Abstract
Objective:
To evaluate the impact of the National Cholesterol Educational Program Adult Treatment Panel III (ATP III) and the Framingham Offspring Study on Omani diabetic subjects.
Methods:
221 subjects with type 2 diabetes (86 females and 135 males) and 156 non-diabetic subjects (70 females and 86 males) aged 30–70 years attending Sultan Qaboos University Hospital between 1999–2002 were recruited. Lipid profile, glucose, %HbA1c, apoproteinA-1 and apoproteinB were measured. Low density lipoprotein was calculated using the Friedwald formula. ATP-III and Framingham Offspring Study guidelines were used to classify lipid parameters into coronary heart disease-risk categories.
Results:
Diabetic compared to non-diabetic subjects had significantly higher triglycerides of >1.7 mmol/L (p=0.01) and lower low density lipoprotein cholesterol of >4.2 mmol/L (p=0.012) and, in female subjects only, lower high density lipoprotein cholesterol of <1.15 mmo/L for (p<0.0001). In addition, 57% of diabetic subjects had abnormal aplipoproteinB of >1.2 g/L compared to 49% of non-diabetic subjects. Combined raised levels of triglycerides, apolipoproteinB and low levels of high density lipoprotein were found in 42% of diabetic compared to 26% of the non-diabetic subjects (p=0.05). Diabetic subjects had significantly higher (p=0.008) NCEP risk-score for coronary artery disease, however, only 34% conformed to a NCEP 10-year-risk score of >10%.
Conclusion:
A substantial proportion of the Omani diabetic subjects were dyslipidaemic according to the ATP III guidelines. This study recommends the implementation of a lower cut-off threshold for starting lipid-modifying agents for Omani diabetics when using the 10-year Framingham Risk Scoring equation.
Keywords: Lipids, Lipoproteins, diabetes mellitus, Oman, NCEP risk score
Diabetes Mellitus is a growing health problem in Oman. According to the national Omani survey carried out in 1991, the prevalence was 10% for type 2 diabetes and 13% for impaired glucose tolerance (IGT).1 This study also showed that the prevalence of diabetes in Oman rose with age and may exceed 50% in the seventh and eighth decade of life, in females and males respectively. Recently, it was reported that the prevalence of diabetes in Oman had increased over the past decade reaching 16.1% of the population aged 30–64 years old.2 Furthermore, a cross-sectional random sample survey reported that approximately 20% of the population had high fasting plasma glucose.3
Type 2 diabetes is associated with an increased risk for cardiovascular disease.4 This risk in part is due to diabetic dyslipidaemia.4,5 The report from National Cholesterol Educational Program Adult Treatment Panel III (NCEP ATP III) recognized the importance of early detection of diabetic dyslipidaemia. According to the ATP-III report, the presence of diabetes or of multiple risk factors with a 20% high 10-year risk for coronary artery disease (CAD) events are now considered to be CAD equivalents, requiring aggressive treatment as for established CAD and other types of atherosclerotic diseases.5 ATP III had further endorsed the importance of including high density lipoprotein cholesterol (HDL-C) as part of their guidelines for assessing the risk of CAD and had re-categorized some of the cut-off values of atherogenic lipoproteins. Furthermore, NCEP ATP III had recommended a scoring system based on the Framingham Risk equation using clinical and lipid data. Thus, it helps to calculate the 10-year absolute CAD risk i.e. the percentage probability of having a CAD event in 10 years and to identify certain subjects with multiple (2+) risk factors for intensive treatment Therefore, this study was aimed to determine the impact of the NCEP ATP-III on Omani subjects with type 2 diabetes in comparison with non-diabetic subjects
RESEARCH DESIGN AND METHODS
PATIENT SELECTION
The study recruited 221 patients (86 females and 135 males) with type 2 diabetes and compared them with BMI and age matched 156 (70 females and 86 males) non-diabetic patients from patients attending Diabetic and Lipid Clinics at the Sultan Qaboos University Hospital, between 1999–2002. All the subjects diagnosed as type 2 diabetics fulfilled the World Health Organization criteria for diagnosis of type 2 diabetes mellitus either by an abnormal oral glucose tolerance test (OGTT), or two abnormal fasting blood glucose tests (>7.0 mmol/L). None of the diabetic subjects were on insulin or Thiazolidinediones (TZDs) therapy. 40% of the diabetic subjects were on dietary control and the rest on oral hypoglycaemic agents, as follows: Glibenclamide (39%), Glipizide (24%), Gliclazide (6%), biguanides (13%, Metformin) and combined therapy (10%).
Patients were excluded from the study if they had a myocardial infarction in the three months prior to entry to the study, or uncontrolled thyroid disease (hypo or hyperthyroidism), macro-proteinuria (positive urine protein dip-stick x2), severe hepatic impairment (known subject with chronic active liver disease or those individuals with obstructive liver pattern) or renal impairment (creatinine level >114 ųmol/L) and those on lipid modifying agents. This study was approved by the Medical Ethics and Research committee at College of Medicine and Health Sciences, Sultan Qaboos University and patients gave informed consent prior to the study.
CLINICAL AND LABORATORY ASSESSMENT
All subjects underwent a clinical physical examination and any abnormality was documented. The subjects’ blood pressure was measured to the nearest even digit using a sphygmomanometer, with the subject in the sitting position after a 5–10 minutes rest. Subjects were labeled hypertensive if blood pressure was equal or greater than 140/90 mmHg on two repeated occasions or by 24 BP monitoring and those on anti-hypertensive therapy. BMI was calculated as weight (kg) divided by height (m2) and used as an index of adiposity. Subjects were labeled CAD if they had had a previous myocardial infarction or had a stable angina pectoris with positive thallium stress test or coronary angiogram or Percutaneous Coronary Angioplasty (PTCA).
Following a 10-hour overnight fast, blood samples were taken for measurement of HbA1c, TC, TG, HDL-C, apoA-1, and apoB. Cholesterol, TG and glucose measurements were performed using timed endpoint enzymatic methods on the Synchron CX system (Beckman, Brea, USA). The within-run and between-run precisions for cholesterol (4.3 mmol/L) were 3% and 4.5%, respectively; for TG (2.0 mmol/L) 3% and 4%, respectively; for glucose (5.5 mmol/L) 2 and 3%, respectively.
HDL-C was determined using a timed-endpoint direct homogenous assay on the same system, with-run of 2.5% and between-run of 3% precisions for HDL-C of 1.2 mmol/L. apoA-1 and apoB were determined using rate nephelometric immunochemistry assay by the IMMAGE system (Beckman). The within-run and between-run precision profiles for apoB (1.2 g/L) were 2.5% and 2.9%, respectively; for apoA-1 (1.05 g/L) they were 3.4% and 3.9%, respectively. Both the apoB and apoA-1 methods used have been standardized according to the International Federation of Clinical Chemistry.6 LDL-C was calculated using the Friedwald formula and was not calculated when TG level was >4.0 mmol/L. The Department of Biochemistry at the Sultan Qaboos University Hospital is engaged in an external quality control scheme of the Royal College of Pathologists, Association of Clinical Biochemistry, Australia.
The NCEP ATP III (2001) guidelines were used to classify lipoproteins concentration into CAD risk categories.5 The LDL-C levels were defined as low (<3.4 mmol/L), borderline (3.4–4.19 mmol/L), borderline-high (4.2–5.0 mmol/L), and high (>5.0 mmol/L) risk categories. The risk categories of HDL-C levels were defined as high (<1.05mmol/L), borderline (1.05–1.15 mmol/L), and low (>1.15 mmol/L). However, no gender cut-off value was used. As far triglycerides, the cut-off value of >1.7 mmol/L was used as borderline-high risk level. The borderline high risk categories for cut-off values were <1.2 g/L for apoA-1 and >1.2 g/L for apoB. These cut-off values were selected from the Framingham Offspring study and the International Federation of Clinical Chemistry guidelines.7 The 10-year-risk for CAD was determined using the Framingham risk scoring system as recommended by the NCEP ATP III
Descriptive analysis including the estimation of mean values and the SEM for continuous variables were documented. Prevalence and frequencies were expressed as percentages. Skewed parameters were logarithmically transformed when a parametric test was used. Categorical variables were compared by the χ2 statistic with the Yates correction or the exact Fisher test when appropriate. Binary regression analysis was carried out to study the influences of diabetes and gender on the probability of having high-risk categories for CAD for the different lipid and lipoprotein parameters, after adjustment of the other observational variables. ANOVA was used to determine differences in subject characteristics. ρ value (two-tailed) <0.05 was considered as statistically significant. All data was analyzed with the SPSS.
RESULTS
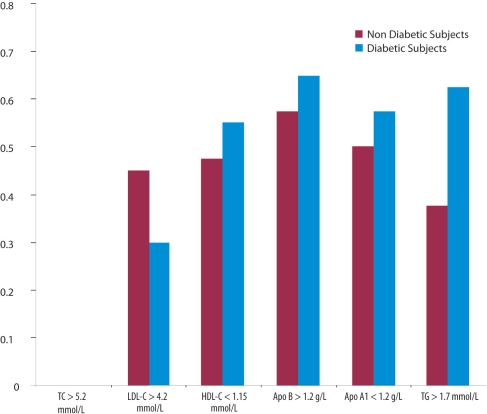
A comparison of clinical parameters and the means of fasting lipid profile between diabetic and non-diabetic subjects showed that diabetics had significantly higher mean levels of TG (p<0.0001) and lower of HDL-C (for female gender only, p<0.0001) and LDL-C (p=0.04) [Table 1]. Using NCEP-ATPII cut-off values for high borderline and high risk categories [Figure 1] indicated that 29% of diabetic subjects had significantly lower LDL-C level of (>4.2 mmol/L), compared to 43% of non-diabetic subjects ((p=0.012)). On the other hand, 57% of diabetic subjects had abnormal apoB of >1.2 g/L compared to 49% non-diabetic subjects. A significantly higher level (p=0.01) of TG (>1.7 mmol/L), was found in diabetic subjects (63%) compared to non-diabetic subjects (43%), irrespective of gender. The percentage of diabetic (56%) and non-diabetic (48%) subjects showed no significant difference on abnormal level of <1.15 mmol/L HDL-C when the same cut-off value was used for both genders as recommended by NCEP ATP III guidelines. However, despite a significantly larger percentage of male subjects, compared to female subjects, who had an HDL-C levels of <1.15 mmol/L, (p=0.001), there was a significantly higher (p=0.05) percentage of female diabetic subjects with abnormally low HDL-C levels compared to nondiabetic subjects. Borderline high-risk of apoA-1 1.0–1.2 g/L and high-risk of apoA-1 <0.9 g/L were detected in 60% of males compared to 48% of females subjects; such a difference was statistically significant (p=0.01).
Table 1 :
The clinical and metabolic characteristics of subjects with type 2 diabetes compared to non-diabetic subjects
| Non-diabetic subjects | Subjects with type 2 diabetes | pvalue Diabetic vs. non-diabetic | |||||
|---|---|---|---|---|---|---|---|
| Women | Men | Both | Women | Men | Both | ||
| Number | 70 | 86 | 156 | 86 | 135 | 221 | |
| Age | 48.3 (1.8) | 45.7 (1.5) | 46.6 (1.3) | 47.8 (1.1) | 49.5 (1.0) | 47.9 (0.91) | 0.30 |
| BMI | 31.2 (0.76) | 31.3 (0.61) | 31.2 (0.5) | 30.2 (0.98) | 30.9 (0.8) | 30.2 (0.61) | 0.45 |
| TG (mmol/L) | 1.29 (1.03–1.6) | 1.41 (1.23–1.61) | 1.36 (1.21–1.57) | 1.77 (1.65–2.01) | 1.86 (1.75–2.09) | 1.82 (1.69–1.98) | <0.0001 |
| TC (mmol/L) | 6.2 (0.21) | 5.8 (0.15) | 5.93 (0.11) | 5.92 (0.18) | 5.75 (0.14) | 5.85 (0.11) | 0.79 |
| LDL-C (mmol/L) | 4.10 (0.19) | 4.2 (0.13) | 4.2 (0.10) | 3.8 (0.11) | 3.6(0.12) | 3.7 (0.09) | 0.04 |
| HDL-C (mmol/L) | 1.47 (1.27–1.58) | 1.19 (1.05–1.24) | 1.24 (1.13–1.37) | 1.17 (1.15–1.26) | 1.10 (1.05–1.14) | 1.12 (1.10–1.18) | 0.10 |
| Apo B (g/L) | 1.33 (0.05) | 1.29 (0.04) | 1.30 (0.03) | 1.31(0.04) | 1.23 (0.03) | 1.25 (0.02) | 0.53 |
| ApoA1 (g/L) | 1.3 (0.04) | 1.13 (0.02) | 1.18 (0.02) | 1.24 (0.04) | 1.15 (0.02) | 1.18 (0.02) | 0.89 |
| Hypertension (%) | 35 | 36 | 35 | 61 | 60 | 60 | <0.0001 |
| CAD % | 15 | 25 | 20 | 22 | 40.8 | 34 | <0.0001 |
| Fasting glucose (mmol/L) | 5.4 (0.12) | 5.2 (0.11) | 5.3 (01) | 5.8 (0.22) | 6.0 (0.21) | 5.9 (0.2) | 0.006 |
| HBA1c (%) | 5.5 (0.05) | 5.4 (0.06) | 5.4 (0.05) | 8.09 (0.3) | 7.6 (0.22) | 7.7 (0.16) | <0.0001 |
| NCEP risk-score | 9.8 (0.8) | 8.1 (0.9) | 8.7 (0.8) | 11.2 (0.8) | 10.8 (0.5) | 10.98 (0.4) | 0.008 |
Data are number, means +/− SEM, 95% CI for Log transformed variables and %. TG and HDL-c
Figure 1:
Percentage of diabetic subjects with abnormal lipids and lipoproteins levels compared to non-diabetic subjects
Logistic regression analysis of the influence of type 2 diabetes and gender on the probability of having serum lipids that were outside the recommended targets adjusted for age, BMI and HbA1c, showed that diabetic compared to non-diabetics subjects had a significantly higher likelihood ratio of having TG of >1.7 mmol/L and a lower likelihood ratio of having an abnormal, LDL-C of >4.2 mmol/L, irrespective of gender [Table 2]. Female diabetics had a significantly higher likelihood ratio of having an abnormal HDL-C level of <1.15 mmo/L compared to female non-diabetic subjects. Furthermore, female subjects had a significantly higher likelihood ratio of having an LDL-C of >4.2 mmol/L and a lower likelihood ratio of having an HDL-C of <1.15mmol/L and apoA-1 of <1.2 g/L compared to males.
Table 2:
Logistic regression analysis examining the influence of diabetes and gender on the probability of having LDL-C, HDL –C, TG, apo B, and apo A-1 outside the recommended target
| Probability | LDL -C> 4.2 mmol/L | pvalue | TG >1.7 mmol/L | pvalue | HDL-C <1.15 mmol/L | pvalue | Apo B >1.2 g/L | pvalue | Apo A-1 <1.2 g/L | pvalue |
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetic subjects vs. non-diabetic subjects | 0.572 (0.357–0.918) | 0.02 | 2.183 (1.344–3.548) | 0.002 | 1.334 (0.836–2.181) | 0.25 | 1.334 (0.776–2.205) | 0.31 | 1.205 (0.170–2.023) | 0.48 |
| Diabetic women vs. non-diabetic women | 0.581 (0.259–1.256) | 0.15 | 2.520 (1.129–5.627) | 0.024 | 3.724 (1.458–9.522) | 0.006 | 1.529 (0.662–3.760) | 0.35 | 1.846 (0.752–4.536) | 0.18 |
| Diabetic women vs. diabetic men | 2.472 (1.485 4.115) | <0.0001 | 0.660 (0.357–1.221) | 0.18 | 1.052 (0.589–1.945) | 0.87 | 1.508 (0.780–2.910) | 0.22 | 0.718 (0.379–1.377) | 0.30 |
| Diabetic men vs. non-diabetic men | 0.495 (0.265–0.926) | 0.03 | 2.021 (1.096–3.725) | 0.024 | 0.860 (0.466–1.586) | 0.63 | 1.200 (0.631–2.282) | 0.57 | 0.978 (0.509–1.879) | 0.95 |
| Women vs. men | 2.320 (1.440–3.745) | 0.001 | 1.275 (0.819–1.984) | 0.28 | 0.468 (0.296–0.742) | 0.001 | 1.170 (0.746–1.834) | 0.49 | 0.658 (0.417–0.989) | 0.04 |
Data are odd ratio (95% CI). Adjusted for age, HBA1c, and BMI
Combined dyslipidaemia of two or more lipid abnormalities was observed in 89% of diabetic and 76% of non-diabetic subjects. Combined raised levels of TG and apoB and raised TG and low level of HDL-C was highly prevalent (p=0.05) among diabetic compared to non-diabetic subjects, [Table 3].
Table 3 :
Proportion of subjects having combined dyslipidemia, borderline-high risk categories for CAD, according to the NCEP ATP III and the Framingham Offspring study
| Combined dyslipidaemia | Non-diabetic(%) | Diabetic (%) |
|---|---|---|
| LDL-C (>3.4 mmol/L) and TG (>1.7 mmol/L)* | 24 | 34 |
| TG (>1.7 mmol/L) and HDL-C (<1.15 mmol/L)* | 23* | 37* |
| LDL-C (>3.4 mmol/L) and HDL-C (<1.15 mmol/L) | 30 | 30 |
| All the three | 15 | 19 |
| TG (>1.7 mmol/L) and Apo B (>1.2 g/L) | 27* | 43* |
| TG (>1.7 mmol/L), Apo B (>1.2 g/L) and HDL-C (<1.15 mmol/L) | 18 | 25 |
Statistically significant, p value = 0.04
Omani subjects with type 2 diabetes had significantly higher (p=0.008) NCEP risk-score compared to non-diabetics [Table 1]. The Framingham scoring divides subjects with multiple risk factors into those with 10-year-risk for CAD of >20%, 10%–20%, and <10%. The percentage of diabetic subjects (34%) with a 10-year risk for CAD of >10%–20% was significantly higher (p=0.05), compared to non-diabetics (24 %,).
DISCUSSION
Detection and treatment of dyslipidaemia are means of reducing the risk of CAD associated with type 2 diabetes.4,8 When applying the recent guidelines released by NCEP ATP III and the Framingham Offspring Study to classify lipoprotein concentrations, a large proportion of studied Omani diabetic subjects turned out to be dyslipidaemic. Combined raised levels of apoB, triglycerides and low levels of HDL-C were highly prevalent among Omani diabetic subjects compared to non-diabetic subjects, despite the fact that the proportion of Omani diabetic subjects with borderline-high and high-risk categories of LDL-C for CAD was significantly lower compared to non-diabetic subjects. The NCEP recommends an optimal goal of LDL cholesterol to be <2.6 mmol/L for diabetics.4 When using this clinical end point, a substantial percentage of the diabetics would require intervention and ongoing monitoring to ensure that the recommended LDL-C goal is reached and maintained.
Although the gender comparisons for HDL-C risk categories were statistically significant, female subjects with type 2 diabetes had a six to seven-fold increased likelihood of having high-risk category levels of HDL-C for CAD compared to non-diabetics. This may explain why the protective effect of gender against CAD is not evident in diabetic women. However, a similar proportion of males with borderline-high and high-risk categories was noticed in both diabetic and non-diabetic subjects. The latter finding could in part explain the high risk of CAD among the males compared to the females. Given the recent findings of the beneficial effect of increasing HDL-C levels, these data indicate that increasing this lipoprotein, along with lowering LDL-C levels, should be an important target for intervention among Omani subjects irrespective of the diabetic status.9, 10 The 10-year absolute risk-score for CAD was calculated for all subjects studied using the Framingham risk-scoring equation. This study indicated that Omani subjects with type 2 diabetes had significantly higher risk-score for CAD compared to non-diabetics and were more significant among those diabetic subjects with established CAD. However, only a small proportion of Omani diabetic subjects showed a risk-score for CAD of >20%. A similar finding was reported by Durrington in 2001, who highlighted the above point and suggested an CAD risk score of >15% over 10-year as a threshold for starting statin therapy.11
Recognizing that diabetes confers a cardiovascular risk equivalent to that of established atherosclerotic disease, the ATP III set the same LDL-C target (<2.6 mmol/L) for diabetic patients and, therefore, recommended aggressive treatment with lipid lowering drugs to achieve this target. Clinical trials have confirmed the benefits of LDL-C lowering as an effective primary prevention strategy for diabetic patients8,12 and recommended the use of statin without a particular threshold level of LDL-C as the sole arbiter of which patients with type 2 diabetes should receive statins. However, in many patients with diabetes and cardiovascular disease, it will be difficult to attain an LDL-C goal of <1.8 mmol/L since approximately 25% of patients will require more than two lipid-lowering drugs at maximal doses to attain this goal, assuming 100% tolerance of lipid-lowering medications.13 There remains an important opportunity to improve the quality of care for these high-risk patients if the management of dyslipidemia is set optimally to achieve guideline-recommended lipid targets14, which may be achieved by the combination of drugs15 and/or rosuvastatin.16 Indeed, an economic simulation model suggested that increasing the use of rosuvastatin can result in cardiovascular event reduction and cost savings.17 This implies that our diabetic patients who have high prevalence of dyslipidemia, but low calculated CAD risk, may benefit substantially from earlier intervention and therefore be prevented from having cardiovascular events.
Acknowledgments
We would like to thank Dr. Ali Al-Hinai, consultant cardiologist, for his support in patients recruitment and the finncial support by Sultan Qaboos University grant; IG/MED/BIOC/00/0
Abbreviations:
- TC
total cholesterol
- LDL-C
Low Density Lipoprotein cholesterol
- HDL-C
High Density Lipoprotein cholesterol
- TG
triglycerides
- apoB
apolipoprotien B
- apoA-1
apolipoprotein A-1
- CAD
coronary artery disease
- OGTT
Oral Glucose Tolerance Test
- NCEP
National Educational Cholesterol Program
- ATP III
Adult Treatment Panel III
- BMI
Body Mass Index.
REFERENCES
- 1.Asfour MG, Lambourne A, Soliman A, et al. High prevalence of diabetes mellitus and impaired glucose tolerance in the Sultanate of Oman: Results of the 1991 national survey. Diabet Med. 1995;12:1122–1125. doi: 10.1111/j.1464-5491.1995.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Lawati A, Riyami A, Mohammed A, Jousihati P. Increasing prevalence of diabetes mellitus in Oman. Diabet Med. 2002;19:954–957. doi: 10.1046/j.1464-5491.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Lawati JA, Mohammed AJ, Al-Hinai HQ, Jousilahti P. Prevalence of the metabolic syndrome among Omani adults. Diabetes Care. 2003;26:1781–1785. doi: 10.2337/diacare.26.6.1781. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetic Association Standards of Medical care for Patients with Diabetes Mellitus. Diabetes Care. 2003;26:S33–S50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 5.Third Report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner El. Apolipoprotein B and A-1 values in 127,576 Swedish males and females, standardized according to the World Organization–International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998;44:1641–1649. [PubMed] [Google Scholar]
- 7.Contois JH, McNamara JR, Lammi-Keefe CJ, Wilson PW, Massov T, Schaefer EJ. Reference intervals for plasma apolipoprotein B determined with a standardized commercial immunoturbidimetric assay: Results from the Framingham Offspring Study. Clin Chem. 1996;42:515–523. [PubMed] [Google Scholar]
- 8.Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 9.Haim M, Benderly M, Brunner D, Behar S, Graff E, Reicher-Reiss H, et al. Elevated serum triglyceride levels and long-term mortality in patients with coronary heart disease: the Bezafibrate Infarction Prevention (BIP) Registry. Circulation. 1999;100:475–482. doi: 10.1161/01.cir.100.5.475. [DOI] [PubMed] [Google Scholar]
- 10.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of High-Density Lipoprotein Cholesterol Interventional Trial Study Group. N Engl J Med. 1999;341:410–18. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 11.Durrington P. Calculation of coronary risk in Type II diabetes. Clinical Science. 2001;101:681–682. [PubMed] [Google Scholar]
- 12.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy AG, MacLean CD, Littenberg B, Ades PA, Pinckney RG. The challenge of achieving national cholesterol goals in patients with diabetes. Diabetes Care. 2005;28:1029–1034. doi: 10.2337/diacare.28.5.1029. [DOI] [PubMed] [Google Scholar]
- 14.Yan AT, Yan RT, Tan M, Hackam DG, Leblanc KL, Kertland H, et al. Vascular Protection (VP) and Guidelines Oriented Approach to Lipid Lowering (GOALL) Registries Investigators. Contemporary management of dyslipidemia in high-risk patients: targets still not met. Am J Med. 2006;119:676–683. doi: 10.1016/j.amjmed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Carmena R. Type 2 diabetes, dyslipidemia, and vascular risk: rationale and evidence for correcting the lipid imbalance. Am Heart J. 2005;150:859–870. doi: 10.1016/j.ahj.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Guthrie RM. Rising to the challenge of treating high-risk patients. Am J Manag Care. 2006;12:S318–S324. [PubMed] [Google Scholar]
- 17.Huse DM, Song X, Ozminkowski RJ, et al. Impact of rosuvastatin use on costs and outcomes in patients at high risk for cardiovascular disease in US managed care and medicare populations: A data analysis. Clin Ther. 2006;28:1425–1442. doi: 10.1016/j.clinthera.2006.09.019. [DOI] [PubMed] [Google Scholar]