Abstract
Nodular lymphocyte predominant Hodgkin’s lymphoma (NLPHL) is a recently described type of Hodgkin’s lymphoma (HL) and accounts for 5–6% of all the cases of HL. Here we report the case of an elderly man who presented to Sultan Qaboos University Hospital, Oman, with severe hypercalcemia, and was diagnosed to have stage IV NLPHL. Although the incidence of hypercalcemia is estimated to be between 1–5% in classical HL, to our knowledge this is the first report of NLPHL presenting with severe hypercalcemia. The patient responded to the anti-CD20 monoclonal antibody, Rituximab, and has been in clinical remission for more than 3 years.
Keywords: Hodgkin’s Lymphoma, Hypercalcemia, Thrombocytopenia, Monoclonal antibody, Rituximab, Case Report, Oman
A 75 year old Omani gentleman, presented with a 6 month history of weakness, weight loss of 20 kg, loss of appetite and a two month history of low grade fever, cough and difficulty in walking. The past medical history was significant for hypertension for 20 years (treated with atenolol), Type II diabetes mellitus (treated with oral hypoglycemics), chronic renal failure for 8 years; hypertrophic cardiomyopathy diagnosed 2 years previously; and osteoarthritis with a right knee joint replacement. General physical examination was unremarkable except for generalized body weakness and an ejection systolic murmur suggestive of aortic sclerosis.
Laboratory investigations revealed: Hb 13g/dl, WBC 6.5 x 109/L, platelets 66 x 109/L; creatinine 313 μmol/L; Na+ 132 mmol/L; K+ 3.8 mmol/L; Cl 100 mmol/L; CO2 19 mmol/L; corrected serum calcium 4.08 mmol/L; PO4 1.88 mmol/L; alkaline phophatase 94 U/L. Serum para-thyroid hormone (PTH) levels were appropriately reduces to 0.7 pmol/L (1.3–7.6 pmol/L), whereas, serum para-thyroid hormone related peptide (PTH-rp) < 1.0 pmol/L (<1.3 pmol/L); was within the normal limits; however, 1,25 dihydroxyvitamin D3 [1,25 (OH) D3] was elevated at 411 pmol/L (43–148 pmol/L). An assessment of thyrombocytopenia, renal failure and severe hypercalcemia secondary to elevated 1,25 (OH) D3 was made.
An upper GI endoscopy revealed severe hemorrhagic gastritis and reflux esophagitis. The parathyroid scan was reported normal. A CT (computed tomography) scan of the chest and abdomen revealed a left axillary lymph node measuring 4x2 cm, which was not palpable on clinical examination.
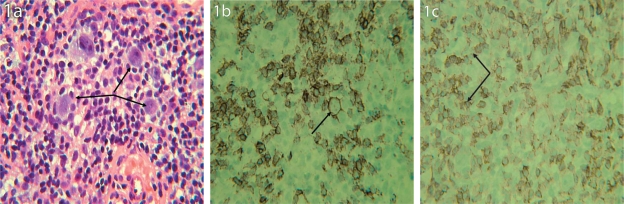
A core biopsy of the lymph node was done which revealed an infiltrate consisting of occasional atypical large cells (positive for CD20, CD79a, and CD45, weakly positive in a few cells for CD30, and negative for CD5, CD10, CD15, bcl-1, and epithelial membrane antigen). These large atypical cells were interspersed within a background of small lymphocytes (positive for CD3 and CD5), some of which were arranged in a rosette fashion (positive for CD57) around the CD20 positive large atypical lymphocytes. Overall, the histopathological picture was suggestive of either a lymphocyte predominant Hodgkin’s lymphoma (NLPHL) or a T-cell/histiocyte rich B-cell lymphoma (T/HRBCL). The bone marrow biopsy showed increased cellularity and scattered large atypical lymphocytes, staining positively for the CD20 antigen.
The patient was diagnosed to have NLPHL/T/HRBCL, stage IV. Although the histopathology was more in favor of NLPHL, the clinical presentation (age and stage IV disease) was more in keeping with T/HRBCL. A second opinion, sought from a reference pathology centre (Armed Forces Institute of Pathology, Washington, USA), was consistent with the original impression of NLPHL. However, in view of the CD57 positive small lymphocytes arranged in a rosetting manner around the large malignant cells, a diagnosis of NLPHL was favoured.
The patient was treated with zolendronic acid resulting in normalization of elevated calcium levels in the next 48 hours. Subsequently, the patient was treated with anti-CD20 antibody (Rituximab) weekly for 4 weeks. Platelet count improved and reached normal limits during the course of treatment with Rituximab. The 1,25 (OH) D3 returned to 45 pmol/L in 2 weeks. Re-evaluation CT scans at 2 months revealed a partial remission (PR) and at 6 months revealed complete remission (CR). A bone marrow biopsy 2 months later was reported to be within the normal limits.
DISCUSSION
NLPHL is a rare type of B-cell lymphoma with unique pathologic and clinical features that distinguish it from classical Hodgkin’s lymphoma.1–3 Patients with NLPHL tend to be younger males who present with indolent and asymptomatic lymphadenopathy limited to peripheral lymph nodes. The immunophenotype of the malignant lymphocytic and/or histiocytic cells forms the basis of the pathologic distinction from the subtypes of classical Hodgkin’s disease.1 Despite an excellent response to an initial combined-modality treatment, patients with NLPHL tend to relapse.2 The benign nature of these relapses and the incidence of late treatment-related toxicity have raised questions about the need for an aggressive upfront approach. However, recent insights into the molecular pathogenesis of NLPHL and the development of novel targeted therapies promise to improve future treatment.3
Although, in our case, a diagnosis of NLPHL was entertained, T/HRBCL was a close differential. NLPHL and T/HRBCL are distinct tumors and are treated differently. They are linked by a morphologic and probably a biologic continuum, which renders the differential diagnosis difficult.4, 5 The NLPHL is characterized by atypical lymphocytic and histiocytic (L&H) or ‘popcorn’ cells, embedded in the background of progressively transformed follicles. The atypical cells are CD45+, express the B-cell associated antigens CD19, CD20, CD22, CD79a, and epithelial membrane antigen (EMA), but lack the expression of CD15 and CD30.6 While tumor cells in NLPHL and T/HRBCL are immunophenotypically similar, the background composition differs. In NLPHL, small B cells are CD3+ CD4+ CD57+ T cells, whereas in T/HRBCL, CD8+ T cells and histiocytes dominate. While CD57+ T cells surrounding the ‘popcorn cells’ are typically seen in NLPHL, the absence of these cells in the rosettes form does not argue against the diagnosis.3 Tumor cells either are loosely scattered or formed clusters, thus resembling areas of either T/HRBCL or inflammatory diffuse large BCL (DLBCL) within the nodules.5
On the other hand, diffuse large B cell lymphoma (DLBCL) can arise in patients previously known to have, or have been treated for NLPHL. For example, the Nebraska group has reported 21 patients in whom DLBCL was diagnosed either concurrently or subsequent to a diagnosis of NLPHL.7 Of the ten patients who presented with nodal DLBCL only, six patients presented with both nodal and extra-nodal involvement, and five presented with only extra-nodal DLBCL. The median overall survival and failure-free survival of the entire group was 35 months and 11 months. Importantly, there were no significant differences in the survival outcomes between patients with DLBCL arising in NLPHL and age-and sex-matched patients with de novo DLBCL. Our patient remained progression-free after 6 months of treatment, and is on regular follow up.
The vast majority of patients with NLPHL present with stage I/II disease and bone marrow involvement is rare.2, 3 However, a small subset of patients in whom lymph node biopsy shows NLPHL with a nodular pattern also may have lymphoma in the bone marrow. Khoury et al. reported the incidence of bone marrow involvement to be 2.5% in a series of 275 patients with classical HL.8 Bone marrow involvement is associated with laboratory, radiological, or morphologic evidence of aggressive disease, and our patient did present with severe hypercalcemia.
Hypercalcemia is not a common feature of Hodgkin’s lymphoma.9, 10 The incidence has been reported to be 0.9%, 1.6%, and 5.4% in different series of patients with classical HL.11 However, little information exists on hypercalcemia in patients with NLPHL. Hodgkin’s lymphoma associated hypercalcemia, although uncommon, has been variously ascribed to be mediated through the secretion of PTH-rp, osteoclast activating factors (OAFs), overproduction of 1,25 (OH) D3 (calcitriol), or due to prostaglandin synthesis.12–15 In our case, serum PTH and PTH-rp levels were either suppressed or within the normal limits. However, serum 1,25 (OH) D3 levels were found to be elevated. Calcitriol has been most consistently implicated as the mediator of hypercalcemia in HL.16 Monocytes and macrophages seem to play a pivotal role in the production of calcitriol in HL and NHL, as is the case in tuberculosis and sarcoidosis. Calcitriol synthesis by macrophages is enhanced by interferon-γ, the expression of which in turn is dependent upon stimulation by interleukin-1 and tumor necrosis factor secreted by the T lymphocytes.17 Calcitriol binds to a specific intracellular receptor present in many tissues, particularly the bone and intestine. Ligand-receptor interaction results in increased intestinal absorption of calcium and phosphate from the intestine, and enhanced osteoclastic activity leading to osteolysis and hypercalcemia.
NLPHL is characterized by indolent nodal disease that tends to relapse after standard radiotherapy or chemotherapy. The malignant cells of NLPHL are CD20+ and therefore Rituximab may have activity with fewer late effects than standard therapy. Recently, two phase II studies and a few case reports have been reported on the use and efficacy of Rituximab in patients with NLPHL. In a phase II study reported by Ekstrand et al.,18 22 patients with CD20+ NLPHL received 4 weekly doses of rituximab at 375 mg/m2. Ten patients had previously been treated for Hodgkin’s disease, while 12 patients had untreated disease. All 22 patients responded to Rituximab (overall response rate, 100%) with complete response in 9 (41%), unconfirmed complete response in 1 (5%), and partial response in 12 (54%). Acute treatment-related adverse events were minimal. With a median follow-up of 13 months, 9 patients had relapsed, and the estimated median freedom from progression was 10.2 months. Progressive disease was biopsied in 5 patients: 3 had recurrent NLPHL, while 2 patients had transformed to NHL. All 3 patients with recurrent NLPHL were retreated with Rituximab again. In a second study reported by Rehwald et al., 19 14 patients were treated in a manner similar to the one described earlier. However, in this series, all patients had received at least one prior chemotherapy regimen. The overall response was 86%, with 8 complete remissions and 4 partial remissions, and 2 patients with progressive disease. At a median follow-up of 12 months, 9 of 12 responders were in remission. Our patient was treated with single agent Rituximab, because of poor performance status at presentation, multiple comorbids including acute renal failure and diabetec neuropathy, and cardiomyopathy, and perceived poor tolerance to combination chemotherapy.
In conclusion, we report the case of an elderly gentleman, with multiple co-morbids, who presented with severe hypercalcemia secondary to NLPHL, which to our knowledge has not been reported before, and was successfully treated with anti-CD20 antibody, and is in complete remission more than 3 years after completion of treatment.
Figures 1:
Photomicrographs of section from the lymph node. (1A) H&E stain. Arrows indicate the atypical cells. (1B) Staining with CD20 antibody. Arrow indicates the membrane staining of the large atypical cell. (1C) Staining with CD57 antibody. Arrows indicate the small lymphocytes arranged in a rosette fashion around the large atypical cells
Acknowledgments
We acknowledge with gratitude the help of medical, nursing and the technical staff involved in the care of this patient at Sultan Qaboos University Hospital, Oman. Our special thanks are due to Dr. Omayma El-Shafie and Dr. Azhar Rizvi for the help in the medical management.
REFERENCES
- 1.Ekstrand BC, Horning SJ. Lymphocyte predominant Hodgkin’s disease. Curr Oncol Rep. 2002;4:424–433. doi: 10.1007/s11912-002-0037-8. [DOI] [PubMed] [Google Scholar]
- 2.Diehl V, Sextro M, Franklin J, Hansmann ML, Harris N, Jaffe E, et al. Clinical presentation, course, and prognostic factors in lymphocyte predominat Hodgkin’s disease and lymphocyte rich classic Hodgkin’s disease: Report from European task force on lymphoma project on lymphocyte predominant Hodgkin’s disease. J Clin Oncol. 1999;17:776–783. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 3.Nogova L, Rudiger T, Engert A. Biology, clinical course and management of noular lymphocyte predominant Hodgkin’s disease. American Society of Hematology Education program book. 2006:266–272. doi: 10.1182/asheducation-2006.1.266. [DOI] [PubMed] [Google Scholar]
- 4.Rudiger T, Gascoyne RD, Jaffe ES, de Jong D, Delabie J, De Wolf-Peeters C, et al. Workshop on the relationship between nodular lymphocyte predominant Hodgkin‘s lymphoma and T cell/histiocyte-rich B cell lymphoma. Ann Oncol. 2002;13:44–51. doi: 10.1093/annonc/13.s1.44. [DOI] [PubMed] [Google Scholar]
- 5.Boudova L, Torlakovic E, Delabie J, Reimer P, Pfistner B, Wiedenmann S, et al. Nodular lymphocyte-predominant Hodgkin lymphoma with nodules resembling T-cell/histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/histiocyte-rich B-cell lymphoma. Blood. 2003;102:3753–3758. doi: 10.1182/blood-2003-02-0626. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostopoulus I, Hansmann ML, Franssila K, Harris M, Harris NL, Jaffe ES, et al. European task force on lymphoma project on lymphocyte predominat Hodgkin’s disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin’s disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96:1889–1899. [PubMed] [Google Scholar]
- 7.Huang JZ, Weisenburger DD, Vose JM, Greiner TC, Aoun P, Chan WC, et al. Nebraska Lymphoma Study Group Diffuse large B-cell lymphoma arising in nodular lymphocyte predominant Hodgkin lymphoma. A report of 21 cases from the Nebraska Lymphoma Study Group. Leuk Lymphoma. 2003;44:1903–1910. doi: 10.1080/1042819031000123528. [DOI] [PubMed] [Google Scholar]
- 8.Khoury JD, Jones D, Yared MA, Manning JT, Jr, Abruzzo LV, Hagemeister FB, et al. Bone marrow involvement in patients with nodular lymphocyte predominant Hodgkin lymphoma. Am J Surg Pathol. 2004;28:489–495. doi: 10.1097/00000478-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Utkan G, Buyukcelik A, Yalcin B, Tek I, Doruk H, Dincol D, et al. Extranodal Hodgkin’s disease presenting with gluteal mass and hypercalcemia. South Med J. 2006;99:1149–1150. doi: 10.1097/01.smj.0000240720.24001.77. [DOI] [PubMed] [Google Scholar]
- 10.Roca B, Simon E. Hodgkin’s disease presenting with hypercalcaemia of unknown origin. Ir Med J. 1998;91:102. [PubMed] [Google Scholar]
- 11.Linde R, Basso L. Hodgkin’s disease with hypercalcemia detected by thallium-201 scintigraphy. J Nucl Med. 1987;28:112–115. [PubMed] [Google Scholar]
- 12.Karmali R, Barker S, Hewison M, Fraher L, Katz DR, O’Riordan JL. Intermittent hypercalcaemia and vitamin D sensitivity in Hodgkin’s disease. Postgrad Med J. 1990;66:757–760. doi: 10.1136/pgmj.66.779.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson JO, Bringhurst FR, Harris NL, Weitzman SA, Aisenberg AC. Humoral hypercalcemia in Hodgkin’s disease. Clinical and laboratory evaluation. Cancer. 1989;63:917–923. doi: 10.1002/1097-0142(19890301)63:5<917::aid-cncr2820630522>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Bolo-Deoku J, Basu S, Lakhani S, Dunne F, Ratcliffe WA, Clarke M, et al. Parathyroid hormone related protein in hypercalcaemia of Hodgkin’s disease. J Clin Pathol. 1992;45:541–542. doi: 10.1136/jcp.45.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laforga JB, Vierna J, Aranda FI. Hypercalcemia in Hodgkin’s disease related to prostaglandin synthesis. J Clin Pathol. 1994;47:567–568. doi: 10.1136/jcp.47.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood. 1993;82:1383–1394. [PubMed] [Google Scholar]
- 17.Rieke JW, Donaldson SS, Horning SJ. Hypercalcemia and vitamin D metabolism in Hodgkin’s disease. Is there an underlying immunoregulatory relationship? Cancer. 1989;63:1700–1707. doi: 10.1002/1097-0142(19900501)63:9<1700::aid-cncr2820630910>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Ekstrand BC, Lucas JB, Horwitz SM, Fan Z, Breslin S, Hoppe RT, et al. Rituximab in lymphocyte-predominant Hodgkin disease: results of a phase 2 trial. Blood. 2003;101:4285–4289. doi: 10.1182/blood-2002-08-2644. [DOI] [PubMed] [Google Scholar]
- 19.Rehwald U, Schulz H, Reiser M, Sieber M, Staak JO, Morschhauser F, et al. German Hodgkin Lymphoma Study Group (GHSG) Treatment of relapsed CD20+ Hodgkin lymphoma with the monoclonal antibody rituximab is effective and well tolerated: results of a phase 2 trial of the German Hodgkin Lymphoma Study. [DOI] [PubMed]