Abstract
Objective:
Chronic pain is associated with increased incidence of hypertension. Sleep deprivation, common in patients with chronic pain, is associated with increased blood pressure and heart rate. This study was designed to determine whether sleep deprivation induces increased cardiovascular responses to pain. In addition; we examined the role of melatonin and endorphins in mediating these responses.
Method:
The study was conducted in Sprague-Dawely rats divided into a control group (n=8) and Rapid Eye Moment sleep deprived (REMSD) group (n=8). REM sleep deprivation was done for three days using the inverted flowerpot technique. Systolic BP and HR were recorded at baseline as well as 5, 10 and 30 minutes after intra-plantar formalin injection. In addition, serum melatonin and endorphin levels were determined.
Results:
Under basal conditions, BP and HR and following acute pain (1st phase of formalin injection) were comparable with non-sleep deprived (non-SD) state. In contrast, the REMSD rats showed significantly greater increases in HR and BP during the 2nd phase of formalin pain as compared to non-SD state. These changes were associated with significant reductions in serum melatonin and endorphin levels in REMSD rats.
Conclusion:
These data indicate that exaggerated blood pressure and HR responsiveness to pain in sleep deprivation could be mediated through reductions in melatonin and endorphin.
Keywords: REM sleep deprivation, Blood pressure, Heart rate, Tonic pain, Melatonin, Endorphins
An adequate amount of sleep is paramount for a healthy and productive lifestyle. Experimental short-term restriction of sleep results in a variety of adverse physiologic effects, including hypertension, activation of the sympathetic nervous system, impairment of glucose control, and increased inflammation.1 The interaction between sleep and pain is generating a considerable research interest as pain and sleep seems to have reciprocal relationships. Patients who suffer from chronic pain experience difficulties in initiating and maintaining sleep and poor sleep can significantly decrease the pain threshold.2 Chronic sleep deprivation has been suggested to be one of the factors which can trigger cardiovascular emergencies such as myocardial infarction.3 However, the mechanisms linking between sleep deprivation, pain and the cardiovascular system are unclear.
Sleep-deprived (SD) rats suffer from desynchronization of physiological and behavioural rhythms.4 The pineal gland, which controls and synchronizes the sleep-awake cycle, is affected by several stresses such as forced swimming and hypoglycemic shock.5 Sleep deprivation also alters the pineal-melatonin system, which has an analgesic action and shares anti-stress activity.6,7 The endogenous opioid peptides have been postulated to have an interaction with melatonin secretion.8 Sleep deprivation is also associated with changes in β-endorphin levels.9 Despite these various studies, the role of melatonin and endorphins levels in altering the cardiovascular responses during stressful stimuli such as pain have not been tested. The aim of this study is to test the effect of Rapid Eye Moment sleep deprivation (REMSD) on blood pressure and heart rate responses to tonic pain in rats. Furthermore, serum melatonin and β-endorphins levels were measured in SD and control rats during tonic pain.
METHOD
ANIMALS
Experiments were carried out on Sprague-Dawley male rats (n=16) approximately weighing 225–250sg. The animals were individually housed in a temperature (22–24°C), humidity (55%) and light-dark cycle (12 hours) controlled room. Food and water were available ad libitum. The rats were randomly divided into 2 groups (N = 8 each). The control group remained in the home cages for the duration of the study, receiving only controlled handling (5–10 minutes/day). The experimental (REMSD) group was subjected to REM sleep deprivation for 72 hours.
REM SLEEP DEPRIVATION MODEL
REM sleep deprivation was induced by using the inverted flowerpot in water tank technique, as mentioned previously.10 Briefly, animals were placed on a small inverted beaker (platform of 6.5cm diameter) surrounded by water reaching to a level of 1cm below the base of beaker. The covers of the cages were modified so as to enable the animal to reach for food and water easily. The water of the tank was changed daily in the morning. Before the animals were subjected to sleep deprivation the rats were trained for 1–2 hours to reside on the platform and they learnt not to fall in the water. After training for 1–2 days, on the day of experiment they were taken from their home cage and placed individually in the sleep deprivation water tank cages. It has been shown that this method deprives the rat of almost 100% of REM sleep.11
BLOOD PRESSURE AND HEART RATE MONITORING
We used a computerized microprocessor based system for recording arterial blood pressure and heart rate. The measurement was performed non-invasively from the tail of the conscious rats with the aid of a pressure cuff (tail cuff method) and piezo-electric pulse transducer (Technical and Scientific Equipment, GmbH). The rats were partially restrained during the period of recording. In order to achieve satisfactory results, rats were warmed for 10–15 minutes prior to recording by placing them in the pre-heated box (32°C). All the rats were trained for 3 consecutive days to adapt them to handling, heating, restraining, inflating and deflating the cuff pressure. We collected 3 recordings each at 1 minute interval; the average of 3 readings was calculated for each record of BP and HR.
STUDY DESIGN
The experiments were conducted in the morning between 10:00–12:00 hours. In the experimental group, all the 8 rats served as their own control. They were subjected to the following protocol of recording BP and HR.
1. Basal records:
The basal records were taken immediately before injection of formalin. These values were used to calculate the respective changes in BP and HR.
2. Formalin injection:
After the basal record, rats were allowed 60 minutes of wash-out period in the home cage. Subsequently, the rats receive a subcutaneous injection (50 μl) of dilute formalin (5%) into the plantar surface of their right hind paw. The pain usually starts within 5 minutes after injection.12 BP and HR were recorded at 5, 10 and 30 minutes after injection.
After completing the entire recording, rats were given pentobarbital anesthesia (40 mg/kg ip) and blood samples were collected in heparinized syringes by cardiac puncture after performing thoracotomy. All samples were collected at 13:00 hours, taking into consideration the light dark cycle as the rate of melatonin production is low at this time. The blood was immediately centrifuged and serum was stored at −20°C for radioimmunoassay (RIA).
MELATONIN AND β-ENDORPHIN MEASUREMENTS
Beta-endorphin was extracted by using Seropack C18 cartridges. A competitive RIA method using antibodies against β-endorphin was used to analyze the extracts. Antibodies have specificity to N-terminal region of β-endorphin with low cross reactivity to β-lipotropin. In order to increase the sensitivity of assay, sequential incubation was performed. Polyethylene glycol was used to separate the bound fraction from unbound fraction. Beta-endorphin has inter-assay coefficient of variance (CV) of 7.2% and intra-assay CV of 7.1%. The specificity of both the hormones was 100%. Melatonin was assayed by enzymatic pretreatment and RIA method (The melatonin assay has inter-assay CV of 4.5% and intra-assay CV of 3.8%, the reference range was < 0.078 nmol/ml). The antibodies to both β-endorphin and melatonin were supplied by Immunobiological Laboratories, Hamburg.
STATISTICAL ASNALYSIS
All the data in the study are expressed as mean ± SEM. Prior to formalin injection, records of BP and HR were obtained to serve as the baseline. The data of nonsleep deprived (non-SD) state were compared with REMSD groups by using unpaired t-test. The level of significance was set at p <0.05.
RESULTS
The basal BP and HR recorded before sleep deprivation and after REMSD were comparable and there was no significant difference, as shown in Table 1.
Table 1.
Effects of REM sleep deprivation on blood pressure and heart rate during acute and tonic pain in rats.
| Basal | Formalin Injection | |||
|---|---|---|---|---|
| 5min | 10min | 30min | ||
| Non SD | ||||
| BP | 148±3 | 183±2 | 162±4 | 149±4 |
| HR | 282±7 | 349±8 | 334±4 | 317±7 |
| REMSD | ||||
| BP | 152±5 | 181±6 | 176±4* | 180±3** |
| HR | 263±8 | 372±9 | 360±7Ж | 358±8 3 |
P<0.02,
P<0.001,
P<0.034, 3P<0.006
Non SD Non Sleep Deprived
REMSED REM Sleep Deprived
THE EFFECT OF FORMALIN INJECTION (TONIC PAIN) ON BP AND HR RESPONSE BEFORE AND AF TER REMSD
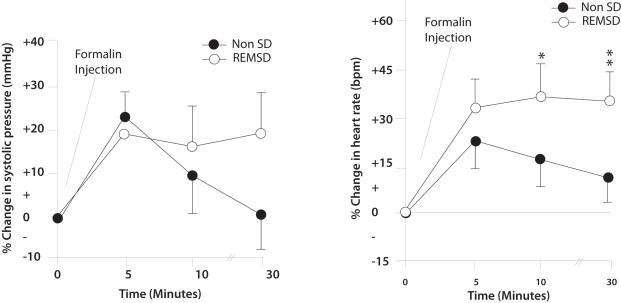
BP and HR responses to formalin injection were recorded at 5, 10 and 30 minutes. In the second tonic phase of pain i.e. after 10 minutes of formalin injection, the BP and HR were maintained at a level higher than basal values. The differences between the BP and HR responses to tonic pain, before and after sleep deprivation (10± 4% vs 17± 5%, respectively) were significant: p < 0.027, P <0.034 respectively. After 30 minutes of tonic pain the BP and HR in non-SD state tended to return towards the basal level. In sleep deprivation, the BP and HR remained elevated with no tendency to return towards the basal level. The differences between the BP and HR responses to 30 minutes of tonic pain in non-SD and after REMSD (1± 0.5% vs 20± 5%) were significant (p=0.001). Similarly, the HR responses before and after REMSD (13% ± 5 vs + 37% ± 5) were also statistically significant (p= 0.006) respectively (Figure 1).
Figure 1.
Line graphs ilustrating the changes in arterial pressure (left) and heart rate (bottom) in response to formalin induced pain in non sleep deprived (REMSD) rats. Data are presented as percentage changes in systolic pressure and heart rate. All data are presented as mean ± SE, *=significance at p<0.05
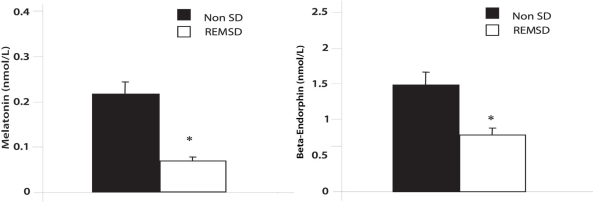
PLASMA MELATONIN AND β-ENDORPHIN LEVELS
Figure 2 shows the plasma levels of melatonin and β-endorphin levels in rats with non-SD and after sleep deprivation. Both these were significantly lower in REMSD rats compared with controls. The melatonin levels in REMSD were (0.07 ± 0.01 nmol/ml) compared with (0.22± .03 pmol/ml) in the controls (p < .001). Beta-endorphin levels in REMSD were (0.86 ± 0.17 pmol/ml) compared with (1.55 ± 0.43 pmol/ml) in the controls (p <0.002).
Figure 2.
Bar graphs illustrating the serum levels of melatonin and endorphins in non-sleep deprived (non-SD) and REM sleep-deprived (REMSD) rats. ± SE, * = significance at P<0.05.
DISCUSSION
We have demonstrated that REM sleep deprivation for 72 hours in rats significantly enhanced and prolonged the BP and HR response to formalin-induced tonic pain. In addition REM sleep deprivation significantly reduced serum melatonin and β-endorphins levels in rats.
The mechanism by which REM sleep deprivation enhances the blood pressure and HR response to pain is probably fairly complex. Possible explanations could be that REM sleep deprivation is associated with hyperalgesia in rats,13 due to lowered melatonin levels, which has a pain modulatory action.7 Melatonin also acts as hypotensive hormone in rats14 and humans.15 It is therefore conceivable that the sleep deprivation that lowered the melatonin levels in SD rats could be responsible for the enhanced cardiovascular response to pain. Examining previous studies the inhibitory role of melatonin on cardiovascular functions indicated that melatonin reduces peripheral norepirephrire release and increases central sympathetic activity.16 Increased sympathetic nervous system activity has been proposed as one of the mechanisms for cardiovascular changes with pain in sleep deprivation.17 However, a study conducted to measure the sympathetic drive after sleep deprivation following stressful stimuli in human subjects failed to show an increased sympathetic activity.18 Melatonin also increases vagal tone by direct action on the hypothalamus, decreases brain serotonin release and is an antioxidant with a vasodilator action directly on blood vessels.16
Another possible explanation for enhanced cardiovascular response in our experiment is reduced β-endorphin levels in REM sleep deprivation. These data support other findings that opioids play a tonic regulatory role on the cardiovascular control system.19,20 However, possible down regulation of opioid receptors in REM sleep deprivation could be involved. The locus ceruleus, the primary noradrenergic pathway for nociceptive cardiovascular response, has opioid receptors in large concentration.21 It is possible that REMSD could have down regulated these receptors as well, resulting in exaggerated blood pressure and HR response in our study. However, there is a lack of available evidence to support this hypothesis.
Although our data indicate that exaggerated BP and heart rate changes with pain in REMSD rats is associated with reduction in melatonin and β-endorphins, the cause and effect relationship is yet to be established. The associated reduction in levels of melatonin and β-endorphins in SD animals suggest possible involvement of these chemicals in regulating cardiovascular response to nociception. Possible future research should be directed at examining whether this exaggerated cardiovascular response can be reversed by treating animals with melatonin or β-endorphins. Extrapolation of these experiments to human studies is still to be determined.
Acknowledgments
We are eternally grateful for the services of female staff (Umairah, Fatima, Nur Insan, Merly and Jerinia) from the Medical Records Department. We also thank Ibrahim Biruar for typing the manuscript.
REFERENCES
- 1.Alvarez GG, Ayas NT. The Impact of Daily Sleep Duration on Health: A Review of the Literature. Prog Cardiovasc Nurs. 2004;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 2.Drewes A. Pain and Sleep Disturbances with Special Reference to Fibromyalgia and Rheumatoid Arthritis. Rheumatology (Oxford) 1999;38:1035–1038. doi: 10.1093/rheumatology/38.11.1035. [DOI] [PubMed] [Google Scholar]
- 3.Tofler GH, Stone PH, Maclure M, Edelman E, Davis VG, Robertson T, Antman EM, Muller JE. Analysis of Possible Triggers of Acute Myocardial Infarction (The MILIS Study) Am J Cardiol. 1990;66:22–27. doi: 10.1016/0002-9149(90)90729-k. [DOI] [PubMed] [Google Scholar]
- 4.Rechtschaffen A, Gilliand MA, Bergmann BM, Winter JB. Physiological Correlates of Prolonged Sleep Deprivation in Rats. Science. 1983;221:182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 5.Champney TH, Steger R, Christie D, Reiter RJ. Alteration in Components of Pineal Melatonin Synthetic Pathway by Acute Insulin Stress in Rat and Syrian Hamster. Brain Res. 1985;338:25–32. doi: 10.1016/0006-8993(85)90244-6. [DOI] [PubMed] [Google Scholar]
- 6.Poeggeler B RR, Tan DX, Chen LD, Manchester LC. Melatonin Hypocotyl Radical-Mediated Oxidative Damage and Aging: A Hypothesis. J Pineal Res. 1993;14:151–168. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 7.Yu CX, Zhu B, Xu SF, Cao XD, Wu CC. The Analgesic Effects of Peripheral and Central Administration of Melatonin in Rats. Eur J Pharmacol. 2000;403:49–53. doi: 10.1016/s0014-2999(00)00421-0. [DOI] [PubMed] [Google Scholar]
- 8.Lissoni P, Esposti D, Esposti G, Mauri R, Resentini M, Morabito F, et al. A Clinical Study on the Relationship Between the Pineal Gland and the Opioid System. J Neural Transm. 1986;65:63–73. doi: 10.1007/BF01249612. [DOI] [PubMed] [Google Scholar]
- 9.Przewlocka B, Mogilnicka E, Lason W, Van Luijtelaar EL, Coenen AM. Deprivation of REM Sleep in the Rat and the Opioid Peptides Beta-Endorphin and Dynorphin. Neurosci Lett. 1986;70:138–142. doi: 10.1016/0304-3940(86)90452-0. [DOI] [PubMed] [Google Scholar]
- 10.Jouvet D, Vimont P, Delorme F, Jouvet M. Study of Selective Deprivation of the Paradoxical Phase of Sleep in the Cat. J Physiol (Paris) 1964;56:381. [PubMed] [Google Scholar]
- 11.Kovalzon VM, Tsibulsky VL. REM-Sleep Deprivation, Stress and Emotional Behavior in Rats. Behav. Brain Res. 1984;14:235–245. doi: 10.1016/0166-4328(84)90191-8. [DOI] [PubMed] [Google Scholar]
- 12.Tjolsen A, Berge OG, Hunskaar JH, Rosland KH. The Formalin Test: An Evaluation of the Method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 13.Onen SH, Abdelkrim A, Didier J, Alain E, Claude D. Effects of Rapid Eye Movement (REM) Sleep Deprivation on Pain Sensitivity in the Rat. Brain Res. 2001;900:261–267. doi: 10.1016/s0006-8993(01)02320-4. [DOI] [PubMed] [Google Scholar]
- 14.He W, Zhu X, Wang H, Qiu Y, Qiu X. Effect of Melatonin on Mean Blood Pressure and Heart Rate in Morphine Withdrawal Rats. Yao Xue Xue Bao. 1998;33:727–730. [PubMed] [Google Scholar]
- 15.Nishiyama K, Yasue H, Moriyama Y, Tsunoda R, Ogawa H, Yoshimura M, et al. Acute Effects of Melatonin Administration on Cardiovascular Regulation in Healthy Men. Am Heart J. 2001;141:E9. doi: 10.1067/mhj.2001.114368. [DOI] [PubMed] [Google Scholar]
- 16.Sewerynek E. Melatonin and the Cardiovascular System. Neuroendocrinol Lett. 2002;1:79–83. [PubMed] [Google Scholar]
- 17.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, Shimizu T. Total Sleep Deprivation Elevates Blood Pressure Through Arterial Baroreflex Resetting: A Study with Microneurographic Technique. Sleep. 2003;26:986–989. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 18.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effect of Sleep Deprivation on Neural Circulatory Control. Hypertension. 2000;35:1173–1181. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 19.Thurston CL, Starnes A, Randich A. Changes in Nociception, Arterial Blood Pressure and Heart Rate by Intravenous Morphine in the Conscious Rat. Brain Res. 1993;612:70–77. doi: 10.1016/0006-8993(93)91645-9. [DOI] [PubMed] [Google Scholar]
- 20.Bhatnagar S, Dallman MF, Roderick RE, Basbaum AI, Taylor BK. The Effects of Prior Chronic Stress on Cardiovascular Response to Acute Restraint and Formalin Injection. Brain Res. 1998;797:313–320. doi: 10.1016/s0006-8993(98)00382-5. [DOI] [PubMed] [Google Scholar]
- 21.Arvidsson U, Riedle M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, et al. Distribution and Targeting of a Mu-Opioid Receptor (MOR1) in Brain and Spinal Cord. J. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]