Abstract
Malignant melanoma is one of the most rapidly increasing cancers and, when it occurs during pregnancy, it can frequently metastasise to the placenta and the foetus. Earlier reports suggested a rapid progress of the disease during pregnancy with a poor prognosis; however, recent controlled studies found that stage for stage, the prognosis of melanoma during pregnancy is similar to that in a non-pregnant state. Early diagnosis and prompt treatment can avoid a tragic outcome.
Keywords: Malignant melanoma, Pregnancy, Metastasis, Case report, Oman
Melanomas are relatively common in white-skinned women of childbearing age and many may have the diagnosis made during pregnancy;1 in dark-skinned races the incidence is reported to be much less. The general incidence of melanoma has been increasing over the last few decades at a rate greater than any other malignancy;2 melanoma is now a major cause of cancer death in women of childbearing age. The incidence in pregnancy has been estimated to range from 0.14 to 2.8 per 1,000 live births and melanoma accounts for about 8% of all malignant tumors arising during pregnancy.3 As there are now an increasing numbers of pregnancies in older women, it is expected that more melanomas will be seen.
For several years, it was assumed that hormonal changes during pregnancy might cause a rapid progress of melanoma with poor maternal and fetal outcomes. However most of the recent studies found no difference in overall survival between pregnant and non-pregnant women with melanoma.4, 5 Additionally, melanoma is known to metastasise to the placenta and the foetus. Metastasis to the products of conception portends a poor prognosis for the mother.6
We report a case of metastatic malignant melanoma diagnosed in the second trimester of pregnancy in a light skinned Asian-Arab lady. The consequences of a delay in diagnosis are discussed.
Case Report
A 28 year old light-skinned lady, gravida 2 and para 1, was referred at 27 weeks gestation to the Department of Obstetrics and Gynaecology, Sultan Qaboos University Hospital for management of a locally advanced malignant melanoma. The history dated back to 12 weeks of gestation when the patient presented with left groin pain. She was found to have left inguinal lymphadenopathy and was treated with antibiotics and subsequently referred to surgeons with no relief. A needle core biopsy from the node at 16 weeks of gestation revealed a metastatic malignant tumor. Subsequently, the slide review and immuno-histochemistry studies done at a tertiary centre showed positive staining for S-100, melanin-A, vimentin and focal positivity for human melanoma black (HMB)-45 confirming the diagnosis of a metastatic malignant melanoma.
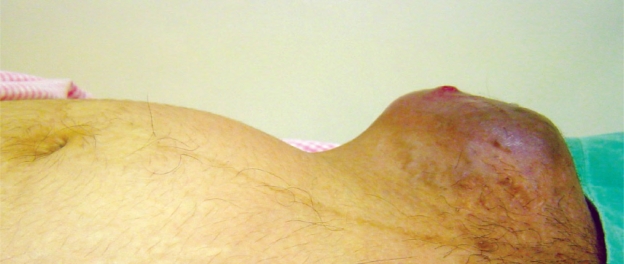
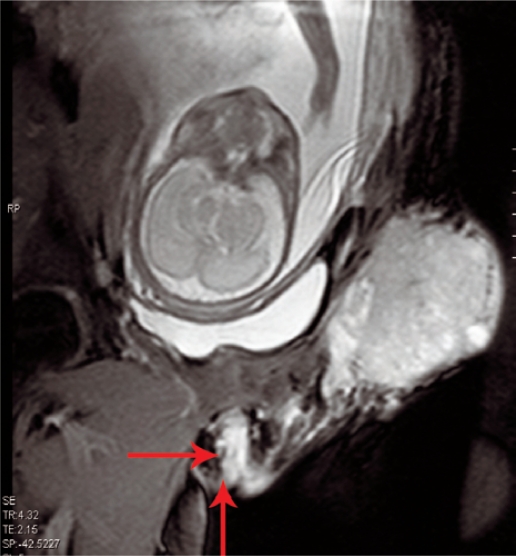
The patient did not arrive at our institution until 11 weeks after the initial biopsy, where the examination revealed a left inguinal dark mass measuring 10 x 7cm size and extending from the left anterior superior iliac spine to the pubic symphysis. The mass was mostly solid and lobulated with some cystic component and was very tender [Figure 1]. A complete physical examination revealed a raised nevus 8 x 7mm size with satellite lesions in the upper lateral part of left thigh. Both breasts had mobile non-tender firm masses: 3 x 3cm in the right breast and 2 x 1cm in the left breast. An ultrasound scan of the foetus revealed a well grown foetus of 27 weeks gestation with normal placenta and amniotic fluid volume. After a multidisciplinary evaluation by the oncologist, the pain management team and the neonatologist, it was decided to deliver the foetus. A magnetic resonance imaging (MRI) of the left groin [Figure 2] was done to ascertain the extent of the tumour in the anterior abdominal wall and the labour was induced at 28 weeks of gestation, resulting in a male baby weighing 1,310gm with an Apgar score of 8 at 1 minute and 9 at 5 minutes. Gross as well as histological examination of placenta did not show any metastasis. A complete physical examination, including an examination of the optic fundi, did not reveal any sign of disease in the newborn. The patient received cabergoline for suppression of lactation.
Figure 1: Pregnant uterus with mass in the left groin.
Figure 2:
T2W coronal oblique magnetic resonance imaging scan of pelvis shows lobulated mass in left inguinal region with infiltration of skin and sub-cutaneous tissues. Dilated lymphatics seen extending up to vulva (arrows)
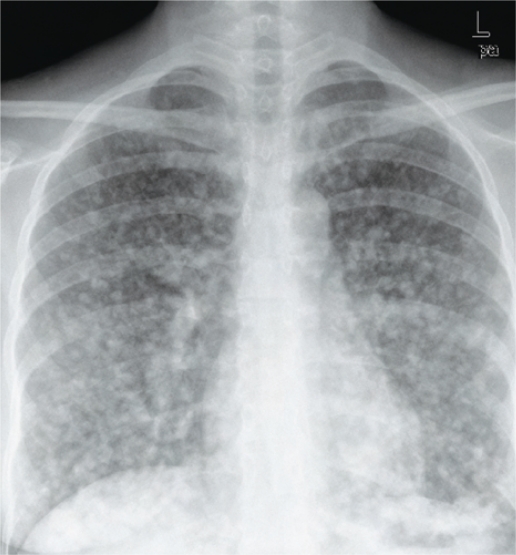
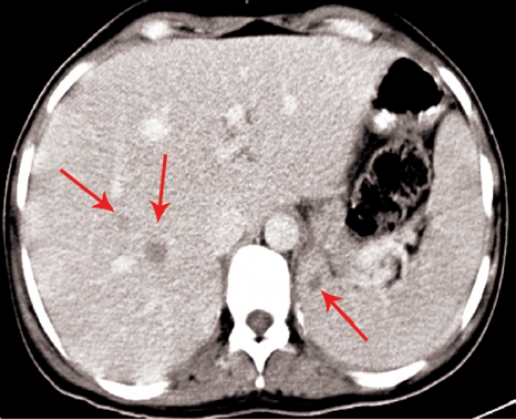
A metastatic workup soon after delivery revealed extensive involvement of the lungs [Figure 3], liver, spleen [Figure 4], and both breasts [Figure 5]. A needle core biopsy from the breast lesion confirmed the presence of metastatic melanoma [Figure 6]. A magnetic resonance imaging (MRI) scan of the brain did not show any metastasis. The patient was started on combination chemotherapy with cisplatin, vinblastine and dacarbazine with subjective improvement following the first cycle. The treatment was complicated and delayed by the refusal of the patient to continue with intravenous chemotherapy. A chest X-ray following 2 cycles of chemotherapy showed a stable disease state. However, once the intravenous chemotherapy was discontinued, the disease progressed and the patient died 4 months after the delivery.
Figure 3:
Chest X-Ray PA view: Shows multiple, scattered, nodular, metastatic lesions in both lungs
Figure 4:
Contrast Enhanced CT Scan shows hypodense, metastatic lesions in right lobe of liver and spleen (arrows)
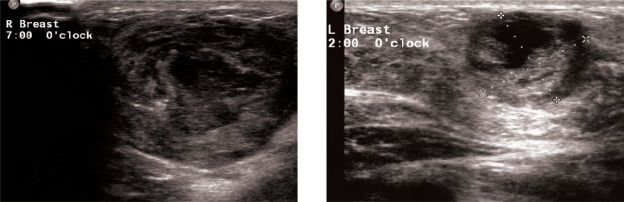
Figure 5:
Ultrasound scan of both breasts showing thick walled necrotic masses
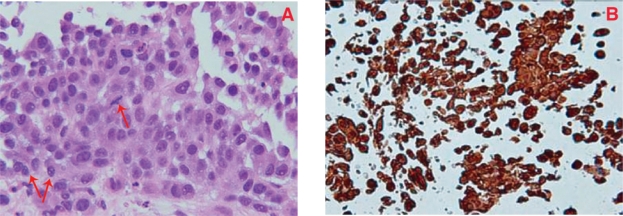
Figure 6:
(A) needle core breast biopsy showing malignant cells with mitotic figures (single arrow) and nuclear inclusions (double arrow), hematoxylin and eosin stain X 60. (B) Immunohistochemistry studies showing strong S-100 positivity
The baby was well for two weeks, then developed a bowel perforation due to necrotising enterocolitis and was transferred to another tertiary care centre with paediatric surgery facilities. Laparotomy revealed multiple bowel perforations; unfortunately, the baby died of septicaemia at 3 months of age.
DISCUSSION
Malignant melanoma is the most aggressive form of skin cancer with an increasing incidence over the past decade. Although it used to be more common in men, it now affects equal numbers in both sexes. The mean age for diagnosis is 52 years, some 10 years earlier than the more common tumors such as breast, lung and prostate. About 30–35% of women with melanoma are at the childbearing age at the time of diagnosis. The most likely aetiology is reported as intermittent, blistering sun exposure among susceptible individuals.7
Common sites of metastasis of malignant melanoma include the lymph nodes, lungs, liver, spleen and the brain. Breast metastasis has been reported in cases of cutaneous melanoma, mainly from the upper limb and trunk. Our patient had bilateral breast metastasis from a primary tumour in the left lower limb which is extremely rare.8
For several years, it was widely held that the prognosis of melanoma was worse during pregnancy and that subsequent pregnancies increased the risk of recurrence.9,10 Also, the use of estrogen containing hormonal preparations was thought to be associated with a worse prognosis. The myths have arisen as a result of the misconception that because skin darkens in pregnancy, melanomas are hormonally sensitive;2 however, recent studies have not found estrogen receptors in melanoma cells.1, 11 These myths about melanoma were strengthened by a report in 1951 from Pack and Scharnagel9 who reviewed 1,050 patients with malignant melanoma. Out of the ten pregnant women in this study, five died within 3 years of diagnosis due to progressive disease. The authors concluded that the women diagnosed with localised melanoma during pregnancy had a worse prognosis, but in this study, only ten pregnant women were included without any comparison group or staging of the disease. Multiple controlled series and investigations have found, however, that stage for stage melanoma is not adversely affected by pregnancy. The prognosis, recurrence and incidence of melanoma seem to be unaffected by pregnancy.4, 5, 12
Melanoma is one of several tumors which metastasise to the placenta, including lymphomas, leukaemias, breast and lung cancer.13 Although melanoma is the most common maternal malignant tumour to metastasise to the placenta, the occurrence is very rare. A review of literature showed 87 cases of placental/foetal involvement with maternal malignancies. Of these 72 (83%) reported placental involvement only, 10 (11%) reported foetal metastasis without placental examination, and 5 (6%) reported both placental and foetal metastasis.6 During the period from 1918 to 2002, melanoma was the cancer most commonly found to involve the placenta and foetus, accounting for 27 of 87 (31%) cases. Microscopic evaluation of the placenta was performed in 24 of 27 patients, and placental involvement was documented in all 24 patients. Six of the 27 reports indicated foetal metastasis, but 3 reports did not document corresponding placental involvement.6
Male infants seemed to be at a higher risk than females for developing metastasis of any maternal cancer. Males comprised 80% of all infants with metastasis of melanoma and 75% with metastasis of all cancers.6
Previously published reviews have calculated an approximate 25% mortality risk to babies born to mothers with placental involvement. Infants developing clinical evidence of maternally derived metastasis have an exceptionally poor prognosis, with death typically occurring within 3 months of diagnosis. The neonates delivered with concomitant placental involvement, but without clinical evidence of the disease, should be considered a high risk population. They should be periodically evaluated for development of melanoma for at least 24 months postpartum. Adjuvant treatment of infants born to women with placental metastasis of melanoma has not been reported.14
The management of melanoma during pregnancy requires several difficult decisions as the disease involves both the mother and the foetus. Decision making should be based on: 1) The impact of pregnancy on the outcome of the metastatic melanoma; 2) the gestational age and the risk of metastasis to the placenta and foetus; 3) the safety of radio diagnostic tests and chemotherapy during pregnancy, and 4) the treatment options for metastatic melanoma during pregnancy.
Although surgery is the definitive therapy for early stage disease, rapidly progressive metastatic disease during pregnancy is difficult to treat. Chemotherapeutic regimens for metastatic disease administered during pregnancy have not demonstrated significant efficacy.15
The decision to deliver our patient at 28 weeks of gestation was to give the mother the best chance of survival. The neonatal survival in our institution at 28 weeks is more than 90%. The dilemma was the mode of delivery; induction of vaginal delivery versus cesarean section. At 28 weeks, the failure of labour induction is very high, but cesarean section was also risky as the tumor was infiltrating into the incision site. Hence we decided to try induction of labour first; fortunately the patient responded.
The delay in the diagnosis of our case may be because of the low index of suspicion; considering that this was a young, Asian-Arab woman, where the risk of melanoma was perceived to be less. The rapid progression of the disease as mentioned may have been due to the natural history of the disease, although it is impossible to know whether hormonal changes during pregnancy had an impact. Whereas most of the data regarding malignant melanoma comes from white-skinned races, its natural history and progression during pregnancy in coloured races, although light-skinned as in our case, may be different.
CONCLUSION
Malignant melanoma is not uncommon during pregnancy, although this is the first case reported from our institution in the past eighteen years. As early diagnosis may lead to cure, it is essential that all clinicians who care for pregnant women understand this disease. Obstetricians and midwives should do a full skin examination of all parts of the body during evaluation of the pregnant women and any suspicious nevi should be biopsied. Treatment of early stage melanoma is the same irrespective of whether or not the patient is pregnant. Delay in the diagnosis and treatment may result in a tragic outcome as in our case.
REFERENCES
- 1.Mackie RM. Pregnancy and exogenous hormones in patients with cutaneous malignant melanoma. Curr Opin Oncol. 1999;11:129–31. doi: 10.1097/00001622-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Katz VL, Farmer RM, Dotters D. Focus on primary care: From nevus to neoplasm: Myths of melanoma in pregnancy. Obstet Gynecol Surv. 2002;57:112–19. doi: 10.1097/00006254-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Ernstoff MS, Christopher PG, Tretter MD, Titus-Ernstoff L. Update: Medical therapy for cutaneous melanoma. Am Soc Clin Oncol. 2003:198–207. [Google Scholar]
- 4.Wiggins CL, Berwick M, Bishop JA. Malignant melanoma in pregnancy. Obstet Gynecol Clin North Am. 2005;32:559–68. doi: 10.1016/j.ogc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Silipo V, Paola DS, Giustino M, Pierluigi B, Angela F, Caterina C. Malignant melanoma and pregnancy. Melanoma Res. 2006;16:497–500. doi: 10.1097/01.cmr.0000232295.91536.09. [DOI] [PubMed] [Google Scholar]
- 6.Nikolin BL, Sveljo O. Metastatic melanoma and pregnancy. Arch Oncol. 2005;13:31–4. [Google Scholar]
- 7.Berwick M, Wiggins C. The current epidemiology of malignant melanoma. Front Biosci. 2006;11:1244–54. doi: 10.2741/1877. [DOI] [PubMed] [Google Scholar]
- 8.Bassi F, Gatti G, Mauri E, Ballardini B, De Pas T, Luini A. Breast metastasis from cutaneous malignant melanoma. Breast. 2004;13:533–5. doi: 10.1016/j.breast.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Pack GT, Scharnagel IM. Prognosis of malignant melanoma in pregnant women. Cancer. 1951;4:324–34. doi: 10.1002/1097-0142(195103)4:2<324::aid-cncr2820040218>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland CM, Loutfi A, Mather FJ, Carter RD, Krementz ET. Effect of pregnancy upon malignant melanoma. Surg Gynecol Obstet. 1983;157:443–6. [PubMed] [Google Scholar]
- 11.Schwartz JL, Mozurkewich EL, Johnson TM. Current management of patients with melanoma who are pregnant, want to get pregnant, or do not want to get pregnant. Cancer. 2003;97:2130–3. doi: 10.1002/cncr.11342. [DOI] [PubMed] [Google Scholar]
- 12.O’Meara AT, Cress R, Xing G, Danielsen B, Smith LH. Malignant melanoma in pregnancy - A population based evaluation. Cancer. 2005;103:1217–26. doi: 10.1002/cncr.20925. [DOI] [PubMed] [Google Scholar]
- 13.Alexander A, Samlowski WE, Grossman D, Bruggers CS, Harris RM, Zone JJ, et al. Metastatic melanoma in pregnancy: Risk of transplacental metastsis in the infant. J Clin Oncol. 2003;21:2179–86. doi: 10.1200/JCO.2003.12.149. [DOI] [PubMed] [Google Scholar]
- 14.Borden EC. Melanoma and pregnancy. Semin Oncol. 2000;27:654–5. [PubMed] [Google Scholar]
- 15.Leachman SA, Jackson R, Eliason M, Larson AA, Bolognia JL. Management of melanoma during pregnancy. Dermatol Nurs. 2007;19:145–52. [PubMed] [Google Scholar]