Abstract
Magnetic resonace cholangiopancreatography (MRCP) was introduced in 1991, as a non-invasive method of imaging the biliary tree. Although endoscopic cholongiopancreatography (ERCP) has been the mainstay for diagnosing and treating pancreatico-biliary disease, complications such as pancreatitis, cholangitis, haemorrhage and duodenal perforation have limited its use as a routine diagnostic test. Although ERCP is still the standard of reference for imaging the pancreatico-biliary system, MRCP is the examination of choice in a setting where ERCP is difficult or impossible. It is useful in cases with severe biliary obstruction to evaluate the ducts proximal to the obstruction. MRCP has specific advantages over ERCP as it is non-invasive, cheaper, uses no radiation, requires no anaesthesia and is less operator dependent. When combined with conventional T1- and T2-weighted sequences, it allows detection of extraductal disease. The technology is still evolving to make the MRCP examination faster, sharper and with higher spatial resolution.
Keywords: Magnetic resonance imaging, Magnetic resonace cholangiopancreatography, Cholangiography, Biliary ducts, Gallstone, Cholangiocarcinoma
Magnetic resonance cholangiopancreatography (MRCP) was introduced in 1991, as a non-invasive method of imaging the biliary tree. Although endoscopic cholongiopancreatography (ERCP) has been the mainstay for diagnosing and treating pancreatico-biliary disease, complications such as pancreatitis, cholangitis, haemorrhage and duodenal perforation have limited its use as a routine diagnostic test.1 In fact, MRCP is the examination of choice in a setting where ERCP is difficult or impossible. Examples include patients with biliary-enteric anastomosis, gastrojejunostomy, obstructive lesions of the oesophagus and stomach. It is also useful in cases with severe biliary obstruction to evaluate the ducts proximal to the obstruction.2 Although ERCP is still the standard of reference for imaging the pancreatico-biliary system, there are specific advantages of MRCP over ERCP; it is a) is non-invasive; b) cheaper; c) uses no radiation; d) requires no anaesthesia; e) is less operator dependent; f) allows better visualisation of ducts proximal to an obstruction and g) when combined with conventional T1- and T2-weighted sequences, allows detection of extraductal disease.3 The disadvantages of MRCP include: a) decreased spatial resolution, making MRCP less sensitive to abnormalities of the peripheral intrahepatic ducts (e.g. sclerosing cholangitis) and pancreatic ductal side branches (e.g. chronic pancreatitis), and b) imaging in the physiologic, nondistended state, which decreases the sensitivity to subtle ductal abnormalities.3 This article will describe the MRCP technique and spectrum of biliary disorders diagnosed using this examination.
Technique of MRCP
The MRCP technique is based on heavily T2-weighted images which result in a dramatic increase in contrast between stationary fluids (bile) and the background (hepatic and pancreatic parenchyma, abdominal fat). As a result, the bile presents a very high signal intensity compared with low signal intensity background. In addition, no signal comes from flowing blood.4
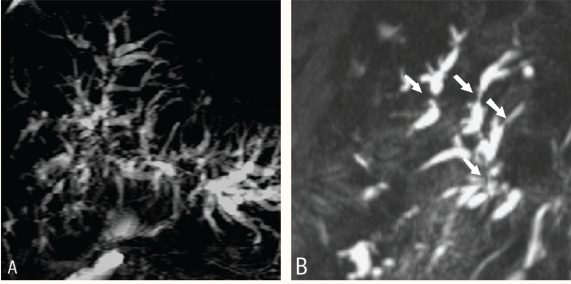
The examination does not require an intravenous contrast agent. It is recommended that patients fast for 3–4 hours before undergoing an MRCP in order to reduce fluid content within the stomach, decrease duodenal peristalsis and promote gall bladder filling. MRCP is performed using breath-hold and non-breath-hold sequences. The breath-hold sequence acquires a single slab of data, between 40 and 80 mm thick, in 1 or 2 seconds. This gives similar projection images to those acquired by ERCP [Figure 1]. Thin slabs (4 mm thick) can also be acquired using breath-hold T2-weighted half Fourier acquisition single-shot turbo spinecho (HASTE) sequences. These are obtained in coronal or oblique coronal views. In addition, the MRCP involves acquiring multiple thin collimation slices, a non-breath-hold, respiratory-triggered 3D turbo spin-echo (TSE) T2-weighted sequence, (1.5 mm) that can be post-processed on an imaging workstation. The commonly used post-processing method is a maximum intensity projection (MIP) algorithm. The source images from a thin collimation multislice acquisition are reviewed in addition to the MIP reconstructions in order to demonstrate small stones or other intraductal pathology that may be obscured by partial volume averaging effects [Figures 2a & 2b].
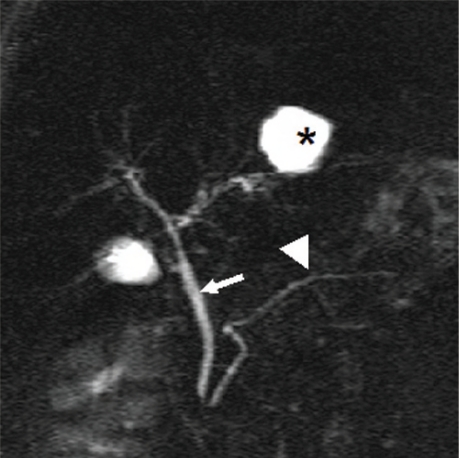
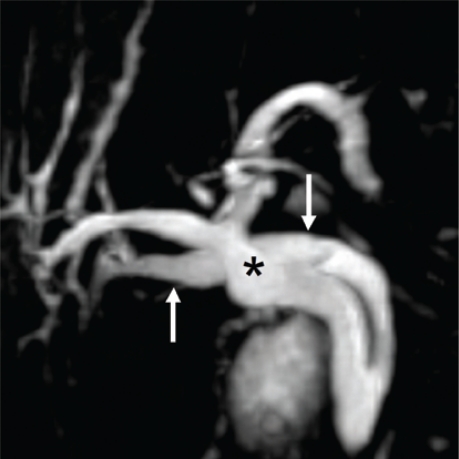
Figure 1:
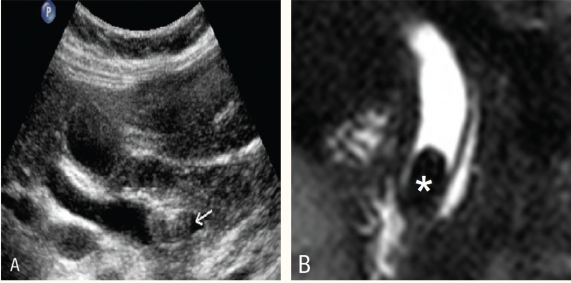
Thick slab image of magnetic resonance cholangiopancreatography RCP examination. The common bile duct (straight arrow) is normal in calibre. The intra-hepatic biliary radicals are not dilated, hence not well visualised. The main pancreatic duct (arrow head) is well delineated. The structure (asterisk) is a hepatic cyst.
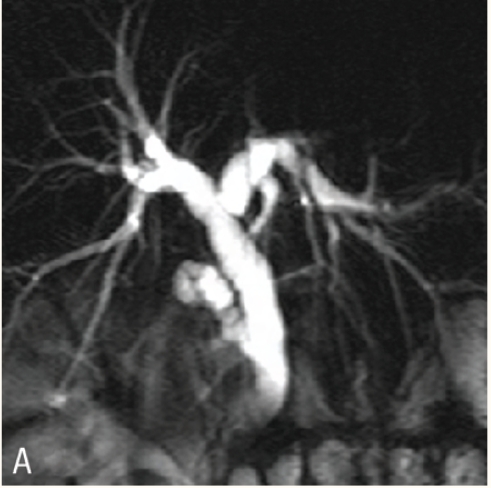
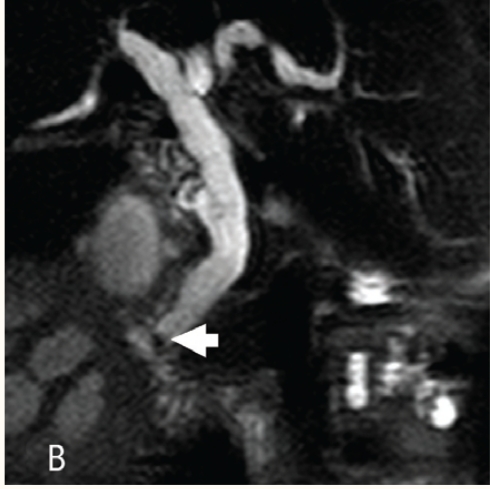
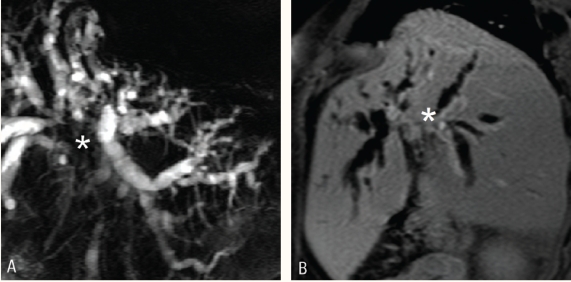
Figure 2a :
Thick slab magnetic resonance cholangiopancreatography shows dilated common hepatic duct and intrahepatic biliary radicals. The cause for the obstruction is not obvious
Figure 2b :
Thin slab magnetic resonance cholangiopancreatography shows a small well defined hypointense lesion in the distal common bile duct (arrow) representing a stone.
Few studies have been conducted exploring the usefulness of doing magnetic resonance (MR) cholangiography using a high field strength of 3 Tesla (T) compared to the commonly used 1.5 T. Compared with MR cholangiography at 1.5 T, MR cholangiography at 3.0 T offers improved contrastto-noise ratio and a higher level of confidence for depicting intrahepatic variants, but without improving image quality significantly. Further development may be achieved with sequence optimisation and improved coil design.5, 6, 7
Enhanced MRCP
There are several MRI contrast agents available on the market which are taken up by hepatocytes and get excreted in the biliary system. Examples of intravenous contrasts with hepatobiliary excretion include mangafodipir trisodium (Teslascan, GE Healthcare Technologies), gadobenate dimeglumine (Gd-BOPTA) (Multihance, Bracco) and gadoliniumethoxybenzyl-diethylene-triamine-pentaacetic acid (Gd-EOB-DTPA) (Primovist, Schering-AG). Three dimensional T1 weighted images are acquired 10–60 minutes after administration of the contrast. This method is good for delineating the biliary anatomy prior to major hepatic surgery or liver donation, assessing the integrity of the bile ducts and differentiating true obstruction from pseudoobstruction.
Secretin Enhanced Dynamic MRCP (Functional MRCP)
The exogenous administration of secretin stimulates the secretion of fluid and bicarbonate by the exocrine pancreas and increases the tone of the sphincter of Oddi. Consequently, the volume of stationary fluid in the pancreatic duct increases and its delineation may be improved at MRCP. After the intravenous administration of 1ml of secretin per 10kg body weight, thick slab MRCP in the coronal plane is performed and repeated every 15–30 seconds for 10–15 minutes. Secretin allows better delineation of the full length of the pancreatic duct and reduces the frequency of false-positive readings of duct strictures as well as better evaluation of the sphincter anatomy and detection of anatomic variants such as pancreas divisum. It depicts the progressive filling of the duodenum with pancreatic fluid assessing indirectly the pancreatic exocrine reserve.8,9
Clinical Applications
IMAGING OF BILIARY CONGENITAL ANOMALIES AND ANATOMIC VARIANTS
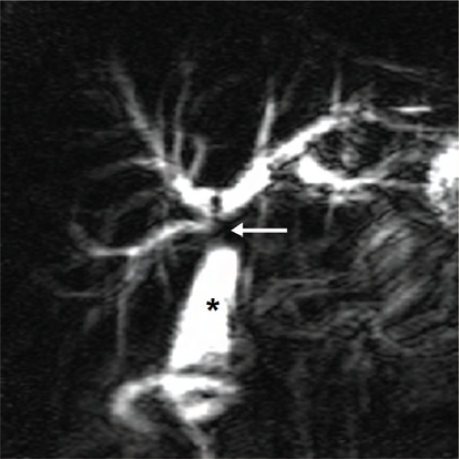
The two major congenital anomalies of the biliary tree include an anomalous pancreaticobiliary junction (APBJ) and congenital biliary cystic disease. Choledochal cysts are congenital anomalies of the bile ducts. They consist of cystic or fusiform dilatations of the extrahepatic biliary tree, intrahepatic biliary radicles, or both. These are classified into five major types using the Todani classification.8 Type 1 is the most common (80–90% of cases) and consists of dilatations of the entire common hepatic and common bile ducts or of segments of each. Complications arising from the cysts include cholelithiasis, choledocholithiasis, carcinoma, pancreatitis, cholangitis, and cyst rupture. MRCP is used to define the extent of the cyst, determine the presence of an anomalous pancreaticobiliary junction (APBJ) and detect associated complications. The MRCP provides information equivalent to that provided with ERCP, but without potential complications, for the preoperative assessment of choledochal cysts.9 An APBJ is an uncommon entity in which the common bile duct and main pancreatic duct are joined outside the duodenal wall with the common channel being greater than 1.5cm. It predisposes patients to choledochal cysts, cholangitis, stones and pancreatitis. It can be associated with biliary tract malignancy in up to one third of the affected individuals. MRCP has been reported to have a sensitivity of approximately 75% and specificity of 100% in the detection of APBJ 10 [Figure 3].
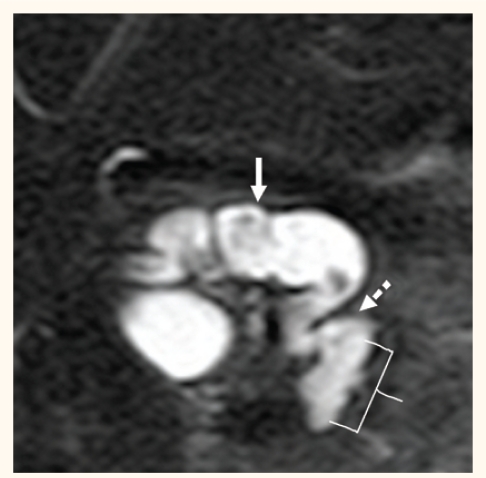
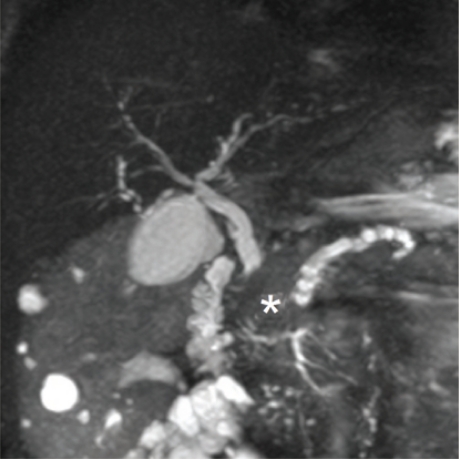
Figure 3:
Choledochal cyst. Maximum intensity projection image showing tortuous dilated common bile duct (solid arrow) without associated intrahepatic biliary ducts dilatation. Note the distal part of the pancreatic duct (dashed arrow). The common biliarypancreatic channel (brace) is elongated and dilated in keeping with anomalous pancreaticobiliary junction.
Variations from the commonly described anatomic pattern of the biliary tree occur in more than 50% of individuals.11 MRCP has been shown to be 98% accurate in diagnosis of aberrant hepatic ducts and 95% accurate in diagnosis of cystic duct variants.12 By demonstrating aberrant anatomy before surgery, the risk of bile duct injury should be reduced, especially during laparoscopic cholecystectomy, which is associated with double the risk of bile duct injury compared with that of open cholecystectomy.13
Anatomic variants with a high potential for injury include an aberrant right hepatic duct with insertion into the common hepatic duct or cystic duct, a long intramural cystic duct parallel to the common hepatic duct, or a cystic duct inserting medially on the common bile duct [Figure 4].
Figure 4:
Aberrant right hepatic duct insertion. Oblique coronal maximum intensity projection image showing the posterior right hepatic duct (solid arrows) coursing posterior to the common hepatic duct (asterisk) and joining the distal portion of the common bile duct.
EVALUATION OF BILIARY DUCT OBSTRUCTIONS
Various studies have shown the sensitivity of MR cholangiography for the detection of focal strictures affecting the bile ducts to be approximately 95%.14,15 To help differentiate between benign and malignant causes of biliary strictures and dilatation, principles that apply to conventional cholangiography may also be applied to MR cholangiography. Malignant lesions usually manifest as irregular strictures with shouldered margins, whereas benign stenosis tends to have smooth borders with tapered margins. However, differentiation may be difficult with MR cholangiography and often depends on discovering a mass or tumour associated with the stricture at cross-sectional T1- or T2-weighted MR imaging, a finding that indicates a malignant cause. Despite this limitation, MR cholangiography helps accurately determine the status of the biliary ductal system in patients with malignant obstruction by identifying the exact site of the obstruction and the length of the stricture. In this way, MR cholangiography can help determine whether a patient should undergo percutaneous transhepatic cholangiography with an antegrade stent placement or retrograde intervention. The unnecessary risks associated with multiple invasive procedures are thereby avoided.16
Malignant obstruction occurring at the porta hepatis is usually secondary to cholangiocarcinoma, metastatic disease of the liver or periportal lymph nodes, invasive hepatocellular carcinoma, or invasive gall bladder carcinoma.17 The extrahepatic suprapancreatic biliary tract may be obstructed by lesions such as lymphadenopathy or by direct extension of malignancies arising in adjacent organs (e.g. gall bladder, pancreas, stomach, colon). Neoplastic obstruction of the intrapancreatic portion of the common bile duct may be caused by carcinoma of the head of the pancreas, cholangiocarcinoma, or ampullary carcinoma.
CHOLANGIOCARCINOMA
Most cholangiocarcinomas are ductal adenocarcinomas that arise from both the intraand extrahepatic bile duct epithelium, and their typical growth pattern can be classified as exophytic, infiltrative, polypoid, or a combination of these.18 The Hilar cholangiocarcinoma (Klatskin tumour) is usually a small lesion and difficult to detect with ultrasonography or computed tomography (CT). MR imaging has been shown to be useful in delineating the size and extent of these tumours. MR cholangiography is useful in depicting the severity of intrahepatic duct dilatation as well as the site and extent of the stricture. Scirrhous tumours tend to demonstrate low signal intensity centrally and variable high signal intensity peripherally, whereas well-differentiated cholangiocarcinomas may exhibit higher signal intensity on T2-weighted images. In general, criteria of unresectability include: 1) bilateral intrahepatic bile duct spread to secondary or segmental biliary radicals; 2) involvement of the main trunk of the portal vein (except in unusual circumstances); 3) bilobar involvement of hepatic arterial and/or portal venous branches; 4) a combination of unilateral hepatic arterial involvement with cholangiographic evidence of extensive contralateral duct spread.19,20
Other abnormalities that may be seen in cholangiocarcinoma (e.g. satellite lesions in the liver, regional lymphadenopathy to pancreaticoduodenal and portocaval nodes, intraductal tumour growth, peritoneal tumour spread) are also well demonstrated with MR imaging and MR cholangiography. Gadolinium contrast material can help define the margins of the tumour [Figure 5]. Enhancement is variable, but often starts at the periphery of the lesion and progresses toward the centre.21,22
Figure 5 :
Cholangiocarcinoma (Klatskin tumor). A) magnetic resonance cholangiopancreatography using coronal thick slab technique demonstrating moderate intrahepatic biliary ducts dilatation due presence of T2 hypointense mass lesion (asterisk) at the junction of the right and left hepatic biliary ducts. B) The hilar mass shows modest enhancement after administration of intravenous gadolinium (asterisk).
INVASIVE GALL BLADDER CANCER
Gall bladder carcinomas can manifest as a polypoid mass with an intraluminal component, a bulky exophytic mass, or a mass infiltrating liver parenchyma and occupying the gall bladder lumen2 [Figure 6]. Biliary obstruction may result from direct extension of the tumour to the porta hepatic or from compression of the extrahepatic bile ducts by enlarged lymph nodes. Biliary stones, which are present in approximately 75% of patients with gall bladder carcinoma, can also be demonstrated with MR imaging. A gall bladder carcinoma may arise in a porcelain gall bladder, a premalignant condition characterised by diffuse calcification of the gall bladder wall.
Figure 6:
Invasive gallbladder cancer. Oblique coronal (A) and axial (B) T2 weighted images show mildly T2 hyper intense mass lesion (asterisk) arising from the gallbladder and invading the adjacent liver. The tumor extends to the liver
PANCREATIC CANCER
Biliary obstruction in the intrapancreatic segment of the common bile duct may be caused by a pancreatic carcinoma, an ampullary carcinoma, or pancreatitis. Differentiation between benign and malignant causes of distal obstruction is difficult with cross-sectional imaging and usually depends on finding a mass associated with the stricture. Irregular or “rat-tail” stenoses are more suggestive of carcinoma than of pancreatitis. A well-known cholangiopancreatographic sign, which can be seen in patients with carcinoma of the head of the pancreas, is the “double duct sign,” which consists of dilatation of the common bile duct and pancreatic duct with biductal strictures in the head of the gland [Figure 7]. Tumours within the head of the pancreas can invade the distal common bile duct simulating strictures caused by cholangiocarcinoma.
Figure 7:
Double duct sign. Thick slab of magnetic resonance cholangiopancreatography study showing dilated common bile duct and main pancreatic duct due presence of pancreatic head mass (*). The mass was proven to be a metastasis from renal cell carcinoma.
CHOLEDOCHOLETHIASIS
Choledocholithiasis accounts for most cases of biliary obstruction and is an important diagnosis in the setting of laproscopic cholecystectomy. Patients who have symptomatic cholelithiasis, acute cholecystitis complicated by jaundice, cholangitis, gall stone pancreatitis or a common bile diameter greater than 6–7mm on sonography are considered at high risk of choledocholithiasis. Patients with choledochlithiasis benefit from ERCP-guided sphincterotomy and stone extraction prior to laprosopic cholecystectomy.23,24
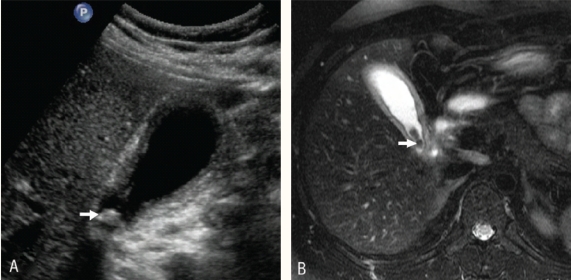
MRCP has a sensitivity of 81% to 93% and specificity of 91% to 95% in the evaluation of common bile stones.25,26,27 It has comparable sensitivity and specificity to ERCP for the evaluation of common bile duct stones. On MRCP, calculi appear as foci of low signal intensity irrespective of their composition [Figure 8]. A combination of thick slab MRCP technique and thin section multislice images increases the sensitivity for detection of large as well as small (1–4mm) stones.28,29
Figure 8 :
Common bile duct stone. A) Ultrasound image showing large stone (arrow) in the distal common bile duct (CBD). The CBD is dilated. B) Coronal magnetic resonance cholangiopancreatography image through the CBD showing impacted stone (asterisk) in the distal duct.
The differential diagnosis for a filling defect in the biliary tree includes calculus, neoplasm, blood clot, air bubble or sludge. Stones have round, oval or angular shape and are located in the dependent part of the bile duct.
BILIARY ENTERIC ANASTOMOSIS
Evaluation of the biliary-enteric anastomosis is difficult due to altered bowel anatomy beyond the level of the duodenum. Long term complications of biliary enteric anastomosis include recurrent obstruction secondary to anastomotic stenosis/stricture, colangitis, intrahepatic stones and dilated bile ducts.16 MRCP can show the site of the anastomosis, status of the intrahepatic ducts, stones and strictures. Thin section source images are the best to depict the site of the anastomosis [Figure 9].
Figure 9:
Biliary-enteric anastomosis stricture: there is a stricture (straight arrow) at the site of the anastomosis between common hepatic duct and jejunum (asterisk) resulting in biliary obstruction.
ACUTE CHOLECYSTITIS
Acute cholecystitis usually results from obstruction of the cystic duct or gall bladder neck. In a patient with suspected acute cholecystitis, ultrasound (US) and/or computed tomography imaging are usually the primary imaging procedure of choice. However, it is often difficult to demonstrate a stone impacted in the cystic duct or gall bladder neck. MR imaging has a higher sensitivity than US for diagnosis of acute cholecystitis.28 MR imaging findings of acute uncomplicated cholecystitis include: a) gallstones, often impacted in the gall bladder neck or cystic duct; b) gall bladder wall thickening (> 3 mm); c) gall-bladder wall oedema; d) gall bladder distention (diameter > 40 mm); e) pericholecystic fluid and f) fluid around the liver, termed the “C sign” (small amount of fluid between the liver and the right hemidiaphragm or the abdominal wall, different from pericholecystic fluid). The presence of one or more of the six criteria is indicative of acute cholecystitis, yielding a sensitivity of 88% and specificity of 89% [Figure 10].
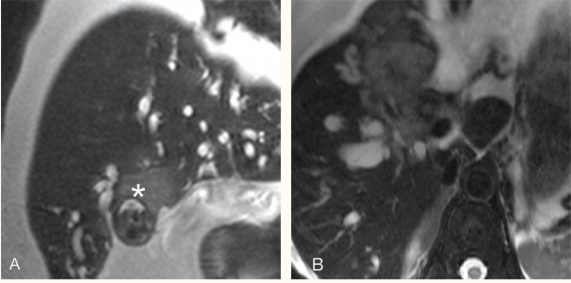
Figure 10 :
Acute cholecystitis: ultrasound image (A) and axial magnetic resonance cholangiopancreatography image (B) through the gallbladder show distended gallbladder. The gallbladder wall is thickened and small stones (white arrows) are seen at the neck of the gallbladder.
POST-SURGICAL COMPLICATIONS
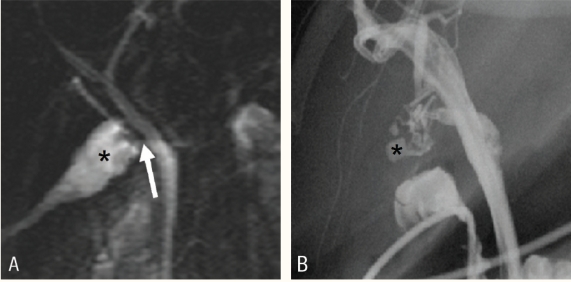
Post-operative bile duct injury can be classified as a leak, stricture, or complete transaction with possible biliary obstruction. Pertinent findings to assess on MRCP include the presence or absence of biliary duct dilatation, stricture, free fluid, fluid collection or non-visualisation of a bile duct segment that may suggest injury 30 [Figure 11].
Figure 11 :
Biliary leak. A) Coronal thin slab of magnetic resonance cholangiopancreatography in a patient who underwent laproscopic cholecystectomy showing fluid collection (asterisk) surrounding the cystic duct stump (arrow). This was suggestive of biliary leak. B) Cholangiogram performed ùsing naso-biliary tube showed small loculated bile leak (asterisk)) from the cystic duct.
POST LIVER TRANSPLANTATION
Despite recent improvements in orthotropic liver transplantation (OLT), largely due to more efficient immunosuppressants, graft preservation solutions and advanced surgical techniques, biliary tract complications remain a frequent cause of morbidity. The biliary tract complications include leak, obstruction, stone formation and strictures.31 Anastomotic biliary leaks and bilomas are common complications in the first 30 days following transplantation. Biliary obstruction is the second most common cause of liver dysfunction after rejection and is usually secondary to a stricture.32 Non-anastomotic strictures are usually due to ischaemia related biliary changes such as those occurring with hepatic artery occlusion. The T tube or plastic biliary stent does not result in artefacts on MRCP.
MRCP can provide equivalent imaging to ERCP and can reliably identify and quantitatively evaluate biliary strictures in post-OLT patients [Figure 12]. MRCP was found in this case to have 87.5–100% sensitivity and 87.5–92.3% specificity.32, 33, 34
Figure 12 :
Post liver transplantation. A) Coronal thick slab and B)Thin 3D sections of magnetic resonance cholangiopancreatography showing multiple short strictures of the intrahepatic biliary ducts (straight arrows).
MRCP can depict the level and degree of obstruction of the ducts below and above the obstruction. Intrahepatic ducts greater than 2mm and extrahepatic ducts greater than 7mm are considered dilated.
PRIMARY SCLEROSING CHOLANGITIS
Primary sclerosing cholangitis (PSC) is an idiopathic, chronic, fibrosing inflammatory disease of the bile ducts that eventually leads to bile duct obliteration, cholestasis, and biliary cirrhosis.35 It is an autoimmune disease and there is a strong association with inflammatory bowel disease, especially ulcerative colitis (70% of cases). Almost 49% of symptomatic patients eventually develop biliary cirrhosis and liver failure. OLT is the only curative therapy for PSC. ERCP is currently the gold standard for diagnosing primary sclerosing cholangitis. ERCP findings usually include multifocal, intrahepatic bile duct strictures alternating with normal calibre ducts, which sometimes produce a beaded appearance. Before the diagnosis of PSC is established, secondary sclerosing and non-sclerosing processes that mimic PSC at cholangiography must be excluded. These include chronic bacterial cholangitis complicating strictures or stones, parasitic infection of the bile, cholangitis related to acquired immunodeficiency syndrome (AIDS), ischaemia due to treatment with floxuridine or hepatic arterial thrombosis complicating liver transplantation, neoplasms including cholangiocarcinoma, metastases, previous bile duct surgery, and congenital biliary anomalies. As the fibrosing process worsens, strictures increase and the ducts become obliterated, and the peripheral ducts cannot be visualised to the periphery of the liver at ERCP, producing a “pruned tree” appearance.
The key cholangiographic features of PSC are randomly distributed annular strictures out of proportion to upstream dilatation. At MRCP, the presence of stenoses is usually inferred when there is prestenotic dilatation.36 Slightly dilated peripheral bile ducts unconnected to the central ducts in several hepatic segments are a characteristic MR sign of primary sclerosing cholangitis.37 Strictures usually occur at the bifurcation of ducts and are out of proportion to upstream ductal dilatation. The peripheral ducts should extend to the periphery of the liver and form acute angles with the central ducts.36,37,38,39
In patients with primary sclerosing cholangitis, MR cholangiopancreatography better shows the bile ducts and can depict more strictures, especially of the peripheral intrahepatic ducts, than ERCP. MR cholangiopancreatography can be used to noninvasively diagnose and follow up patients with primary sclerosing cholangitis.38
Fulcher et al.34 showed that MR cholangiopancreatography had a sensitivity of 85–88% and a specificity of 92–99% in the detection of primary sclerosing cholangitis. Interobserver agreement was excellent (κ = 0.79). The sensitivity and specificity of MR cholangiopancreatography for localising extrahepatic primary sclerosing cholangitis were 83–89% and 83% respectively, and the sensitivity of MR cholangiopancreatography for localising intrahepatic primary sclerosing cholangitis was 83%. Vitellas et al.40 concluded that thick-slab MR cholangiopancreatography is the best technique for depicting normal and strictured bile ducts and allows the differentiation of healthy patients from patients with sclerosing cholangitis. Although endoscopic retrograde cholangiopancreatography was considered the standard, MR cholangiopancreatography was superior for intrahepatic biliary ductal visualisation. Therefore, this technique is of value in the diagnosis and follow-up of patients with sclerosing cholangitis. Thick-slab MR cholangiopancreatography showed good visualisation in more ducts than contrast cholangiography (84% versus 70%; p = 0.10) and showed more strictured ducts than contrast cholangiography (47% versus 36%)
LIMITATIONS OF THE TECHNIQUE
Some of the disadvantages of MRCP include decreased spatial resolution making it less sensitive to abnormalities of the peripheral intrahepatic ducts (e.g. sclerosing cholangitis) and pancreatic ductal side branches (e.g. chronic pancreatitis) and imaging in the physiologic, nondistended state, which decreases the sensitivity to subtle ductal abnormalities. Some of the limitations of the procedure include inability to image patients with pacemakers or claustrophobic patients.
Conclusion
MRCP has important roles in the non-invasive evaluation of the biliary system and should be considered in patients suspected to have cholangiopathies or biliary obstruction. This examination is particularly useful in Oman due to a shortage of ERCP facilities and increased number of MR scanners in the country.
References
- 1.Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 2.Varghese JC, Farrell MA, Courtney G, Osborne H, Murray FE, Lee MJ. Role of MR cholangiopancreatography in patients with failed or inadequate ERCP. AJR Am J Roentgenol. 1999;173:1527–33. doi: 10.2214/ajr.173.6.10584796. [DOI] [PubMed] [Google Scholar]
- 3.Vitellas KM, Keogan MT, Spritzer CE, Nelson RC. MR Cholangiopancreatography of bile and pancreatic duct abnormalities with emphasis on the single-shot fast spin-echo technique. Radiographics. 2000;20:939–57. doi: 10.1148/radiographics.20.4.g00jl23939. [DOI] [PubMed] [Google Scholar]
- 4.Pavone P, Laghi A, Panebianco V, Catalano C, Brillo R, Assael F, et al. Diagnosis of diseases of biliary and pancreatic ducts with magnetic resonance cholangio pancreatography (MRCP) Saudi J Gastroenterol. 1998;4:67–75. [PubMed] [Google Scholar]
- 5.O’Regan DP, Fitzgerald J, Allsop J, Gibson D, Larkman DJ, Cokkinos D, et al. A comparison of MR cholangiopancreatography at 1.5 and 3.0 Tesla. Br J Radiol. 2005;78:894–8. doi: 10.1259/bjr/28094700. [DOI] [PubMed] [Google Scholar]
- 6.Merkle EM, Haugan PA, Thomas J, Jaffe TA, Gullotto C. 3.0- versus 1.5-T MR cholangiography: a pilot study. AJR Am J Roentgenol. 2006;186:516–21. doi: 10.2214/AJR.04.1484. [DOI] [PubMed] [Google Scholar]
- 7.Schindera ST, Miller CM, Ho LM, DeLong DM, Merkle EM. Magnetic resonance (MR) cholangiography: quantitative and qualitative comparison 3 Tesla with1.5 tesla. Invest Radiol. 2007;42:399–405. doi: 10.1097/01.rli.0000261940.98762.20. [DOI] [PubMed] [Google Scholar]
- 8.Lee NJ, Kim KW, Kim TK, Kim MH, Kim SY, Park MS, et al. Secretin-stimulated MRCP. Abdom Imaging. 2006;31:575–81. doi: 10.1007/s00261-005-0118-x. [DOI] [PubMed] [Google Scholar]
- 9.Akisik MF, Sandrasegaran K, Aisen AA, Maglinte DDT, Sherman S, Lehman GA. Dynamic secretinenhanced MR cholangiopancreatography. Radiographics. 2006;26:665–77. doi: 10.1148/rg.263055077. [DOI] [PubMed] [Google Scholar]
- 10.Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–9. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 11.Matos C, Nicaise N, Deviere J, Cassart M, Metens T, Struyven J, et al. Choledochal cysts: comparison of findings at MR cholangiopancreatography and endoscopic retrograde cholangiopancreatography in eight patients. Radiology. 1998;209:443–8. doi: 10.1148/radiology.209.2.9807571. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama M, Baba M, Atomi Y, Hanaoka H, Mizutani Y, Hachiya J. Diagnosis of anomalous pancreaticobiliary junction: Value of magnetic resonance cholangiopancreatography. Surgery. 1998;123:391–7. [PubMed] [Google Scholar]
- 13.Northover JMA, Terblanche J. Applied surgical anatomy of the biliary tree. Clin Surg Int. 1982;5:1–16. [Google Scholar]
- 14.Taourel P, Bret PM, Reinhold C, Barkun AN, Atri M. Anatomic variants of the biliary tree: diagnosis with MR cholangiopancreatography. Radiology. 1996;199:521–7. doi: 10.1148/radiology.199.2.8668805. [DOI] [PubMed] [Google Scholar]
- 15.Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9–14. doi: 10.1016/s0002-9610(05)80397-6. [DOI] [PubMed] [Google Scholar]
- 16.Hall-Craggs M, Allen C, Owens C, Theis BA, Donald JJ, Paley M, et al. MR cholangiography: clinical evaluation in 40 cases. Radiology. 1993;189:423–7. doi: 10.1148/radiology.189.2.8210370. [DOI] [PubMed] [Google Scholar]
- 17.Soto JA, Barish MA, Yucel EK, Siegenberg D, Ferrucci JT, Chuttani R. Magnetic resonance cholangiography: comparison to endoscopic retrograde cholangiopancreatography. Gastroenterology. 1996;110:589–97. doi: 10.1053/gast.1996.v110.pm8566608. [DOI] [PubMed] [Google Scholar]
- 18.Soto JA, Alvarez O, Lopera JR, Múnera F, Restrepo JC, Correa G. Biliary Obstruction: Findings at MR cholangiography and cross-sectional MR imaging. Radiographics. 2000;20:353–66. doi: 10.1148/radiographics.20.2.g00mc06353. [DOI] [PubMed] [Google Scholar]
- 19.Brink JA, Borrello JA. MR imaging of the biliary system. Magn Reson Imaging Clin N Am. 1995;3:143–60. [PubMed] [Google Scholar]
- 20.Parikh AA, Abdalla EK, Vauthey JN. Operative considerations in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7:254–8. doi: 10.1080/13651820500373093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WJ, Lim HK, Jang KM, Kim SH, Lee SJ, Lim JH, et al. Radiologic spectrum of cholangiocarcinoma: Emphasis on unusual manifestations and differential diagnoses. Radiographics. 2001;21:S97–S116. doi: 10.1148/radiographics.21.suppl_1.g01oc12s97. [DOI] [PubMed] [Google Scholar]
- 22.Fulcher AS, Turner MA. HASTE MR cholangiography in the evaluation of Hilar cholangiocarcinoma. AJR Am J Roentgenol. 1997;169:1501–05. doi: 10.2214/ajr.169.6.9393153. [DOI] [PubMed] [Google Scholar]
- 23.Soyer P, Bluemke DA, Reichle R, Calhoun PS, Bliss DF, Scherrer A, et al. Imaging of intrahepatic cholangiocarcinoma: Peripheral cholangiocarcinoma. AJR Am J Roentgenol. 1995;165:1427–31. doi: 10.2214/ajr.165.6.7484579. [DOI] [PubMed] [Google Scholar]
- 24.Soyer P, Gouhiri MH, Boudiaf M, Brocheriou-Spelle I, Kardache M, Fishman EK, et al. Carcinoma of the gall bladder: imaging features with surgical correlation. AJR Am J Roentgenol. 1997;169:781–5. doi: 10.2214/ajr.169.3.9275896. [DOI] [PubMed] [Google Scholar]
- 25.Liu TH, Consorti ET, Kawashima A, Ernst RD, Black CT, Greger PH, et al. The efficacy of magnetic resonance cholangiography for the evaluation of patients with suspected choledocholithiasis before laparoscopic cholecystectomy. Am J Surg. 1999;178:480–4. doi: 10.1016/s0002-9610(99)00224-x. [DOI] [PubMed] [Google Scholar]
- 26.Dwerryhouse SJ, Brown E, Vipond MN. Prospective evaluation of magnetic resonance cholangiography to detect common bile duct stones before laparoscopic cholecystectomy. Br J Surg. 1998;85:1364–6. doi: 10.1046/j.1365-2168.1998.00957.x. [DOI] [PubMed] [Google Scholar]
- 27.Aubé C, Delorme B, Yzet T, Burtin P, Lebigot J, Pessaux P, Gondry-Jouet C, Boyer J. MR cholangiopancreatography versus endoscopic sonography in suspected common bile duct lithiasis: a prospective, comparative study. AJR Am J Roentgenol. 2005;184:55–62. doi: 10.2214/ajr.184.1.01840055. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt S, Chevallier P, Novellas S, Gelsi E, Vanbiervliet G, Tran A, et al. Choledocholithiasis: repetitive thick-slab single-shot projection magnetic resonance cholangiopancreaticography versus endoscopic ultrasonography. Eur Radiol. 2007;17:241–50. doi: 10.1007/s00330-006-0380-5. [DOI] [PubMed] [Google Scholar]
- 29.Reinhold C, Taourel P, Bret PM, Cortas GA, Mehta SN, Barkun AN, et al. Choledocholithiasis: evaluation of MR cholangiography for diagnosis. Radiology. 1998;209:435–42. doi: 10.1148/radiology.209.2.9807570. [DOI] [PubMed] [Google Scholar]
- 30.Park MS, Yu JS, Kim YH, Kim MJ, Kim JH, Lee S, et al. Acute cholecystitis: comparison of MR cholangiography and US. Radiology. 1998;209:781–5. doi: 10.1148/radiology.209.3.9844674. [DOI] [PubMed] [Google Scholar]
- 31.Hakansson K, Leander P, Ekberg O, Hakansson HO. MR imaging in clinically suspected acute cholecystitis: a comparison with ultrasonography. Acta Radiol. 2000;41:322–8. doi: 10.1080/028418500127345587. [DOI] [PubMed] [Google Scholar]
- 32.Khalid TR, Casillas VJ, Montalvo BM, Centeno R, Levi JU. Using MR Cholangiopancreatography to Evaluate Iatrogenic Bile Duct Injury. AJR Am J Roentgenol. 2001;177:1347–52. doi: 10.2214/ajr.177.6.1771347. [DOI] [PubMed] [Google Scholar]
- 33.Linhares MM, Gonzalez AM, Goldman SM, Coelho RD, Sato NY, Moura RM, et al. Magnetic resonance cholangiography in the diagnosis of biliary complications after orthotopic liver transplantation. Transplant Proc. 2004;36:947–8. doi: 10.1016/j.transproceed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Fulcher AS, Turner MA. Orthotopic liver transplantation: Evaluation with MR cholangiography. Radiology. 1999;211:715–22. doi: 10.1148/radiology.211.3.r99jn17715. [DOI] [PubMed] [Google Scholar]
- 35.Kitazono MT, Qayyum A, Yeh BM, Chard PS, Ostroff JW, Coakley FV. Magnetic resonance cholangiography of biliary strictures after liver transplantation: a prospective double-blind study. Magn Reson Imaging. 2007;25:1168–73. doi: 10.1002/jmri.20927. [DOI] [PubMed] [Google Scholar]
- 36.Ward J, Sheridan MB, Guthrie JA, Davies MH, Millson CE, Lodge JPA, et al. Bile duct strictures after hepatobiliary surgery: Assessment with MR cholangiography. Radiology. 2004;231:101–8. doi: 10.1148/radiol.2311030017. [DOI] [PubMed] [Google Scholar]
- 37.Vitellas KM, Keogan MT, Freed KS, Enns RA, Spritzer CE, Baillie JM, et al. Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiographics. 2000;20:959–75. doi: 10.1148/radiographics.20.4.g00jl04959. [DOI] [PubMed] [Google Scholar]
- 38.Schuster DM, Pedrosa MC, Robbins AH. Magnetic resonance cholangiography. Abdom Imaging. 1995;20:353–6. doi: 10.1007/BF00203370. [DOI] [PubMed] [Google Scholar]
- 39.Ernst O, Asselah T, Sergent G, Calvo M, Talbodec N, Paris JC, et al. MR cholangiography in primary sclerosing cholangitis. AJR Am J Roentgenol. 1998;171:1027–30. doi: 10.2214/ajr.171.4.9762990. [DOI] [PubMed] [Google Scholar]
- 40.Vitellas KM, Enns RA, Keogan MT, Freed KS, Spritzer CE, Baillie J. Comparison of MR cholangiopancreatographic techniques with contrast-enhanced cholangiography in the evaluation of sclerosing cholangitis. AJR Am J Roentgenol. 2002;178:327–34. doi: 10.2214/ajr.178.2.1780327. [DOI] [PubMed] [Google Scholar]