURING THE DEVELOPMENT OF XENOPUS LAEVIS, THE SOUTH AFRICAN CLAWED TOAD. J Mol Biol. 1964 May;8:669–687. doi: 10.1016/s0022-2836(64)80116-9. [DOI] [PubMed] [Google Scholar]
- Barbhaiya H., Leverette R. D., Liu J., Maxwell E. S. Processing of U14 small nucleolar RNA from three different introns of the mouse 70-kDa-cognate-heat-shock-protein pre-messenger RNA. Eur J Biochem. 1994 Dec 15;226(3):765–771. doi: 10.1111/j.1432-1033.1994.t01-1-00765.x. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Caizergues-Ferrer M., Mathieu C., Mariottini P., Amalric F., Amaldi F. Developmental expression of fibrillarin and U3 snRNA in Xenopus laevis. Development. 1991 May;112(1):317–326. doi: 10.1242/dev.112.1.317. [DOI] [PubMed] [Google Scholar]
- Chadéneau C., LeMoullac B., Denis M. G. Cloning and analysis of the human S13 ribosomal protein cDNA. Nucleic Acids Res. 1993 Jun 25;21(12):2945–2945. doi: 10.1093/nar/21.12.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B. Nucleotide sequence of the cDNA of a bovine 70 kilodalton heat shock cognate protein. Nucleic Acids Res. 1990 Sep 25;18(18):5569–5569. doi: 10.1093/nar/18.18.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dworniczak B., Mirault M. E. Structure and expression of a human gene coding for a 71 kd heat shock 'cognate' protein. Nucleic Acids Res. 1987 Jul 10;15(13):5181–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger D. L., Pieniazek N. J., Lammie P. J. Nucleotide sequence of Brugia pahangi 17.4 kD protein. Nucleic Acids Res. 1989 Dec 11;17(23):10121–10121. doi: 10.1093/nar/17.23.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragapane P., Prislei S., Michienzi A., Caffarelli E., Bozzoni I. A novel small nucleolar RNA (U16) is encoded inside a ribosomal protein intron and originates by processing of the pre-mRNA. EMBO J. 1993 Jul;12(7):2921–2928. doi: 10.1002/j.1460-2075.1993.tb05954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L. B., Dworniczak B. P., Bautz E. K. Developmental regulation of a constitutively expressed mouse mRNA encoding a 72-kDa heat shock-like protein. Dev Biol. 1988 Jan;125(1):200–207. doi: 10.1016/0012-1606(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Ares M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5' external transcribed spacer of yeast pre-ribosomal RNA and impairs formation of 18S ribosomal RNA. EMBO J. 1991 Dec;10(13):4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanin P., Gigot C., Philipps G. cDNA nucleotide sequence and expression of a maize cytoplasmic ribosomal protein S13 gene. Plant Mol Biol. 1993 Feb;21(4):701–704. doi: 10.1007/BF00014553. [DOI] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- Kiss T., Filipowicz W. Small nucleolar RNAs encoded by introns of the human cell cycle regulatory gene RCC1. EMBO J. 1993 Jul;12(7):2913–2920. doi: 10.1002/j.1460-2075.1993.tb05953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. J., Sanders J. F., Waugh R., Shaw P., Brown J. W. Molecular characterisation of plant U14 small nucleolar RNA genes: closely linked genes are transcribed as polycistronic U14 transcripts. Nucleic Acids Res. 1994 Dec 11;22(24):5196–5203. doi: 10.1093/nar/22.24.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverette R. D., Andrews M. T., Maxwell E. S. Mouse U14 snRNA is a processed intron of the cognate hsc70 heat shock pre-messenger RNA. Cell. 1992 Dec 24;71(7):1215–1221. doi: 10.1016/s0092-8674(05)80069-8. [DOI] [PubMed] [Google Scholar]
- Li H. D., Zagorski J., Fournier M. J. Depletion of U14 small nuclear RNA (snR128) disrupts production of 18S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Mar;10(3):1145–1152. doi: 10.1128/mcb.10.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Maxwell E. S. Mouse U14 snRNA is encoded in an intron of the mouse cognate hsc70 heat shock gene. Nucleic Acids Res. 1990 Nov 25;18(22):6565–6571. doi: 10.1093/nar/18.22.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J., Simanis V. Cloning of the gene for ribosomal protein S13 from the fission yeast Schizosaccharomyces pombe. Nucleic Acids Res. 1992 Aug 11;20(15):4094–4094. doi: 10.1093/nar/20.15.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell E. S., Fournier M. J. The small nucleolar RNAs. Annu Rev Biochem. 1995;64:897–934. doi: 10.1146/annurev.bi.64.070195.004341. [DOI] [PubMed] [Google Scholar]
- Morrissey J. P., Tollervey D. Yeast snR30 is a small nucleolar RNA required for 18S rRNA synthesis. Mol Cell Biol. 1993 Apr;13(4):2469–2477. doi: 10.1128/mcb.13.4.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag M. K., Thai T. T., Ruff E. A., Selvamurugan N., Kunnimalaiyaan M., Eliceiri G. L. Genes for E1, E2, and E3 small nucleolar RNAs. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9001–9005. doi: 10.1073/pnas.90.19.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoloso M., Caizergues-Ferrer M., Michot B., Azum M. C., Bachellerie J. P. U20, a novel small nucleolar RNA, is encoded in an intron of the nucleolin gene in mammals. Mol Cell Biol. 1994 Sep;14(9):5766–5776. doi: 10.1128/mcb.14.9.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peculis B. A., Steitz J. A. Disruption of U8 nucleolar snRNA inhibits 5.8S and 28S rRNA processing in the Xenopus oocyte. Cell. 1993 Jun 18;73(6):1233–1245. doi: 10.1016/0092-8674(93)90651-6. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L., Crosio C., Campioni N., Loreni F., Pierandrei-Amaldi P. Different forms of U15 snoRNA are encoded in the introns of the ribosomal protein S1 gene of Xenopus laevis. Nucleic Acids Res. 1994 Nov 11;22(22):4607–4613. doi: 10.1093/nar/22.22.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Campioni N., Beccari E., Bozzoni I., Amaldi F. Expression of ribosomal-protein genes in Xenopus laevis development. Cell. 1982 Aug;30(1):163–171. doi: 10.1016/0092-8674(82)90022-8. [DOI] [PubMed] [Google Scholar]
- Rubin D. M., Mehta A. D., Zhu J., Shoham S., Chen X., Wells Q. R., Palter K. B. Genomic structure and sequence analysis of Drosophila melanogaster HSC70 genes. Gene. 1993 Jun 30;128(2):155–163. doi: 10.1016/0378-1119(93)90558-k. [DOI] [PubMed] [Google Scholar]
- Savino R., Gerbi S. A. In vivo disruption of Xenopus U3 snRNA affects ribosomal RNA processing. EMBO J. 1990 Jul;9(7):2299–2308. doi: 10.1002/j.1460-2075.1990.tb07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanab G. M., Maxwell E. S. Proposed secondary structure of eukaryotic U14 snRNA. Nucleic Acids Res. 1991 Sep 25;19(18):4891–4894. doi: 10.1093/nar/19.18.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B. Novel intron-encoded small nucleolar RNAs. Cell. 1993 Nov 5;75(3):403–405. doi: 10.1016/0092-8674(93)90374-y. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Olvera J., Wool I. G. The primary structure of rat ribosomal protein S13. Biochem Biophys Res Commun. 1990 Sep 14;171(2):519–524. doi: 10.1016/0006-291x(90)91176-s. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Misumi Y., Shiokawa K., Yamana K. Determination of the rate of rRNA synthesis in Xenopus laevis triploid embryos produced by low-temperature treatment. J Exp Zool. 1983 Mar;225(3):489–495. doi: 10.1002/jez.1402250317. [DOI] [PubMed] [Google Scholar]
- Thiébaud C. H., Fischberg M. DNA content in the genus Xenopus. Chromosoma. 1977 Feb 3;59(3):253–257. doi: 10.1007/BF00292781. [DOI] [PubMed] [Google Scholar]
- Tollervey D., Guthrie C. Deletion of a yeast small nuclear RNA gene impairs growth. EMBO J. 1985 Dec 30;4(13B):3873–3878. doi: 10.1002/j.1460-2075.1985.tb04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh-Rohlik Q., Maxwell E. S. Homologous genes for mouse 4.5S hybRNA are found in all eukaryotes and their low molecular weight RNA transcripts intermolecularly hybridize with eukaryotic 18S ribosomal RNAs. Nucleic Acids Res. 1988 Jul 11;16(13):6041–6056. doi: 10.1093/nar/16.13.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Steitz J. A. A small nucleolar RNA is processed from an intron of the human gene encoding ribosomal protein S3. Genes Dev. 1993 Jul;7(7A):1176–1190. doi: 10.1101/gad.7.7a.1176. [DOI] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Steitz J. A. Requirement for intron-encoded U22 small nucleolar RNA in 18S ribosomal RNA maturation. Science. 1994 Dec 2;266(5190):1558–1561. doi: 10.1126/science.7985025. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr The structure and biogenesis of yeast ribosomes. Adv Genet. 1991;29:63–118. doi: 10.1016/s0065-2660(08)60107-8. [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Wisniewski J., Shworak N. W., Schieman S., Misra S., Gedamu L. Molecular cloning and characterization of a constitutively expressed heat-shock-cognate hsc71 gene from rainbow trout. Eur J Biochem. 1992 Mar 1;204(2):893–900. doi: 10.1111/j.1432-1033.1992.tb16709.x. [DOI] [PubMed] [Google Scholar]