Abstract
Objectives:
This study investigates the in vitro effect of the antioxidant drug, N-acetyl-L-cysteine (NAC), on cytokine production by peripheral blood mononuclear cells (PBMC).
Methods:
PBMC were isolated by Ficoll-Hypaque, and stimulated with anti-CD3 antibodies, phytohaemagglutinin (PHA), lipopolysaccharide (LPS) for 24 hours in the presence or absence of 5 mM NAC. The cytokines produced were measured by enzyme-linked immunosorbent assay (ELISA).
Results:
Treatment with NAC significantly up-regulates the secretion of IL-1β, IL-5 (interleukin) and IFN-γ (interferon) and down regulates IL-10 production, after anti-CD3 or PHA (p<0.05), but not after LPS stimulation. NAC also significantly increased total IL-12 secretion after anti-CD3 (but not PHA or LPS) stimulation and IL-12p40 after anti-CD3, PHA, and LPS stimulation (p <0.05).
Conclusion:
These results indicate that NAC up-regulated the production of pro-inflammatory cytokines, and down regulated anti-inflammatory cytokine production by PBMC, in a process which may be associated with increased levels of glutathione (GSH). Further work is required to determine whether this increase or decrease in cytokine production is due to direct effect of NAC.
Keywords: Antioxidant, N-acetylcysteine, Cytokines, Peripheral blood mononuclear cells
N-acetyl-L-cysteine (nac) is a well-known medication which is commonly used as a mucolytic agent in a variety of respiratory illnesses, in the treatment of paracetemol overdose, and in conditions characterised by decreased antioxidants or oxidative stress such as HIV infection, cancer, heart disease and cigarette smoking. This drug works as a free radical scavenger, as a glutathione (GSH) precursor, or as structural analogue of GSH, enriching the GSH pool in the cellular environment. The beneficial effects of NAC are attributed either to its ability to reduce extracellular cysteine to cysteine, or as a source of sulfhydryl (SH) metabolites, which are important for GSH synthesis.1,2
There are limited studies investigating the effects of NAC on cytokine production by peripheral blood mononuclear cells (PBMC). A study by Viora et al., using PBMC, demonstrated that NAC up-regulated the secretion of interleukin, IL-1β, IL-2, IL-12, and IL-15 after phytohaemagglutinin (PHA) stimulation.3 Several reports have shown that NAC treatment induced a significant up-regulation of IL-12 production by isolated monocytes.4,5 In addition, pre-treatment with NAC reduced lipopolysaccharide (LPS)-mediated secretion of TNF-α (tumour necrosis factor) and IL-6 by human alveolar macrophages6 and by rat epithelial cells.7
Based on the hypothesis that antioxidant drugs such as NAC can act as modulators of the immune response and can be used in antioxidant therapy in many human diseases with oxidative imbalance,1,2 we studied the in vitro effect of NAC (as an active antioxidant agent) on the production of some of pro/anti-inflammatory cytokines by peripheral blood mononuclear cells isolated from normal healthy individuals. Also, we examined whether NAC can influence several cell-activation pathways using different stimuli.
METHODS
Peripheral blood (5ml) was collected in sodium heparin-containing tubes from 14 normal volunteers (10 males and 4 females, aged between 23–37 years) at the College of Medicine and Health Sciences, Sultan Qaboos University (SQU), Muscat, Oman. All the volunteers provided informed consent, and completed a questionnaire to assess their health status. The study was approved by the Research Committee of the College of science Medicine at SQU.
PBMC were isolated by Ficoll density gradient centrifugation (Sigma Diagnostics, USA) following the manufacturer’s instructions. Buffy coats were harvested and cells were washed twice (centrifuged at 300g for 5 minutes) in Hanks solution (GIBCO, Paisley, UK) and resuspended in an RPMI-1640 medium (GIBCO) supplemented with 10% heat-inactivated fetal calf serum (FCS), (Sigma Diagnostics, USA), L-glutamine and penicillin/streptomycin (GIBCO, USA, UK) (complete medium).
The antioxidant compound NAC (Sigma Diagnostics, USA) was dissolved in RPMI1640, the pH was adjusted to 7.4 by addition of sodium hydroxide (NaOH), and used at final concentration of 5 mM, after addition to cell suspension. The trypan blue dye exclusion test was performed in order to exclude any drug toxicity.
All the materials for this assay were purchased from Sigma Diagnostics (USA). Total glutathione (reduced and oxidized GSH) was measured in PBMC lysates after 24 hour incubation with 5 mM NAC. Cells were lysed in 2ml of 0.05% v/v Triton-X-100 in buffer (125 mM sodium phosphate, 6.3 mM sodium ethylenediamine tetra acetic acid (Na-EDTA), adjusted to pH 7.5). The samples were assayed by enzymatic recycling procedure as described previously.8
PBMC were resuspended at concentration of 1 x 106 cell/ml (for IL-1β, IL-1ra, IL-10, IL-12p40, and IFN-γ) or 5 x 106 cell/ml (for IL-5 and IL-12) in complete medium, and cultured in 24-well plates (1ml/well). Cells were stimulated with mouse anti-human CD3 (Serotec, UK, MCA 463XZ; 10μg/ml), PHA, Sigma Diagnostics; 10μg/ml), or lipopolysaccharide (LPS, Escherichia coli serotype 055:B5, Sigma Diagnostics; 200ng/ml), in the presence or absence of 5 mM NAC. Cells were incubated for 24 hr at 37°C / 5% CO2. Supernatants were harvested and the cytokine content (except for IL-1β) was measured using commercially available sandwich enzyme-linked immunosorbent assays (ELISA) (R&D Systems, USA), performed according to the manufacturer’s instructions.
Monocytes were isolated by an adherence method as previously described.9 The plastic-adherent cells were cultured for 24 hours in a complete medium containing 100 ng/ml LPS in the presence or absence of 5 mM NAC.
A sandwich ELISA was developed in our laboratory using a method previously described.10 ELISA was performed to determine the production of IL-1β in the supernatant of the cell cultures (PBMC and isolated monocytes).
The SPSS (Statistical Package for the Social Sciences) software programme was used for data analysis. As all the data were normally distributed, pair-wise group (NAC-treated cells and un-treated cells) comparison was carried out using Student’s t-test. P values of less than 0.05 were considered statistically significant.
RESULTS
Incubation of PBMC with 5mM NAC for 24 hours resulted in a two-fold increase in total GSH levels in all treated samples. The range of GSH concentration was 0.56 – 0.85 and 1.2 – 1.5nmol/106 cells before and after treatment with 5mM NAC respectively.
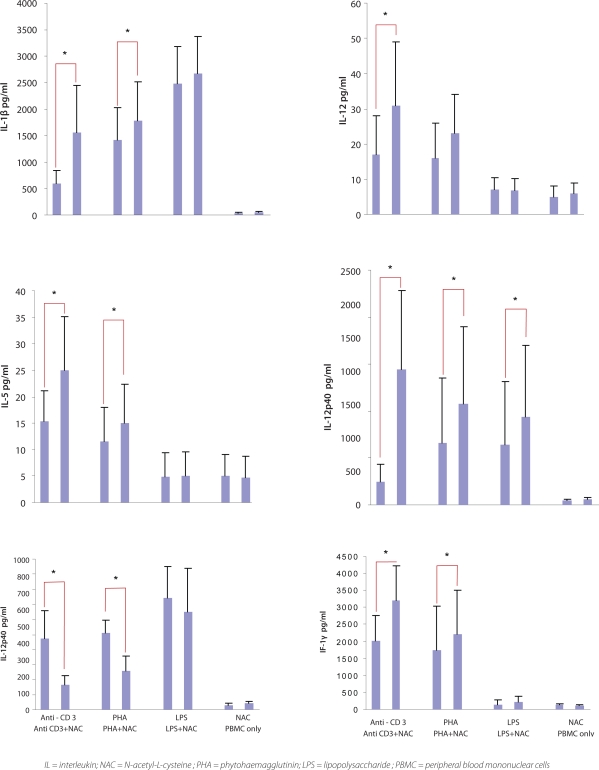
As shown in Figure 1, NAC induced significant secretion of IL-1β after anti-CD3 and PHA stimulation (p <0.05), and statistically insignificant secretion after LPS stimulation. Incubation of plastic-adhered monocytes with NAC in the presence of LPS resulted in increased IL-1β production. Although this increase is not statistically significant, it was a consistent in all five experiments (p value = 0.061).
Figure 1:
N-acetyl-L-cysteine (NAC) modulates cytokine production. Anti-CD3/PHA/LPS-stimulated peripheral blood mononuclear cells (PBMC) were treated with 5 mM NAC for 24 hr. Results are expressed aspg/ml. Data presents the mean value ± S.D. of at least six experiments for six different blood volunteers. * = Significant p value <0.05.
Low concentrations of IL-5 were detected after stimulation of PBMC with anti-CD3 (mean value of 15pg/ml), PHA, (12pg/ml) and LPS (5pg/ml) when compared to other cytokines. In the presence of NAC, significant IL-5 secretion was further increased after anti-CD3 or PHA (but not with LPS) stimulation, this achieved statistical significant (p <0.05) [Figure 1].
NAC had an inhibitory effect on IL-10 secretion by PBMC. In the presence of NAC, a significant decrease (p <0.05) in IL-10 production was observed after anti-CD3, and PHA stimulation, but not after LPS stimulation. IL-10 production, following LPS stimulation in the absence or presence of NAC, was twice the concentration achieved after anti-CD3 or PHA stimulation [Figure 1].
The levels of IL-12 obtained after all three stimulants were low, similar to those of IL-5. However, stimulation with anti-CD3 or PHA induced better IL-12 secretion than with LPS. The addition of NAC caused a significant increase in IL-12 production after anti-CD3 (p <0.05); a slight increase was observed after PHA stimulation, but this was not significant. There was no significant change in Il-12 levels after LPS stimulation [Figure 1].
Unlike total IL-12, stimulation of PBMC with anti-CD3, PHA, or LPS induced substantial amounts of IL-12p40. The presence of NAC with all three stimulants elicited higher levels (p <0.05) of IL-12p40. This was highest after anti-CD3 stimulation [Figure 1].
Stimulation of PBMC with anti-CD3 or PHA (but not LPS), induced high levels of IFN-γ, compared to un-stimulated cells. LPS (100 ng/ml) failed to induce a significant amount of IFN-γ. As shown in Figure 1, exposure of PBMC to NAC elicited a significant upregulation (p <0.05) of IFN-γ after anti-CD3 or PHA stimulation and a slight increase after LPS stimulation.
DISCUSSION
NAC significantly up-regulated IFN-γ, IL-1β, IL-5, IL-12, and IL-12p40, and significantly down-regulated IL-10 production by PBMC after cellular stimulation. A number of studies investigated the effect of both NAC on cytokine production by several cell types including alveolar epithelial cells,4 alveolar macrophages,11 and PBMC.3 Some of our results (increase in IL-1β and decrease in IL-10 production) are in partial agreement with a study carried out Viora et al. which demonstrated a significant up-regulation of IL-1β, IL-2, IL-12 and IL-15 production and an insignificant increase in IL-10 and IFN-γ by PHA-stimulated PBMC in presence of NAC.3 This discrepancy in the production of IL-10, may be related to the genetic variation among individuals, hence, immense differences in response to various stimulations and cytokine production was observed.
T cells and monocytes are major sources of inflammatory mediators and can communicate with each other as well as with other cells12 This communication is mediated by soluble factors and by direct cell-cell contact.12 As traditionally known, potent cytokine polypeptides have pleiotropic activities and functional redundancy, acting in a complex intermingled network where one cytokine can influence the production of, and response to, many other cytokines.13 For example, Th1 cells release IFN-γ, and to a lesser extent IL-2 and IL-12, promoting a proinflammatory response. Th2 cells secrete IL-4, and sometimes IL-5, IL-6, IL-10 and IL-13, and enhance anti-inflammatory responses.14 Therefore, we were not able to prove that the decrease in IL-10 or the increase of other inflammatory cytokines in our study is due to the direct effect of NAC.
Moreover, our data showed that an increase in IL-1β production after anti-CD3 activation, is most likely due to the effect of T lymphocytes activation in this system.
The effects of NAC on cytokine production are still controversial. On the one hand, several studies (including this study), showed that, NAC up-regulated proinflammatory cytokines.3 On the other hand, some studies reported that NAC down regulated pro-inflammatory cytokines such IL-6, TNF-α IL-1β, and IL-8.7,15,16 In addition, Stanislaus et al. reported that NAC treatment blocked induction of TNF-α, IL-1β, and IFN-γ.17 From the above discussion, it appears that, the effect of NAC on cytokine production depends on cell types.
CONCLUSION
NAC up-regulates the production of pro-inflammatory cytokines and down regulates anti-inflammatory cytokines by PBMC, in a process associated with increased levels of GSH. Also this study indicated a side effect of NAC when used as an anti-inflammatory drug. Further studies are required to confirm whether this increase or decrease in cytokine production is due to a direct effect of NAC.
Acknowledgments
We gratefully thank the Medical Research and Ethics Committee (MREC), College of Medicine and Health Sciences, Sultan Qaboos University, for financially supporting this research. We appreciatively thank Dr. Abdullah Al-Maniri and Dr. Shyam S Ganguly for their help in statistical analysis.
Footnotes
CONFLICT OF INTEREST: None declared
SOURCE OF FUNDING: College of Medicine and Health Sciences, Sultan Qaboos University, Grant No.
REFERENCES
- 1.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–27. [PubMed] [Google Scholar]
- 3.Viora M, Quaranta M, Straface E, Vari R, Masella R, Malorni W. Redox imbalance and immune functions: opposite effect of oxidized low-density lipoproteins and N-acetylcysteine. Immunol. 2001;104:431–8. doi: 10.1046/j.1365-2567.2001.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobashi K, Aihara M, Araki T, Shimizu Y, Utsugi M, Iizuka K, et al. Regulation of LPS induced IL-12 production by IFN-γ and IL-4 through intracellular glutathione status in human macrophages. Clin Exp Immunol. 2001;124:290–6. doi: 10.1046/j.1365-2249.2001.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utsugi M, Dobashi K, Koga Y, Shimizu Y, Iizuka K, Hamura J, et al. Glutathione redox regulates lipopolysaccharide-induced IL-12 production through p38 mitogen-activated protein kinase activation in human monocytes: role of glutathione redox in IFN-gamma priming of IL-12 production. J Leukoc Biol. 2002;71:339–47. [PubMed] [Google Scholar]
- 6.Gosset P, Wallaert B, Tonnel AB, Fourneau C. Thiol regulation of the production of TNF-alpha, IL-6 and IL-8 by human alveolar macrophages. Eur Respir J. 1999;14:98–105. doi: 10.1034/j.1399-3003.1999.14a17.x. [DOI] [PubMed] [Google Scholar]
- 7.Haddad JJ. The involvement of L-γ-glutamyl-l-cysteinyl-glycine (glutathione/GSH) in the mechanism of redox signalling mediating MAPKp38-dependent regulation of pro-inflammatory cytokine production. Biochem Pharmacol. 2002;63:305–20. doi: 10.1016/s0006-2952(01)00870-x. [DOI] [PubMed] [Google Scholar]
- 8.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 9.Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol. 2004;172:3399–405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shukaili A, Al-Kaabi J, Hassan B. A comparative study of interleukin-1beta production and P2X7 expression after ATP stimulation by peripheral blood mononuclear cells isolated from rheumatoid arthritis patients and normal healthy controls. Inflammation. 2008;31:84–90. doi: 10.1007/s10753-007-9052-0. [DOI] [PubMed] [Google Scholar]
- 11.Coan C, Hideg K, Mehlhorn RJ. Protein sulfhdryls are protected from irreversible oxidation by conversion to mixed disulfides. Arch Biochem Biophys. 1992;295:369–78. doi: 10.1016/0003-9861(92)90530-a. [DOI] [PubMed] [Google Scholar]
- 12.Burger D, Dayer JM. The role of human T-lymphocytemonocyte contact in inflammation and tissue destruction. Arthritis Res. 2002;4:S169–76. doi: 10.1186/ar558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad JJ. Cytokines and related receptor-mediated signaling pathways. Biochem Biophys Res Commun. 2002;297:700–13. doi: 10.1016/s0006-291x(02)02287-8. [DOI] [PubMed] [Google Scholar]
- 14.Parris K. Th1/Th2 balance: The hypothesis. Its limitations and implication for health and diseases. Alt Med Rev. 2003;8:223–46. [PubMed] [Google Scholar]
- 15.Peristeris P, Clark BD, Gatti S, Faggioni R, Mantovani A, Mengozzi M, et al. N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cell Immunol. 1992;140:390–9. doi: 10.1016/0008-8749(92)90205-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim do Y, Jun JH, Lee HL, Woo KM, Ryoo HM, Kim GS, et al. N-acetylcysteine prevents LPS-induced proinflammatory cytokines and MMP2 production in gingival fibroblasts. Arch Pharm Res. 2007;30:1283–92. doi: 10.1007/BF02980269. [DOI] [PubMed] [Google Scholar]
- 17.Stanislaus R, Gilg AG, Singh AK, Singh I. N-acetyl-L-cysteine ameliorates the inflammatory disease process in experimental autoimmune encephalomyelitis in Lewis rats. J Autoimmune Dis. 2005;2:4–15. doi: 10.1186/1740-2557-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]