Abstract
Objective:
This project aimed to assess the antibacterial potential of various brands of honey sold in Muscat area on some isolates of H. pylori and to determine if there is any synergy between honey and amoxycillin or clarithromycin used in the treatment of H. pylori gastritis and duodenal ulcer.
Methods:
Eight samples of commercial honey were used in the experiment after they were checked for purity by sub-culturing on blood agar and incubating for 48 hours at 37°c. Honey samples showing gross contamination were discarded. Purified culture isolates of H. pylori from our laboratory stock cultures were swabbed on chocolate plate using 1x 104 cfu/ml. One hundred microlitres (100μl) of various honey samples were placed on each plate which was subsequently incubated microaerophilically at 37ºc for 3 days. The presence or absence of growth inhibition zones on each plate was noted and an average zone size of each honey was taken. Honey samples with high zone sizes were further diluted from 1:2–1:8 to find the end-points of their growth inhibition concentrations and the experiment was repeated in triplicates. The synergistic effect between honey, amoxycillin and clarithromycin was done in triplicates by placing honey at various distances between each antibiotic after swabbing chocolate agar with 1x 104 cfu/ml of H. pylori. The plates were incubated as before.
Results:
All honey samples produced growth inhibition zones with H. pylori no at dilution of honey but had different zone sizes at 1:2–1:8 dilutions. Black Forest honey had the highest antibacterial activity followed by Langnese honey. None of the honeys had a synergistic effect with either clarithromycin or amoxycillin.
Conclusion:
We conclude that, in vitro, some honey brands possess antibacterial activity against H. pylori and that no synergy or antagonism was observed between honey and clarithromycin or honey and amoxicillin using H. pylori as a test organism. Though no synergy or antagonism was observed between honey, amoxicillin or clarithromycin, it has been suggested that the use of honey with triple therapy regimen may help shorten the time required to eliminate H. pylori from stomach lining of patients with gastritis or duodenal ulcer caused by H. pylori infection
Keywords: Antibacterial, Amoxicillin, Clarithromycin, Honey, H. pylori, Triple therapy, Synergy
The history of Helicobacter pylori dates back to 1875 when the German scientists first found spiral bacteria in the lining of the human stomach.1 Walery in 1899 published a paper on spiral shaped bacteria from the sediment of human gastric washings, but his publication was in Polish so it attracted little or no attention.2
The work of the German and Polish scientists was not rediscovered till 1979 when Warren saw curved and spiral bacteria in the biopsy material from gastric mucosa. He and Marshall isolated and cultured the organism and named it Campylobacter pyloridis.3 They contended that most stomach ulcers and gastritis were caused by colonization with this bacterium. Following DNA sequences, the organism in 1989 was renamed Helicobacter pylori by Warren and Marshall.4
However, the association of H. pylori with gastritis or ulcers was distrusted since it was believed that no bacterium could survive for long in the acid pH of the stomach. To prove their point, Marshall infected himself and had gastritis. The organism was subsequently isolated from his stomach lining.5 Marshall and Warren were awarded the Nobel Prize in medicine in 2005 for their discovery of H. pylori and its role in gastritis and peptic ulcer disease.6
Currently, it is estimated that half the world’s population harbours H. pylori and that while it exits as normal flora in some people, in others, it causes gastritis and ulcers.7
Malfertheiner et al.8 recommended the use of triple therapy which includes an anti-ulcer agent such as ranitidine, bismuth citrate, or a proton pump inhibitor, omeprazol or pantoprazole and a combination of two antibiotics from either metronidazole, clarithromycin or amoxicillin for elimination of H. pylori from the stomach. Though this recommendation was made and is applied in the treatment of H. pylori infection, a significant problem associated with the treatment is the organism’s increasing resistance to antibiotics and the failure of the clinicians to follow up the course of treatment with repeated isolations and sensitivity pattern of the original isolate. Hiyana9 in Japan found most of his H. pylori isolates to be resistant to clarithromycin and metronidazole while Crone et al.10 isolates from children and adolescent, in vitro, showed resistance to clarithromycin.
Honey has been used as a medicine since ancient times in many countries.11,12,13 Its use is recorded in Sumerian clay tablets estimated to be 4000 years old and in Egyptian papyri from 1900 to 1250 B.C.11 The revelation in the Holy Koran and documentation in the Hadith clearly referred to the effectiveness of honey in the healing of diseases for mankind.14 Abu Taib et al.14 reported that growth of H. pylori was inhibited by 20% concentration of natural honey while Al Somal et al,15 Mcgovern et al.16 found, in vitro, that manuka honey solution possessed antibacterial properties against H. pylori.
Our project aimed to determine the antibacterial potential of various brands of honey sold in Muscat area on H. pylori and to find out if there is synergy between honey and amoxycillin or clarithromycin which are two antibiotics currently in use in the treatment of H. pylori infection in Sultan Qaboos University Hospital (SQUH).
METHODS
CULTURES OF HELICOBACTER PYLORI
These were provided from departmental stock cultures. The organisms were checked for purity by inoculating them onto Campylobacter and chocolate agar media and incubating microaerophilically (CO2 10%, Oxygen 10%, Nitrogen 80%) for 5 days at 37°C. Grown colonies were Gram stained and observed for spiral shape, oxidase and urease activities. Spiral shaped, urease and oxidase positive colonies typical of H. pylori were used in the experiment.
HONEY SAMPLES
Thirteen brands of commercial honey available in Muscat area of Oman were collected for the study. They were checked for purity by inoculating onto blood agar and incubating aerobically at 37°C for 48 hours. Samples showing growth of bacteria or growth of more than 4–5 colonies of yeasts were excluded from the study. Only eight samples were found good for our study [Table 1].
Table 1:
Honey samples and their sources
| Honey sample | Sources |
|---|---|
| Black Forest | Germany |
| Langnese Forest | Germany |
| Langnese Natural Bee | Germany |
| Black Forest | Germany |
| Blossom bee honey | Switzerland |
| Al-Shifa natural honey | Iran |
| Al-Nada chestnut honey | Oman |
| Al-Nada clove honey | Oman |
CHECKING THE HONEY SAMPLES FOR ANTIBACTERIAL ACTIVITY
The honeys were initially checked for antibacterial activity by using Staphylococcus aureus (NCTC 6571) as a control organism. This strain of S. aureus is known to be sensitive to most antimicrobial agents and is used in our laboratory for the control of our antibiotic sensitivity pattern. An inoculum containing 1x104 cfu/ml of the organism was prepared using McFarland tube 0.5. A dry swab immersed into it was pressed on the side of the bottle to remove excess fluid and was subsequently used to swab a dry diagnostic sensitivity test (DST, Oxoid, UK) plate. One hundred microlitres (100μl) of honey was placed on the surface of each plate using a chipped sterile tip. This process was applied for each honey sample. The plates were incubated at 37°C overnight and zones of growth inhibition produced by each honey with the test organism were subsequently taken.
DETERMINATION OF ANTIBACTERIAL ACTIVITY OF HONEY ON H. PYLORI
Inocula containing 1x 104 cfu/ml of each H. pylori were prepared and smeared on chocolate as done with S. aureus control. One hundred microliters (100μl) of each individual honey were placed on the surface of each plate using a chipped sterile tip as before. The plates were incubated microerophilically (CO2 10%, Oxygen 10%, Nitrogen 80%) at 37°C for 3 days. Honey samples producing zones of inhibition of more than 8mm were double diluted from 1:2 to 1:8 to obtain the final end-points of antibacterial activity.
SYNERGISTIC TEST BETWEEN HONEY AND SOME ANTIBIOTICS
The antibiotics used in this experiment were those used for the treatment of cases of H. pylori infection at SQUH. Amoxycillin (Panpharma, France) and clarithromycin (Panpharma, France) obtained from the Pharmacy Department of the SQUH at concentrations of 250mg powder were diluted to 10μg and 15μg respectively using sterile distilled water. These concentrations are contained in the antibiotic discs routinely used at Sultan Qaboos University Hospital for the determination of H. pylori sensitivity. Efforts to obtain the antibiotics as pure liquid concentrates proved impossible.
Two isolates of H. pylori out of five used in this experiment were employed. An inoculum of each H. pylori was prepared and smeared on plates as mentioned earlier in the text. Fifty microlitres (50μl) of honey and antibiotics were placed at 15, 20 and 25mm apart from each other using three sets of plates per distance and for each antibiotic. They were allowed to seep into the media and incubated microaerophilically (CO2 10%, Oxygen 10%, Nitrogen 80%) at 37ºC for 3 days. Synergy exists if the growth inhibition zone produced by honey in combination with any of the antibiotics is greater than growth inhibition zones produced by either alone.17
RESULTS
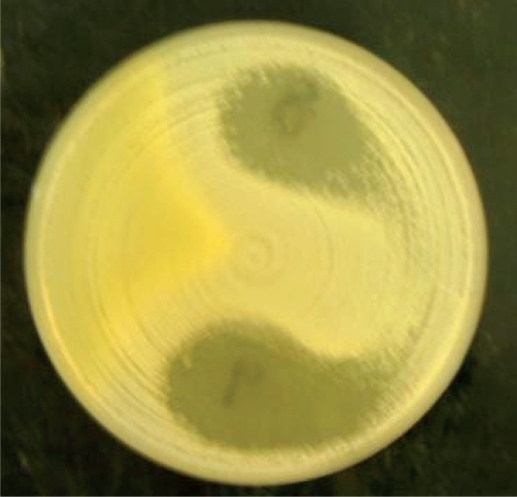
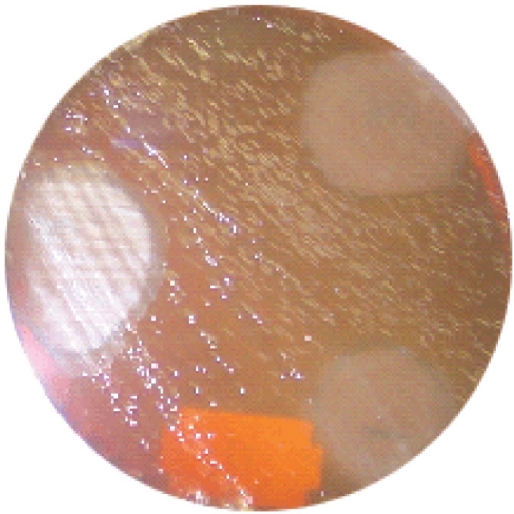
Table 1 shows the types of honey and their sources while figure 1 is a micrograph of the growth inhibition zones produced by some honey on S. aureus (control organism). Table 2 shows the inhibition zone sizes produced by various honeys at various dilutions of the honeys. The highest growth inhibition zone was produced by Black Forest (sample 1) followed by Langnese honey and natural honeys (samples 2 and 3). Other honeys used in the investigation produced zones only at no dilution. Figure 2 represents the micrograph of the growth inhibition zones produced by some of the honey samples on H. pylori on chocolate agar.
Figure 1:
Zone of inhibition of honey on Staphylococcus aureus (NCTC6571).
Table 2:
The zone of growth inhibition of the honey samples on H. pylori
| Code | Honey samples |
Zone of inhibition (mm) at various dilutions |
||
|---|---|---|---|---|
| Neat | 1:2 | 1:4 | ||
| 1 | Black Forest | 25 | 19 | 18 |
| 2 | Langnese Forest | 25 | 17 | 17 |
| 3 | Langnese Natural Bee Honey | 28 | 11 | Zero |
| 4 | Black Forest | 25 | Zero | Zero |
| 5 | Blossom Bee Honey | 15 | ND* | ND |
| 6 | Al-Shifa Natural Honey | 17 | ND | ND |
| 7 | Al-Nada Clove Honey | 29 | Zero | Zero |
| 8 | Al-Nada Chestnut Honey | 26 | ND | ND |
Not done (insufficient amount of honey)
Figure 2:
Zone of inhibition of honey on Helicobacter pylori on chocolate agar.
No synergy was observed between honey and clarithromycin or honey and amoxicillin.
DISCUSSION
Honey is known to possess antimicrobial activity and has been used to cure many skin infections.11,12,13,18,19 In our experiment, we observed that all the honey samples examined possessed antibacterial activity against H. pylori with the natural Black Forest honey from the Black Forest in Germany showing the highest antibacterial activity. This is followed by Langnese honey also from Germany.
The elements responsible for the antibacterial activity of honey are not fully known, but have been attributed to various polyphenolic compounds found in honey (propolis, flavonoids, flavones, tannins) and to glucose oxidase and osmosis.11 Honey is a product of various plant nectars and differences exist between one nectar and the other based on plant species, the season of the year and the geographical location of the plant.11 Molan11,19 attributed the antibacterial activity of honey to its content of glucose oxidase, which liberates hydrogen peroxide when it is diluted. The hydrogen peroxide so liberated is antibacterial besides encouraging epithelial proliferation.
Burdon20 emphasized that the normal route of wound healing is the stimulation of the growth of fibroblasts and epithelial cells by the hydrogen peroxide produced as a result of injury or infection. Somerfield21, Condon22 and Osato23 ascribed the antibacterial effect of honey to the osmotic effect of its sugar content. Honey contains 38% fructose, 31% glucose and 17% water while its pH is 3.9.24 Its low content of water encourages wound healing by hygroscopic absorption of water molecules on wound surfaces and by soothing of the wound.12,13
The findings of our work agree with the observation of many earlier researchers14,15,16,19,23,25 who, in vitro, found honey to possess antibacterial activity against H. pylori.
In SQUH, amoxicillin and clarithromycin are used in the treatment of Helicobacter infection using triple regimen.26 The use of triple regimen is based on the fact that antibiotics will eliminate H. pylori allowing gastric or duodenal ulcers to heal naturally.8
This condition is not always obtained for some patients fail to comply with the treatment regimen, thereby creating a lack of sufficient concentration of antibiotics in the stomach over a period required to eliminate the organism. In addition, there is an increase in gastric pH which affects the activity of some of the drugs. The low content of the drugs in the stomach results in bacterial mutation, increase of bacterial load and subsequent emergence of intracellular and dormant forms of the bacterium which renders the drugs ineffective.27
Though in our experiment, we found no synergy existing between amoxicillin or clarithromycin and honey, suggestions were made that the combination of triple regimen with any of the agents known, in vitro, to have antibacterial effect against H. pylori could help eliminate the organism from the stomach.19,27 In this respect, suggestions were advanced of combining the triple therapy regimen with fresh or fermented milk, propolis, broccoli sprouts, ginger, honey, or mangava-brava.27,28,29,30,31,32,33 Fresh milk contains lactoferrin, fermented milk (lactoferrin and lactobacilli), propolis (polyphenols), broccoli sprouts (sulforaphane) ginger (gingerols), honey (glucose oxidase and polyphenols), mangava-brava (ellagic acid). These substances, individually, are antibacterial and are known in vitro, to stop the growth of H. pylori, but whether, in combination with the triple therapy, more effective elimination of H. pylori is ensured is subject to further experiment, though synergy has been shown to exists between honey and some antibiotics.34
However, the advantage which honey has over other substances suggested is that honey has a pH of 3.9.24 and its dilution by gastric juices in the stomach will activate its glucose oxidase content. The glucose oxidase so activated will liberate hydrogen peroxide which is both antibacterial and an activator of fibroblasts and epithelial cells needed in the healing process of the ulcer caused by H. pylori. Helicobacter pylori thrives in acid pH, but produces infection under alkaline pH due to its ability to hydrolyze urea from mucosa cells liberating ammonia27. The acid pH of honey can neutralize this thereby helping the healing process of ulcer caused by the organism. The drawback is that it is difficult to quantify the dosage, but it is worth experimenting on.
In our experiment, we found the use of the surface diffusion method better than well diffusion. In surface diffusion, contaminants in honey had more surface area for growth and were clearly visible as contaminants but this was absent in the well diffusion method. Moreover, viscous honey samples diffused poorly in the well diffusion method resulting in little or no growth inhibition zones being produced.
CONCLUSION
In summary, we have tried to show that in vitro many honey samples sold in Muscat area of Oman possess antibacterial activity against H. pylori and that the degree of this antibacterial activity varies from sample to sample. The differences in antibacterial activity, in our opinion, are dependent on where the nectars were obtained by the bees, the mode of preparation and preservation of the honeys and how long the honeys stayed on the shelves before use. We found that no synergy existed between honey and amoxicillin or clarithromycin used in the treatment of H. pylori infection. Though no synergy was observed between honey and the antibiotics used in our experiment, we suggest that honey used in combination with triple therapy may help speed up the rate of elimination of H. pylori from cases of gastritis and duodenal ulcer patients.
Acknowledgments
The authors are most grateful to Dr. Akbar Rafay for providing all the stock cultures of H. pylori used in the experiment, the Pharmacy Department of SQUH for providing the antibiotics for synergy experiment and to the staff of Microbiology and Immunology for their cooperation through out the period of the investigation.
REFERENCES
- 1.Blasser MJ. An endangered species in the stomach. Sci Am. 2005;292:38–45. doi: 10.1038/scientificamerican0205-38. [DOI] [PubMed] [Google Scholar]
- 2.Konturek JW. Discovery by Jaworski of Helicobacter pylori and its pathogenic role in peptic ulcer, gastritis and gastric cancer. J Physiol Pharmacol. 2003;54:23–41. [PubMed] [Google Scholar]
- 3.Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 4.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 5.Helicobacter pylori in peptic ulcer disease NIH Consensus Statement online Jan 7, 1994; 12:1023. [PubMed] [Google Scholar]
- 6.The Nobel Prize in Physiology or Medicine awarded to Marshall BJ and Warren JR “For their discovery of the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer disease”. Nobelprize.org/med/laureates/2005/index.html, Accessed 9th September 2006.
- 7.Angela CM, Charles WP. Helicobacter pylori. In: Sussman Max., editor. Molecular Medical Microbiology. London: Academic Press; 2002. pp. 1331–1347. [Google Scholar]
- 8.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: The Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 9.Hiyana T, Tanaka S, Masuda H, et al. Prevalence of Helicobacter pylori resistance to clarithromycin and metronidazole determined by 23s ribosomal RNA and rdxA gene analyses in Hiroshima, Japan. J Gastroenterol Hepatol. 2003;18:1202–1207. doi: 10.1046/j.1440-1746.2003.03140.x. [DOI] [PubMed] [Google Scholar]
- 10.Crone J, Granditsch G, Huber WD, et al. Helicobacter pylori in children and adolescent: Increase of primary clarithromycin resistance 1997–2000. J Paediatr Gastreenterol Nutr. 2003;36:368–371. doi: 10.1097/00005176-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Molan PC. The antibacterial activity of honey: 1 The nature of the antibacterial activity. J Bee World. 1992;73:5–28. [Google Scholar]
- 12.Efem SE. Recent advances in the management of Fournier’s gangrene: Preliminary observations. J Surg. 1993;113:200–204. [PubMed] [Google Scholar]
- 13.Efem SE. Clinical observation on the wound healing properties of honey. Br J Surg. 1988;75:679–681. doi: 10.1002/bjs.1800750718. [DOI] [PubMed] [Google Scholar]
- 14.Abu Taib MM, Chowdhury MN, Al-Humayyd M. Inhibitory effect of natural honey on Helicobacter pylori. Trop Gastroenterol. 1991;12:139–143. [PubMed] [Google Scholar]
- 15.Al-Somal N, Coley KE, Molan PC, Hancock BM. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J Royal Soc Med. 1994;87:9–13. doi: 10.1177/014107689408700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mc-Govern PB, Abbas SZ, Vivian G, Dalton HR. Manuka honey against Helicobacter pylori. J Royal Soc Med. 1999;92:439. doi: 10.1177/014107689909200832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miles RS, Amyes SGB. Laboratory control of antimicrobial therapy. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney, Practical Medical Microbiology. 14th Ed. Edinburgh: Churchill Livingstone; 1996. p. 175. [Google Scholar]
- 18.Dunford C, Cooper RA, Molan PC. Using honey as a dressing for infected skin lesions. Nurs. 2000;96:7–9. [PubMed] [Google Scholar]
- 19.Molan PC. Why honey is effective as a medicine:The scientific explanation of its effects. Bee World. 2001;82:22–40. [Google Scholar]
- 20.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 21.Somerfield SD. Honey and healing. J R Soc Med. 1991;84:179. doi: 10.1177/014107689108400327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condon RE. Curious intersections of bugs and bees. Surg. 1993;113:234–235. [PubMed] [Google Scholar]
- 23.Osato MS, Reddy SG, Graham DY. Osmotic effect of honey on growth and viability of Helicobacter pylori. Dig Dis Sci. 1999;44:462–464. doi: 10.1023/a:1026676517213. [DOI] [PubMed] [Google Scholar]
- 24.White JW, et al. Composition of American honeys. USDA Tech Bull. 1962. p. 1261.
- 25.Ali AT, Chowdhury MN, Al Humayyd MS. Inhibitory effect of honey on Helicobacter pylori Trop Gastroenterol. 1991;12:139–143. [PubMed] [Google Scholar]
- 26.Scrimgeour EM, Alexander PC, Rafay Akbar, Al-Riyami Kassin. Antibiotic Handbook. 6th Ed. Oman: Courtesy of Glaxo Smith Kline; 2003. [Google Scholar]
- 27.McLoughlin R, Racz I, Buckley M, O’Connor HJ, O’Morain C. Therapy of Helicobacter pylori. Helico. 2004;9:42–49. doi: 10.1111/j.1083-4389.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- 28.Da Mota Menezea V, Atallah AN, Lapa AJ, Catapani WR. Assessing the therapeutic use of Lafoensia pacari St. Hil. Extract (Mangavabrava) in the eradication of Helicobacter pylori: double blind randomized clinical trial Helico. 2006;11:188–95. doi: 10.1111/j.1523-5378.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 29.Pantoflickova D, Corthesy-Theulaz I, Dorta G, et al. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment Pharmacol Ther. 2003;18:805–813. doi: 10.1046/j.1365-2036.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 30.Di Mario F, Aragona G, Bo ND, Ingegnoli A, Cavestro GM, et al. Use of lactoferin for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;17:125–129. [Google Scholar]
- 31.Boyanova L, Dereji S, Koumanova R, Katsarov N, Gergova G, Mitov I, Nikolov R, Krastev Z. Inhibition of Helicobacter pylori growth in vitro by Bulgarian propolis: preliminary report. J Med Microbiol. 2003;52:417–419. doi: 10.1099/jmm.0.04895-0. [DOI] [PubMed] [Google Scholar]
- 32.Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of cagA+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699–3702. [PMC free article] [PubMed] [Google Scholar]
- 33.Galan MV, Kishan AA. Silverman Al Oral broccoli sprouts for the treatment of Helicobacter pylori infection: A preliminary Report. Dig Dis Sci. 2004;49:1088–1090. doi: 10.1023/b:ddas.0000037792.04787.8a. [DOI] [PubMed] [Google Scholar]
- 34.Al Jabri AA, Al Hosni SA, Nzeako BC, Al Mahrooqi ZH, Nsanze H. Antibacterial activity of Omani honey alone and in combination with gentamicin. Saudi Med J. 2005;26:767–771. [PubMed] [Google Scholar]