Abstract
Amiodarone is an iodine rich agent widely used for the treatment of ventricular arrhythmias, paroxysmal supraventricular tachycardia, atrial fibrillation and flutter. However 14–18% of patients treated with amiodarone develop overt thyroid dysfunction in the form of either amiodarone-induced thyrotoxicosis (AIT) or amiodaroneinduced hypothyroidism (AIH). Two different types of AIT have been recognised and designated as Type 1 and Type 2. Distinguishing between the two is often difficult, but necessary for instituting appropriate treatment. We report a case of a 56 year-old male patient who was started on amiodarone for atrial fibrillation and then developed AIT. The challenges in the diagnosis and management of these patients are discussed.
Keywords: Amiodarone, Thyroid, Thyrotoxicosis, Apathetic hyperthyroidism, Arrhythmias, Case Report
Amiodarone is an iodinated benzofuran derivative widely used in the treatment of a variety of arrhythmias. However, in patients on prolonged treatment with this agent, a variety of thyroid abnormalities are observed primarily resulting from an inhibition of 5 deiodinase activity. The complex effects of this drug on the thyroid range from subclinical abnormalities in thyroid function to overt hypo or hyper function of the thyroid. Amiodarone-induced thyrotoxicosis (AIT) develops in 3% of patients treated with amiodarone and 10% of those in iodine deficient areas. The different mechanisms leading to development of AIT results in two types of thyrotoxicosis labelled as Type 1 and Type 2. Differentiation of the types can be achieved using a combination of clinical, laboratory and radiological means and is important in deciding the appropriate treatment for the patient.
Case Report
A 56 year-old male patient presented to the outpatient service of Armidale Rural Referral Hospital, Australia, with complaints of extreme lethargy, tiredness and a lack of initiative experienced over the preceding three months. In 2001, the patient had been diagnosed as having atrial fibrillation and was on a maintenance dose of amiodarone 200mg daily. Clinical examination revealed him to be in atrial fibrillation with a heart rate of 90/minute. There was mild enlargement of the thyroid and minimal exophthalmos. There was no tenderness of the thyroid on palpation. On suspicion of amiodarone-induced thyroid dysfunction, an urgent thyroid function test was done which revealed surprisingly hyperthyroidism. It was decided to admit the patient with a provisional diagnosis of amiodarone-induced thyrotoxicosis for further workup.
The patient underwent a full evaluation which included a repeat thyroid function test, inflammatory markers, thyroid autoantibodies and radiological evaluation of the thyroid using an ultrasound and nuclear scan study [Table 1].
Table 1:
Laboratory values at the time of admission
| Full blood count | Inflammatory markers | Thyroid function tests | Renal function tests | Thyroid antibodies |
|---|---|---|---|---|
| Hb - 12.7mg/dl (13–15) | ESR - 40mm/hr | TSH - 0.06mU/L(0.30–4.50) | Blood urea - 9 mmol/L (3.6–8.4) | Anti-thyro globulin- Negative |
| Total count - 7900 cells/mm3(4–11000) | CRP - 30 mg/L | Free T4 - 67.4 pmol/L (7–17) | Creatinine – 119 mmol/L (60–120) | Anti-microsomal antibody- Negative |
| Platelet - 280000 cells/mm3 (1.5–450000) | Free T3 - 7.6pmol/L (3.8–6) | Sodium - 140 mmol/L (136–144) | Anti-thyroid peroxidise antibody- Negative | |
| Potassium 3.5 mmol/L (3.5–5) |
Legend: ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; TSH = thyroid stimulating hormone;
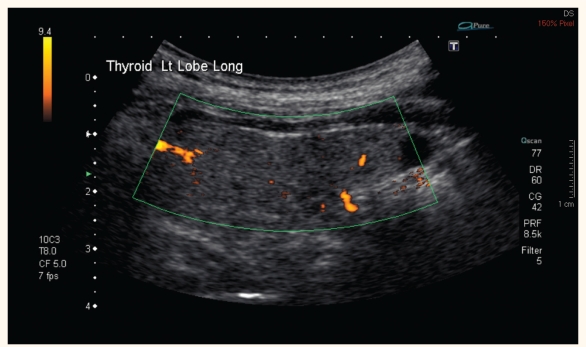
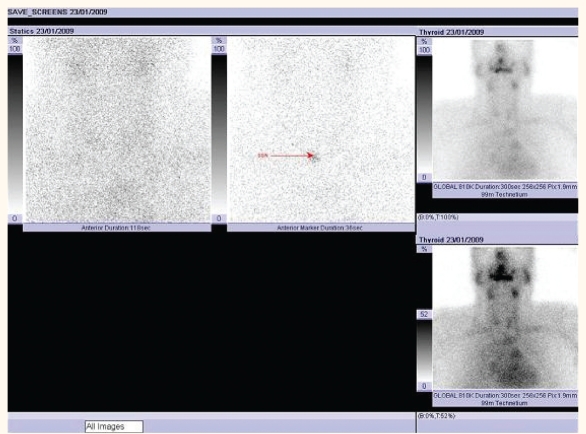
The ultrasound study of the thyroid did not reveal any enlargement of the gland or increased vascularity [Figure 1]. The nuclear study showed no uptake in activity consistent with amiodarone-induced thyroiditis [Figure 2]. Inflammatory markers were mildly elevated with an erythrocyte sedimentation rate (ESR) of 40mm/hour and a C-reactive protein (CRP) of 30mg/L.
Figure 1:
Doppler ultrasound of the thyroid showing no increased vascularity of the thyroid
Figure 2:
Radionuclide scan of the thyroid showing no uptake activity
The clinical presentation, laboratory features and radiological evaluation was suggestive of Type 2 AIT or a mixed picture. After discussion with the endocrinologist, it was decided to start the patient on propylthiouracil (PTU) 100mg three times daily with a plan to add steroids if the thyroid function did not revert to normal. Amiodarone was discontinued and the patient started on metoprolol, adopting a rate control strategy for his atrial fibrillation. On follow-up after one month, the patient reported an improvement in his clinical symptoms. However, his thyroid functions continued to be in the toxic range with a thyroid stimulating hormone (TSH) of 0.03 mU/L, free T4 of 44 pmol/L and free T3 4.9 pmol/L. In view of these results, it was decided to start the patient on oral steroids with a dose of 30mg prednisolone per day. PTU was continued at the same dosage.
After a further month, the patient reported, during a follow-up visit, that there was a significant reduction in his symptoms. A repeat blood test revealed normalisation of his thyroid functions with a TSH of 1.65mU/L and free T4 of 14 pmol/L. It was decided to taper his PTU to a maintenance dose of 50mg twice daily. Steroids were tapered and discontinued over the next week. The final diagnosis was Type 2 amiodarone-induced thyrotoxicosis responding to antithyroid drugs and steroids.
Discussion
Amiodarone is a benzofuranic acid derivative with a high content of iodine and a structural formula that resembles T4. It is a widely-used anti-arrhythmic drug. The maintenance dose of amiodarone varies from 200 to 600mg per day releasing approximately 7 to 21mg of iodide. Considering the daily requirement of iodine to be 150 to 200mcg, amiodarone treatment releases daily a 50 to 100 fold excess of iodine.2 In addition, amiodarone inhibits Type 1 5′-deiodinase enzyme activity thereby decreasing the peripheral conversion from T4 to T3. Amiodarone is predominantly metabolised by dealkylation into desethylamiodarone (DEA) which is known to have anti-arrhythmic properties. A total of 75% of the drug is excreted through the bile and faeces. The elimination half-life of amiodarone is unusually long at an average of 58 days.3
Chronic treatment with amiodarone can have several effects on thyroid function. Over half of the patients treated with this agent can develop innocent changes in thyroid hormone levels. A total of 14 to 18% of patients treated with amiodarone develop overt thyroid dysfunction.3 This can be in the form of acute transient suppression of thyroid function, hypothyroidism in patients susceptible to the inhibitory effects of a high iodine load or thyrotoxicosis which can be due to two different mechanisms. Type 1 AIT is seen in patients with preexisting or latent thyroid disorders and is caused by unregulated hormonal synthesis (the Jod-Basedow phenomenon). Type 2 AIT occurs in patients who had normal thyroid function and is probably due to an increased release in the hormones from an inflammatory process resulting in thyroiditis.3, 4
Differentiation of these two types of AIT is often difficult, but important for deciding promptly upon the appropriate therapy. In addition, one often finds a mixed form with features from both types, further compounding differential diagnosis. Although the differentiation can be extremely difficult, there are several clinical and laboratory clues which help the clinician in making a distinction. The initial task of the physician is to establish whether the patient had a pre-existing thyroid disorder. Clinical examination should focus on looking for signs of thyroid illness such as goitre, exophthalmos or other peripheral signs of thyrotoxicosis, the presence of which would make the probability of Type 1 AIT more likely. The presence of fever and tenderness over the thyroid gland may indicate ongoing thyroiditis and suggest a Type 2 AIT picture. It is important to remember that patients with AIT may lack the classical signs of thyrotoxicosis because of the cardioinhibitory effects of amiodarone.5 Other clinical features of thyrotoxicosis such as weight loss, muscle weakness or irritability can predominate in these patients. Our patient presented with features atypical of hyperthyroidism and fitted into the clinical syndrome of apathetic thyrotoxicosis. The apathetic presentation of thyrotoxicosis is not unusual, especially in older persons. In patients with this syndrome, the usual hyperactivity and restlessness of thyroid hyper-function is replaced with apathy and depression. The presence of thyroid autoantibodies such as antimicrosomal antibody or antithyroglobulin antibody may indicate a preexisting autoimmune thyroid disease.6 Normal or increased values of radioactive iodine uptake in the nuclear studies indicate Type 1 AIT whereas in Type 2 AIT, the uptake is usually reduced or nil.7 Inflammatory markers such as ESR and CRP may be elevated in Type 2 AIT indicating an ongoing thyroiditis. Interleukin-6 (IL-6) appears to be a better marker for thyroid gland destruction and is usually elevated in Type 2 AIT.8 However, falsely low IL-6 levels are often encountered in Type 2 AIT limiting its usefulness. In addition, several preexisting disorders of thyroid such as Grave’s disease can give rise to high IL-6 levels. At present, the role of interleukin in differentiation of Type I and Type 2 AIT remains unclear. Colour flow sonography is another promising radiological modality which can help to distinguish between Type 1 and Type 2 AIT. An increased vascularity of the thyroid gland is often evident in Type 1 AIT, whereas Type 2 AIT usually shows absent or uneven patchy parenchymal flow.9
The treatment of AIT is often a challenge for the clinician. Mild AIT can subside spontaneously in approximately 20% of patients.10 It is important to distinguish between the types of AIT as the treatment differs for each. It is also often difficult to make a decision about the continuation of amiodarone as many patients will require this drug from the cardiac point of view. However, immediate stoppage of amiodarone should not make a difference as this drug has a very long elimination half life.
Type 1 AIT is often treated with antithyroid agents with a view to reducing the thyroid hormone synthesis. The addition of potassium perchlorate to this regimen helps to achieve a faster euthyroid state by inhibiting iodine uptake by the thyroid.11 Type 2 AIT is usually treated with a course of glucocorticoids for its anti-inflammatory and membrane stabilising effects. The usual dose required is between 30 and 50mg per day which is tapered over two to three months. Another agent which has been tried in the treatment of Type 2 AIT is lithium carbonate which inhibits the thyroid hormone secretion. A further method of treatment which can be considered is a total thyroidectomy especially in patients who would need to continue amiodarone.12 Patients with AIT were found to have a high incidence of mortality and morbidity in a study conducted by Conen et al.13
Very often clinical and laboratory features suggest a mixed mechanism for AIT. In such situations, the physician often has to combine both modalities of treatment. Our patient had clinical features suggestive of Type 1 AIT whereas the laboratory and radiological investigations suggested a thyroiditis-like picture. In view of this, we decided to combine antithyroid drugs with corticosteroids resulting in a better outcome for the patient.
Conclusion
This report shows that AIT continues to be a diagnostic and therapeutic dilemma for clinicians. However, vigorous efforts to distinguish between the two types of AIT are warranted in order to provide optimal treatment. It is important for health care providers, who regularly prescribe amiodarone, to be aware of the consequences on thyroid function in long-term users of this medication. Patients who are likely to be on this drug on a long-term basis should have a baseline thyroid examination including thyroid hormone levels and antibodies. Repeat thyroid examinations and hormone level tests are recommended every six months or sooner if clinical signs of hypo- or hyperthyroidism develop in patients on amiodarone.
References
- 1.Martino E, Safran M, Aghini-Lomardi F, Rajatanavin R, Lenziardi M, Fay M, et al. Environmental iodine intake and thyroid dysfunction during chronic amiodarone therapy. Ann Internal Medicine. 1984;101:28–34. doi: 10.7326/0003-4819-101-1-28. [DOI] [PubMed] [Google Scholar]
- 2.Martino E, Bartalena L, Bogazzi F, Braverman LE. The Effects of amiodarone on thyroid. Endocr Rev. 2001;22:240–54. doi: 10.1210/edrv.22.2.0427. [DOI] [PubMed] [Google Scholar]
- 3.Rajeswaran C, Shelton RJ, Gilbey SG. Management of amiodarone-induced thyrotoxicosis. Swiss Med Wkly. 2003;133:579–85. doi: 10.4414/smw.2003.10296. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MD, Erickson DZ, Carney JA, Bahn RS. Nongoitrous (type 1) amiodarone-associated thyrotoxicosis: evidence of follicular disruption in vitro and in vivo. Thyroid. 1995;5:177–83. doi: 10.1089/thy.1995.5.177. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas GA, Gustavo AC, Cabral JM, Jose MC, Leslie CA, Camilo AL. Amiodarone-induced thyrotoxicosis: Diagnostic and therapeutic strategies. Cleve Clinic J Med. 2003;70:624–31. doi: 10.3949/ccjm.70.7.624. [DOI] [PubMed] [Google Scholar]
- 6.Iudica-Souza C, Burch HB. Amiodarone-induced thyroid dysfunction. Endocrinologist. 1999;9:216–227. [Google Scholar]
- 7.Martino E, Bartalena L, Marriotti S, Aghini-Lombardi F, Ceccarelli C, Lippi F, et al. Radioactive iodine thyroid uptake in patients with amiodarone-iodineinduced thyroid dysfunction. Acta Endocrinol. 1988;119:167–73. doi: 10.1530/acta.0.1190167. [DOI] [PubMed] [Google Scholar]
- 8.Bartalena L, Grasso L, Brogioni S, Aghini-Lombardi F, Braverman LE, Martino E. Serum Interleukin-6 in amiodarone induced thyrotoxcosis. J Clin Endocrinol Metab. 1994;789:423–7. doi: 10.1210/jcem.78.2.8106631. [DOI] [PubMed] [Google Scholar]
- 9.Daniels GH. Clinical Review 120: Amiodarone-I induced thyrotoxicosis. J Clin Endocrinol Metab. 2001;86:3–8. doi: 10.1210/jcem.86.1.7119. [DOI] [PubMed] [Google Scholar]
- 10.Eaton SE, Euinton HA, Newman CM, Weetman AP, Bennet WM. Clinical experience of amiodarone induced thyrotoxicosis over a 3-year period: role of colour-flow Doppler sonography. Clin Endocrinol. 2002;56:33–8. doi: 10.1046/j.0300-0664.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 11.Wolff J. Perchlorate and thyroid gland. Pharmacol Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- 12.Williams M, LoGerfo P. Thyroidectomy using local anaesthesia in critically ill patients with amiodaroneinduced thyrotoxicosis: a review and description. Thyroid. 2002;12:523–5. doi: 10.1089/105072502760143926. [DOI] [PubMed] [Google Scholar]
- 13.Conen D, Melly L, Kaufmann C, Bilz S, Ammann B, Schaer B, et al. Amiodarone-induced thyrotoxicosis: clinical course and predictors of outcome. J Am Coll Cardiol. 2007;49:2350–5. doi: 10.1016/j.jacc.2007.02.054. [DOI] [PubMed] [Google Scholar]