Abstract
Huntington disease (HD) has been reported in Arab families in several Middle East countries including Saudi Arabia, Oman, Syria, Lebanon, and Egypt and in non-Arab populations in other countries in the region. It is probably under-reported, and until now, has not been recorded in Yemen, the United Arab Emirates, Jordan or in Iraq. The Middle East has always been on the crossroads of trade and travel, and HD was probably introduced to some of these countries in previous times. The prevalence rate in Middle Eastern Arabs is estimated to vary from 3 to 4 per 100,000. Although the HD gene which codes for the protein huntingtin has been identified, the function of this protein is not known. At present, no treatment has been found to delay the onset of HD or to treat it effectively. Although relatively rare, HD has increasingly become a focus of international gene research, with the support and collaboration of the International Huntington Association (IHA). The IHA has been represented in Saudi Arabia and Oman.
Keywords: Huntington, Chorea, Middle East, Arab race
Genetic aspects of heredity and disease have made enormous advances in recent decades especially in regard to our understanding of the molecular basis of gene expression. This has had a significant impact in every branch of medicine, and induced most of us to keep abreast of the fundamentals of basic genetics and its terminology. In brief, a gene is a portion of deoxyribonucleic acid (DNA) encoded on a chromosome, which contains a particular set of instructions usually coding for a single protein. Each somatic cell contains 23 paired chromosomes, one of each pair derived from each parent. Each chromosome (a single very long DNA helix) encodes thousands of genes. Much of the variation in our body traits such as height, intelligence and blood pressure among ‘normal’ people can be accounted for by our remarkable genetic variability (polymorphism). These genetic differences also influence our ability to meet environmental challenges including those that produce disease. Ultimately, all human disease can be considered to result from interaction between our genetic make up and the environment. An example is the development of Type 2 diabetes mellitus in susceptible subjects exposed to a high carbohydrate diet.
In genetic disorders, the genetic component is so overwhelming that disease manifests without the necessity for environmental challenge. Disorders caused by the transmission of a single mutant gene follow three different patterns of inheritance. In autosomal dominant disorders, the individual inherits a single dose of the mutant gene (mutant allele) from one parent (i.e. he or she is heterozygous for the gene) and the disease will be expressed. Examples include familial hypercholesterolaemia and Marfan’s syndrome. In recessive disorders, the mutant gene is inherited from each parent (i.e. both alleles are mutant) and the patient is homozygous for the gene. Again, the disease will be expressed. In populations in which consanguineous marriages occur frequently, i.e. arranged marriages between cousins, certain recessive conditions are more common. Familiar examples include sickle cell anaemia and β-thalassemia.
In X-linked disorders, the mutant gene is located on the X chromosome. Since a female has two X chromosomes, she may be either heterozygous or homozygous for a mutant gene; therefore, the trait may manifest as either dominant or recessive expression. As a male has only one X chromosome, if he inherits the mutant gene, the full syndrome will always be manifested e.g. haemophilia A and Duchenne's muscular dystrophy.
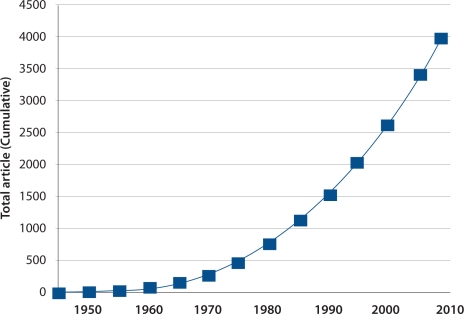
An example of a relatively rare, but now prominent autosomal dominant condition, is Huntington disease (HD) or Huntington’s chorea as it was formerly termed. HD is named after an American general practitioner, George Huntington, who in 1872 described inherited chorea in families living on Long Island, New York.1 His single publication earned him enduring recognition, and led ultimately to a worldwide association of medical workers, scientists and affected families (the International Huntington’s Association), and a massive and intensive research programme which continues today. The number of publications annually is increasing rapidly [Figure 1].
Figure 1:
Publications on Huntington disease listed in the Entrez Pubmed website of the National Library of Medicine, Washington DC, USA. (www.ncbi.nlm.nih.gov/pubmed)
CHARACTERISTICS AND PREVALENCE OF HUNTINGTON DISEASE
Research on the molecular biology of HD has increased our understanding of aspects of cellular function and gene expression, and incidentally this has shed light on unrelated diseases such as Alzheimer’s. The discovery of the general location of the HD gene on chromosome 4 in 1993 using DNA marking methods was an important first step toward the Human Genome Project.2
HD is an unique neurodegenerative disease which has three characteristic features: the onset of chorea or choreoathetosis, usually in middle-age, dominant inheritance and progressive dementia. The average doctor in the Middle East may never encounter or recognise a case, yet affected families have been documented in Arabs in Oman,3 Saudi Arabia,4–6 Syria,4 Lebanon,4 and Egypt,4 and HD has also been recorded in a Sudanese family living in Jeddah, Saudi Arabia 7 and in non-Arab communities in Turkey,8 Israel,9 and Iran.10 It is probable that foci of HD occur in Yemen, the United Arab Emirates, Jordan and Iraq, although they do not appear to have been yet identified or reported.
Although HD may present in early childhood or in the elderly, commonly it presents in middle life, by which time the patient has probably married, and has adolescent children who have viewed the distressing evolution of the clinical disease. Involuntary movements of face (grimacing), and chorea or choreoathetosis (stereotyped, repetitive twitching and jerking movements) of limbs and digits appear, affecting speech and gait. Subsequently over a decade or more, intellectual impairment develops with memory loss and behavioural and psychiatric disturbances. The progression is remorseless leading to incapacitation and decease usually within 15 to 20 years. Prior to decease, the patient is typically mute, bedfast, and affected by terminal intercurrent infections such as pneumonia. The presentation is so characteristic, that it is relatively easy to suspect the diagnosis when one has already seen a case or observed a videotape of a patient (There is a videotape of an Omani patient, ‘Maryam’ in the Medical Library of Sultan Qaboos University Hospital in Muscat, Oman, and there are also several good videoclips of HD patients listed in the Google.com website).
Every child born to an affected mother or father has a 50% chance of inheriting the anomalous gene, and all gene-positive subjects will ultimately develop the disease unless they die before it presents. Genetic studies suggest that spontaneous mutation is rare in HD, among the lowest for any similarly inherited condition.11 Transmission from a gene-positive individual is therefore the usual explanation for the occurrence of a case including an apparently sporadic case in a family with no history of HD.
The prevalence rate in persons of Western European descent is between 3 and 7 cases per 100,000, with the Netherlands and the United Kingdom historically, being high-prevalence regions. Studies have shown that emigration in the 18th and 19th centuries of individuals from Western Europe introduced the disease to North America, South Africa and Australia and elsewhere.12 The prevalence rate in individuals of Asian or African descent on the other hand, is much lower, in the order of 1 per 1,000,000. The prevalence rates for HD for the Middle East are likely to vary from 3 to 4 per 100,000 for the whole region, but there may well be foci of higher prevalence rates adjacent to ports or in isolated communities. For Oman, with an Arab population of some 3,000,000 persons, it is likely that there are about 100 HD cases in Arabs countrywide, of which only about a dozen cases (including anecdotal reports) are known.
When a focus of the disease is found in non-Caucasian populations, eg Black Africans in Tanzania 13 and Zimbabwe,14 or Melanesians in Papua New Guinea,15 there may be evidence that it was introduced by Caucasian visitors in earlier times. The relatively high prevalence foci of HD in Melanesian families in the remote islands of New Britain, and Malaita in the New Hebrides can probably be explained by the numerous visits by whaling ships from New England, USA, to hunt sperm whales in the coastal waters off these islands in the 18th and 19th centuries. Ship’s logs record shore visits by many American sailors to both islands.16 Many of the crewmen bore surnames from well-known HD families from New England, a high-prevalence area of the disease.12 In Venezuela, it is believed the disease was introduced to the Lake Maracaibo region between 1860 and 1870 by a Spanish sailor.17 In this isolated population, an exceedingly high prevalence rate of up to 700 per 100,000 developed, and as a consequences of intermarriage, some 30% of patients were subsequently identified as being homozygous for the gene.
The Middle East has always been on the crossroads of trade and travel, and it is not surprising that foci of HD occur in local populations throughout the region. Over the years, immigration has brought many individuals from afar to reside in the region, among whom there are likely to have been individuals carrying the trait for HD. In Jeddah, the individual who introduced HD to a Saudi family, was an Afghan migrant.5 Although HD has not been reported in Afghanistan, the disease certainly occurs there: the individual who introduced HD to Australian Aboriginal families in the tropical Kimberley region of Western Australia in the late 1880s, was also an Afghani, a cameleer who married seven indigenous women.18 In the case of the Oman studies, no foreign antecedent could be identified in recent preceding generations. However, with its rich history of past settlement, trade and colonisation (the Portuguese ruled Muscat and coastal Oman during the 17th and first half of the 18th centuries), there must have been many opportunities for introduction of HD.3 Oman’s long association with Zanzibar and coastal East Africa, which it administered until the early 1970s, and the migration of thousands of Arab families to Oman when independence was achieved in Tanzania and Kenya, would have been another opportunity for introduction of the disease: HD occurs in Tanzania.13
DEVELOPMENT OF THE DISEASE
The gene which produces the cytoplasmic protein we call ‘huntingtin’ (which is present in every normal cell), i.e. the Huntingtin gene (HTT), was identified in 1993 on the short arm of chromosome 4 (4p.16.3) by the Huntington’s Disease Collaborative Research Group.19 This study was conducted in the high prevalence region of Venezuela referred to previously. The gene contains a sequence of three DNA bases, cytosine-adenine-guanine (CAG) repeated multiple times (i.e. CAGCAG etc). CAG is the genetic code for the amino acid glutamine. The range of glutamine repeats in normal huntingtin (Htt) is less than 27. Like all proteins, this is translated and then affects biological functions, but although in mouse models, it is essential for development and survival, in man its precise function is still unknown. In HD, the abnormal or mutant gene (mHTT) produces a sequence of 36 or more glutamines (polyglutamines) in huntingtin protein, which results in protein aggregation and neuronal degeneration in parts of the brain.20, 21 Astrogliosis and loss of neurones especially in the striatum, frontal and temporal cortices are the main sequelae The subthalamic nuclei in the striatum sends control signals to the globus pallidus which initiates and modulates motion, and their impairment, results in the characteristic involuntary movements.
The more CAG repeats that are present, the earlier HD develops. Thus with relatively few repeats, i.e. 36–39, the disease may only appear in late old age, but with very large repeats, it can present before 20 years-of-age (7% of cases) when it is called juvenile HD or the Westphal variant. Our 6 year old patient in the Oman study had 92 CAG repeats, and had the typical associated features of rigidity and dystonia.3
The development of the transgenic mouse model for HD (the R6 line) in 1996 (when a CAG repeat expansion was inserted into a mouse genome) has been a significant step in investigating the disease,22 and, last year, a new transgenic rhesus monkey model of HD was developed by Emory University, Atlanta, Georgia with a view to improving treatment modalities.23 It is an arresting sight to observe animals with the characteristic involuntary movements of the classical disease.
DIAGNOSIS AND FOLLOW-UP
The diagnosis of HD is usually confirmed by a neurologist or a psychiatrist. Until the availability of DNA diagnosis, the differential diagnosis of HD included various conditions including hepatolenticular degeneration, tardive dyskinesia following the use of phenothiazine drugs, Parkinson’s disease when overdosed with levodopa and hereditary acanthocytosis. However, with the definitive DNA test, the diagnosis can easily be established, with supportive neuroradiology, if available. In the latter, magnetic resonance (MRI) imaging or computerised tomography (CT scan) of the brain demonstrates caudate nuclei atrophy with the bicaudate diameter exceeding the normal range of 12.5–15mm. At this point, predictive genetic testing by a blood test of family members at risk of the disease is available to individuals at 18 or more years of age, following full counselling. At no time are individuals encouraged to be tested, it must always be entirely at their own volition. Currently, prenatal diagnosis of HD in maternal plasma is being evaluated.24
It is essential in a hereditary disease of this serious nature, that patients, affected families and individuals at-risk (as well as the medical staff looking after cases), are fully informed about the condition. At all times confidentiality and anonymity for patients and families must be assured. Preliminary explanation may be provided by the diagnosing doctors, but this is best followed up by provision of detailed information booklets with representative diagrams of family pedigrees, which the family can study at their leisure. Such booklets are provided by the International Huntington Association (IHA) or its regional branches (or related organisations) in English and other major languages. The IHA was formed in 1979 in Oxford, England, and is now a worldwide organisation mostly of lay people and family members who promote social support for families and patients, collaborate in research projects, act as fund-raisers, and disseminate knowledge of the condition.
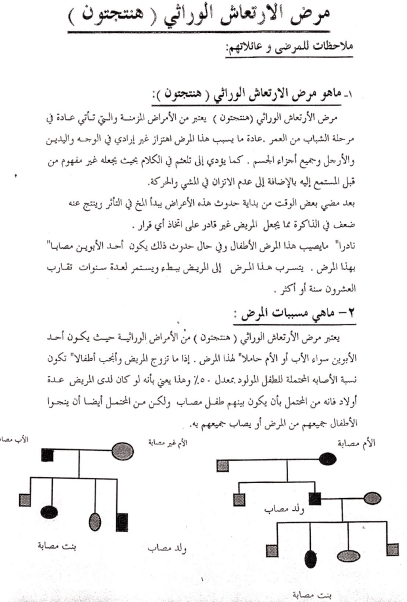
Formerly, the IHA did not provide information booklets in Arabic, and accordingly, in Saudi Arabia and in Oman, a 3-page Arabic information booklet was prepared for local use. This booklet which had representative diagrams of family pedigrees was discussed at length and in detail with family members. It explained the hereditary nature and cause of the disease in simple, non-technical terms, and emphasized that at present there was no curative or preventative medication [Figure 2]. This is important as families are likely to consider any resource to find a solution. Usually several sessions of explanation and discussion were required before the families felt they were adequately informed about HD.
Figure 2:
Example of first page of basic information notes about Huntington disease in Arabic used in Jeddah, Saudi Arabia and in Oman
In Saudi Arabia and in Oman, affected families were relieved that a definitive diagnosis and explanation had been reached. In the case of one of the Saudi families, the family had long been under the impression that the illness was caused by sorcery and malevolent jinns. Although impoverished, they had been attending traditional healers and had tried various expensive, futile remedies. Following counselling sessions, they realised that traditional treatment was futile. There are times when the families concerned may reject the diagnosis and seek a second opinion. In Jeddah, one family sought a second opinion in UK, and after further neuroradiology and DNA studies, still rejected the diagnosis. In the Oman study,3 the parents of an affected child refused to accept the diagnosis. One of the parents was mildly symptomatic, but both refused DNA tests, and left to seek advice elsewhere. They were lost to follow-up.
TREATMENT OPTIONS
Unfortunately, despite world-wide trials and research for many decades, no treatment has been found to delay or prevent the onset or to effectively modify the symptoms of this formidable disease.25 Medications in use for movement disorders include those which may reduce chorea including haloperidol and fluphenazine, and tetrabenazine (a dopamine depleter).26 For rigidity (eg. in the Westphal variant), L-dopa (a dopamine precursor) and pramipexole have been used. When required, antidepressants eg, amitriptyline and mirtazapine, and antipsychotics eg risperidone, haloperidol and buspirone are available. However, the beneficial effects of these neuroleptics have to be balanced with side-effects: there is a saying among HD patients that there are two occasions when they thank the physician, first when a new medication is started, and then when it is stopped. Various substances are under investigation for possible neuroprotection, including coenzyme Q10 (boosts energy in cells and acts as an antioxidant), minocycline (which inhibits apoptosis) and unsaturated fatty acids. Unfortunately, a recent report showed no benefit using an omega-3 fatty acid during 6 months of placebo-controlled evaluation.27
Another neurotrophic factor under investigation is brain-derived neurotrophic factor (BDNF) which promotes neurogenesis and is elevated by selective serotonic reuptake inhibitor antidepressants such as citalopram.28 Preclinical studies of fibroblast growth factor 2, which promotes neurogenesis and extends survival time in transgenic mice, and caspase 6 inhibitor, which appears to delay or prevent the onset of clinical HD in transgenic mice, are underway.29 Apart from numerous pharmaceutical trials in progress, other approaches to the management of patients include nutritional supplements, and intellectual and enhanced voluntary physical activity (exercise delays some symptoms in the transgenic mouse model). It is a feature of pharmacological and indeed all trials in HD, that many subjects and controls are required for extended longitudinal studies. Most trials follow the format of preclinical trials, then phase I, phase II, and phase III studies which may take a year or more to evaluate. It is a notable feature of HD families that they are always eager to participate in research projects and drug trials and like to be well-informed about results.
MANAGEMENT OF THE DISEASE
The life-long management of the many thousands of patients and families affected by HD remains a major problem worldwide. Since there is no effective remedy, following diagnosis further medical care is often delegated to general physicians, and general practitioners with the support of social workers. When available, support from physiotherapists or occupational therapists is often valuable. In developing countries, inevitably, impoverished families in rural or outlying areas, have no follow-up or medical care (no access to medications or clinics), and families have to look after patients until the end. Considerable financial hardship is common when the breadwinner of the family is affected. In Jeddah, the Sudanese patient was a taxi-driver, and the necessity to abandon work posed serious financial difficulties for his youthful wife and numerous children.7 In most countries especially in developing countries, it is rare to find a doctor or medical worker who can keep in touch with families over many years and visit them from time to time.
For those at risk, the implications of a positive gene test are formidable: a young man or woman has observed the effects of the disease in his or her parent unfolding over a decade or more, and knows that there is no effective treatment or prevention. Some individuals have committed suicide, become socially irresponsible, or lapsed into substance abuse. The response in many Western countries has been the development of ‘gene-positive’ social groups. In Perth, Western Australia there is a large group of young gene-positive individuals who frequently meet socially and discuss their problems together. They are a remarkably cheerful and positive group. Facing the disease together clearly boosts morale, and they can keep up to date with progress in research and treatment. When it comes to marriage, a gene positive individual can elect to avoid having children.
In regard to personal accident or life insurance or obtaining mortgage or other loans, gene positive individuals or those at risk of HD, are at a major disadvantage. Insurance or lending institutions have routinely rejected applications from such individuals.30 The IHA has endeavoured to find ways in which these individuals can receive some insurance coverage.
The IHA has been represented until recently in Saudi Arabia and Oman, but at present the position of Local Representative is vacant in both countries. This is a most rewarding role, as HD families everywhere are deeply appreciative of medical workers who devote time and interest to their problems. Home visits are always warmly received. Doctors and health workers who become involved with HD families frequently become long lasting confidants and friends with them. It has been said by some workers, that involvement with HD is addictive. The author has kept in touch by correspondence with one HD family in Papua New Guinea (last visited in 1988) for over 20 years.
The IHA and the World Federation of Neurology Research Group combine every two years in the World Congress on Huntington’s disease to bring associations and researchers together to share ideas and research. The first meeting took place in Toronto in 2003, and the next meeting will be in Vancouver, Canada, 12–15 September, 2009. This meeting will no doubt record the outstanding achievements of the past few years, stimulate much discussion and anticipate further developments which may finally offer curative solutions to this formidable condition.
CONCLUSION
Although HD may be a relatively rare neurodegenerative disease, it has become a leading focus for international genetic research. This has led to many significant advances in our understanding of gene function, but despite intensive research over the past two decades, the function of the huntingtin protein, which is produced by the normal Huntington gene and which is present in every cell in our body, is still not understood, and no effective treatment has been found for the disease. To those of us who have devoted many years to the study of HD, and have met dozens of patients and many, many families affected by this most devastating disease, there could be no greater hope than that one day, the clinical disease will be arrested, the signs and symptoms reversed or treated effectively, and the onset of the disease in gene positive individuals, wholly prevented. It will no longer be necessary to be the unwelcome bearer of the dreaded news that a gene positive person has inherited a fatal and incurable condition.
REFERENCES
- 1.Huntington G. On chorea. Med Surg Rep. 1872;26:317–21. [Google Scholar]
- 2.Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, et al. “A polymorphic DNA marker genetically linked to Huntington’s disease”. Nature. 1983;306:234–8. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 3.Scrimgeour EM, Koul RL, Chand PR, Tharakan JKJ. Juvenile onset of Huntington’s disease in an Omani chld with asymptomatic, at risk parents. J Med Genet. 1997;34:701. doi: 10.1136/jmg.34.8.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kremer K, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J, et al. A worldwide study of Huntington’s disease mutation. N Engl J Med. 1994;330:1401–6. doi: 10.1056/NEJM199405193302001. [DOI] [PubMed] [Google Scholar]
- 5.Scrimgeour EM, Tahoon SA, Zawawi TH. Huntington’s disease in two unrelated Arab kindreds, and in an Afghan family resident in Saudi Arabia. J Med Genet. 1994;31:819–20. doi: 10.1136/jmg.31.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohlega S, McLean D, Omer S, Al-Kawi MZ, Roos RA, Losekoot M, et al. Huntington’s disease: report on four Saudi families. Saudi Med J. 1996;17:456–9. [Google Scholar]
- 7.Scrimgeour EM, Samman Y, Brock DJH. Huntngton’s disease in a Sudanese family from Khartoum. Hum Genet. 1995;96:624–625. doi: 10.1007/BF00197424. [DOI] [PubMed] [Google Scholar]
- 8.Atac FB, Elibol B, Schaefer F. The genetic analysis of Turkish patients with Huntington’s disease. Acta Neurol Scand. 1999;100:195–8. doi: 10.1111/j.1600-0404.1999.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 9.Gazit E, Lubomirov L, Munakov O, Topper A, Frydman M, Fried K, et al. Distribution of CAG repeats in normal and Huntington’s disease patients in Israel. Clin Genet. 1998;54:250–1. doi: 10.1111/j.1399-0004.1998.tb04296.x. [DOI] [PubMed] [Google Scholar]
- 10.Banoei MM, Houshmand M, Panahi MS, Shariati P, Rostami M, Manshadi MD, et al. Huntington’s disease and mitochondrial DNA deletions: event or regular mechanism for mutant huntingtin protein and CAG repeats. Cell Mol Neurobiol. 2007;27:867–75. doi: 10.1007/s10571-007-9206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden MR. Huntington’s chorea. Berlin & Heidleberg: Springer Verlag; 1981. p. 108. [Google Scholar]
- 12.Hayden MR. Huntington’s chorea. Berlin & Heidleberg: Springer Verlag; 1981. pp. 15–30. [Google Scholar]
- 13.Scrimgeour EM. Huntington’s disease in Tanzania. J Med Genet. 1981;18:200–3. doi: 10.1136/jmg.18.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scrimgeour EM, Pfumojena JW. Huntington disease in Black Zimbabwean families living near the Mozambique border. Am J Med Genet. 1992;44:762–6. doi: 10.1002/ajmg.1320440610. [DOI] [PubMed] [Google Scholar]
- 15.Scrimgeour EM. Huntington’s disease in two New Britain families. J Med Genet. 1980;17:197–202. doi: 10.1136/jmg.17.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scrimgeour EM. Possible introduction of Huntington’s chorea into Pacific islands by New England whalemen. Am J Med Genet. 1983;15:607–13. doi: 10.1002/ajmg.1320150410. [DOI] [PubMed] [Google Scholar]
- 17.Avila-Giron R. Medical and Social Aspects of Huntington’s chorea in the state of Zulia, Venezuela. Advances in Neurology. 1973;1:261–6. [Google Scholar]
- 18.Panegyres PK, McGrath F. Huntington disease in indigenous Australians. Int Med. 2008;38:130–2. doi: 10.1111/j.1445-5994.2007.01484.x. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald M. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disase Collaborative Research Group. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 20.Li SH, Li XJ. Huntingtin and its role in neuronal degeneration. Neuroscientist. 2004;10:467–75. doi: 10.1177/1073858404266777. [DOI] [PubMed] [Google Scholar]
- 21.Benn CL, Sun T, Sadri-Vakili G, McFarland KN, DiRocco DP, Yohrling GJ, et al. Huntingtin modulates transcription, occupies gene promoters in vivo, and binds directly to DNA in a polyglutamine-dependent manner. J Neurosci. 2008;15:10720–33. doi: 10.1523/JNEUROSCI.2126-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathasivam K, Hobbs C, Mangiarini L, Mahal A, Turmaine M, Doherty P, et al. Transgenic models of Huntington’s disease. Philos Trans R Soc Lond B Biol Sci. 1997;54:963–9. doi: 10.1098/rstb.1999.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, et al. Towards a transgenic model of Huntington’s disease in a non-human primate. Nature. 2008;453:863–4. doi: 10.1038/nature06975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bustamente-Aragones A, Trujillo-Tiebas MJ, Gallego-Merlo J, Rodriguez de Alba M, Gonzalez-Gonzalez C, Cantalapiedra D, et al. Prenatal diagnosis of Huntington disease in maternal plasma: direct and indirect study. Eur J Neurol. 2008;15:1338–44. doi: 10.1111/j.1468-1331.2008.02312.x. [DOI] [PubMed] [Google Scholar]
- 25.Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des. 2006;12:2701–20. doi: 10.2174/138161206777698693. [DOI] [PubMed] [Google Scholar]
- 26.Fasano A, Cadeddu F, Guidubaldi A, Piano C, Soleti F, Zinzi P, et al. The long-term effect of tetrabenazine in the management of Huntington disease. Clin Neuropharmacol. 2008;31:313–8. doi: 10.1097/WNF.0b013e318166da60. [DOI] [PubMed] [Google Scholar]
- 27.Dorsey ER, Shoulson I, Leavitt B, Ross C, Bieck CA, de Blieck EA, et al. Randomised controlled trial of etheyl-ecosapentaenoic acid in Huntington disease: the TREND-HD study. Arch Neurol. 2008;65:1582–9. doi: 10.1001/archneur.65.12.1582. [DOI] [PubMed] [Google Scholar]
- 28.Rose E, Saudou F, Caboche J. Pathophysiology of Huntington’s disease: from huntingtin functions to potential treatments. Curr Opin Neurol. 2008;21:497–503. doi: 10.1097/WCO.0b013e328304b692. [DOI] [PubMed] [Google Scholar]
- 29.Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, et al. Proc Natl Acad Sci USA. 2005;102:18189–94. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oster E, Dorsey ER, Bausch J, Shinaman A, Kayson E, Oakes D, et al. Fear of health insurance loss among individuals at risk for Huntington disease. Am J Med Genet. 2008;146A:2070. doi: 10.1002/ajmg.a.32422. [DOI] [PMC free article] [PubMed] [Google Scholar]