Abstract
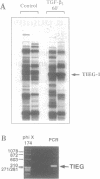
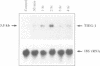
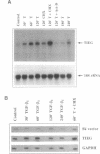
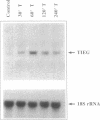
The TGF-beta family of growth factors has been extensively studied and found to play major roles in bone physiology and disease. A novel, TGF-beta-inducible early gene (TIEG) in normal human fetal osteoblasts (hFOB) has been identified using differential-display PCR. Using this differentially expressed cDNA fragment of TIEG to screen a hOB cDNA library, a near full-length cDNA for this gene was isolated. Northern analyses indicated that the steady-state levels of the 3.5 kb TIEG mRNA increased within 30 min of TGF-beta treatment of human osteoblasts and reached a maximum of 10-fold above control levels at 120 min post-treatment. This regulation was independent of new protein synthesis. Computer sequence analyses indicates that TIEG mRNA encodes for a 480 amino-acid protein. The TIEG protein contains three zinc finger motifs, several proline-rich src homology-3 (SH3) binding domains at the C-terminal end, and is homologous in this region to the zinc finger-containing transcription factor family of genes. A growth factor/cytokine-specific induction of TIEG has been shown. TIEG expression in hFOB cells was highly induced by TGF-beta and bone morphogenetic protein-2 (BMP-2), with a moderate induction by epidermal growth factor (EGF), but no induction by other growth factors/cytokines was observed. In addition to osteoblastic cells, high levels of TIEG expression were detected in skeletal muscle tissue, while low or no detectable levels were found in brain, lung, liver or kidney. Because TIEG is an early induced putative transcription factor gene, and shows a growth factor induction and tissue specificity, its protein product might play an important role as a signalling molecule in osteoblastic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Galéra P., Musso M., Ducy P., Karsenty G. c-Krox, a transcriptional regulator of type I collagen gene expression, is preferentially expressed in skin. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9372–9376. doi: 10.1073/pnas.91.20.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. A., Enger R. J., Riggs B. L., Spelsberg T. C. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995 Feb;10(2):178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Type beta transforming growth factor (TGF beta) regulation of alkaline phosphatase expression and other phenotype-related mRNAs in osteoblastic rat osteosarcoma cells. J Cell Physiol. 1987 Dec;133(3):426–437. doi: 10.1002/jcp.1041330303. [DOI] [PubMed] [Google Scholar]
- Noda M., Yoon K., Prince C. W., Butler W. T., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J Biol Chem. 1988 Sep 25;263(27):13916–13921. [PubMed] [Google Scholar]
- Oursler M. J., Cortese C., Keeting P., Anderson M. A., Bonde S. K., Riggs B. L., Spelsberg T. C. Modulation of transforming growth factor-beta production in normal human osteoblast-like cells by 17 beta-estradiol and parathyroid hormone. Endocrinology. 1991 Dec;129(6):3313–3320. doi: 10.1210/endo-129-6-3313. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Riggs B. L., Spelsberg T. C. Glucocorticoid-induced activation of latent transforming growth factor-beta by normal human osteoblast-like cells. Endocrinology. 1993 Nov;133(5):2187–2196. doi: 10.1210/endo.133.5.8404670. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Mundy G. R. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2024–2028. doi: 10.1073/pnas.84.7.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Flanders K. C., Roche N. S., Kondaiah P., Reddi A. H., Termine J. D., Sporn M. B., Roberts A. B. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987 Jul;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi S. C., Belasco J. G., Greenberg M. E. Regulation of proto-oncogene mRNA stability. Biochim Biophys Acta. 1992 Dec 16;1114(2-3):95–106. doi: 10.1016/0304-419x(92)90009-n. [DOI] [PubMed] [Google Scholar]
- Subramaniam M., Oursler M. J., Rasmussen K., Riggs B. L., Spelsberg T. C. TGF-beta regulation of nuclear proto-oncogenes and TGF-beta gene expression in normal human osteoblast-like cells. J Cell Biochem. 1995 Jan;57(1):52–61. doi: 10.1002/jcb.240570107. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Maeno M., Hawrylyshyn B., Yao K. L., Domenicucci C., Sodek J. Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol. 1988 Mar;106(3):915–924. doi: 10.1083/jcb.106.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]