Abstract
Chronic social defeat stress in mice significantly decreases subsequent social interactions and induces other depression-like behaviors. Here we measured and manipulated levels of acetylated histone H3 (acH3), a chromatin mark of transcriptional activation, in the hippocampus and amygdala after ten continuous days of social defeat stress in male C57/Bl6J mice. This form of social stress causes a transient increase, followed by a persistent decrease, in levels of acH3 in hippocampus. By comparison, increased acH3 in amygdala was more robust but also highly transient. The persistent decrease in acH3 in hippocampus may be pathological, since it is reversed by chronic fluoxetine administration. Consistent with this hypothesis, infusion of a histone deacetylase (HDAC) inhibitor MS-275 (100 μM) into hippocampus reverses a defeat-induced deficit in sucrose preference, although it does not restore social interaction behavior. Next, different forms of social enrichment were examined with or without hippocampal infusion of MS-275. After social stress, simple pair-housing with another male C57, or female C57, mouse does not reverse social avoidance. However, when HDAC inhibitors are infused into hippocampus during social housing with another male, social avoidance is attenuated. Interestingly, social avoidance is reversed when MS-275 is infused directly into amygdala. Together, these findings further support the antidepressant potential of HDAC inhibitors, and indicate that temporally overlapping environmental and molecular events are required to optimally reverse specific stress-induced behavioral symptoms.
Keywords: Social stress, depression, hippocampus, amygdala, histone deacetylase inhibitor, social interaction
Introduction
Chromatin modifications are a powerful mediator of gene expression, and a candidate mechanism for elevating mood in clinical depression. Chronic administration of antidepressant drugs alters histone acetylation and other chromatin modifications in specific brain regions [1,2,3,4]. The hippocampus and amygdala are two such regions important for conditioned emotional responses [5,6]. Combined use of antidepressant medications and cognitive-based therapies is most effective for treating clinical depression [7]. Therefore, treatment outcomes may occur best through cognitive therapies that are reinforced by forms of neural plasticity at the molecular level in limbic brain regions. Here, we examined the antidepressant potential of MS-275, a selective inhibitor of class I HDACs, infused directly into hippocampus or amygdala during various conditions of social housing. This was based on the hypothesis that social enrichment after stress might promote the extinction of pathological social avoidance behavior. In fact, social isolation is a form of social stress that promotes depression-like behaviors in rodents and primates [8,9], and increasing histone acetylation in the hippocampus facilitates the learning of new events [10].
Methods
We utilized a social defeat model [11] to explore the effect of chronic social stress on acH3 at Lys 14 (acH3K14) in hippocampus and amygdala of 9-11 week old C57/Bl6J male mice (Jackson Laboratories), following published methods [12]. Briefly, 1 hour, 24 hour, or 10 days after 10 days of social defeat stress or after 20 additional days of fluoxetine administration (20 mg/kg ip daily), the brains of mice (n=6/group) were collected and coronal sections (35 μm) were processed for infrared immunohistochemistry using a Licor system as described previously [12]. Integrated intensities of acH3 and total levels of the histone linker protein, histone H1, were determined using Odyssey software. Results are reported as integrated intensity values/mm2 and are presented as mean ± SEM. H1 values were used as a normalization control.
The antidepressant potential of MS-275 was examined across several behavioral assays. C57/Bl6J mice (n=10-12/group) were surgically implanted with two subcutaneous Alzet® minipumps (model 1002, Durect Corp., Cupertino, CA) containing MS-275 (100 μM, provided by Steven Haggerty, Broad Institute), or 5% hydroxypropyl β-cyclodextrin vehicle (Trappsol®, CTD, Inc), connected to bilateral guide cannulae (28 gauge, Plastics One, Roanoke, VA) as described [12]. The cannulae targeted dorsal hippocampus (AP -2.0, ML +1.4, DV -1.5 mm Bregma) or amygdala (AP -2.0, ML +3.2, DV −4.9, 30° angles). Mice recovered at least 5 days after surgery before behavioral testing (details for each test can be found in [12]). Mice were assessed across four behavioral assays in the following order. Open field: Locomotor activity (distance traveled, cm) was assessed in the same arena used for social interaction testing during a 2.5 minute trial immediately prior to a test for social interaction. Social Interaction: Social interaction was tested as described [11], with a novel CD1 aggressor mouse used as a social target. Sucrose Preference: Twenty-four hours later, sucrose preferences were calculated as a percentage of sucrose (1%)/water consumed and averaged over 2 days. Forced Swim Task: Twenty-four hours after the final sucrose preference assessment, videotracking-based methods were used to record the duration of time spent “immobile” in a beaker of water over a 6-min trial [13].
In a separate series of experiments, 40 C57/Bl6J mice that were socially defeated along with 30 non-stressed controls were used to examine the effect of social housing on social interaction. Twenty-four hours after 10 days of defeat stress or control conditions, social interaction was assessed in all 70 mice. Thirty defeated mice with the lowest interaction scores and the thirty controls were then distributed evenly across three separate housing conditions (i.e., single housing, pair housing with another C57/Bl6J male of the same age and weight, or pair housing with a female C57/Bl6J of the same age, n=10/group). During these housing conditions no overt signs of submissive behaviors (i.e., defensive upright as described in [14]) continued to be observed in experimental mice. After 10 days of social housing all experimental and control mice were again housed singly, and 24 hours later these mice were assessed for social interaction behavior.
In a final experiment, 36 additional C57/Bl6J mice were used to examine the effect of MS-275 in hippocampus during social housing. After 10 days of social defeat stress (n=20) or control conditions (n=16), all mice were tested for social interaction. The 16 most avoidant mice, and 16 controls, were then evenly distributed across two infusion conditions (MS-275 100 μM or vehicle, n=8/group) with intra-hippocampal cannulae, and 48 hours later housed with another male C57/Bl6J mouse of the same age and weight for 10 days prior to being singly housed again, and tested for social interaction behavior 24 hours later. One socially defeated mouse infused with vehicle was removed from the study a few days after cannula implantation due to health concerns.
At the completion of all behavioral experiments cannula placements were visually confirmed for each mouse as previously described [12]. In addition, immunohistochemistry [see 12] was carried out on sections of hippocampal or amygdala tissue directly under the site of infusion to verify increases in acH3 after MS-275 infusion.
Statistics
Ratios of acH3 over total H1 were calculated, and 2 × 3 ANOVAs (stress × time) were carried out for each brain area. The relative density of acH3 after 10 days of in vivo infusion of vehicle or MS-275 was analyzed using an ANOVA. For analyses of depressive-like behaviors, groups of non-defeated controls and socially defeated mice were compared using two-way ANOVAs (stress × drug). For all two-way ANOVAs, Bonferroni post-hocs were used to assess isolated comparisons. All differences were considered significant when P < 0.05.
Results
Immunohistochemical analysis was used to quantify acH3K14 levels in hippocampus and amygdala of chronically defeated or control mice at 1 hour, 24 hour, or 10 days after the last defeat, or after 20 more days of fluoxetine administration (Fig. 1A). Levels of acH3K14 in hippocampus show a small, but significant increase at the 24 hour time point, as indicated by a main effect of time (F2,30 = 8.7, p = 0.001) and an interaction between time and stress (F2,30 = 8.7, p = 0.001) (Fig. 1B). Levels of acH3K14 in hippocampus were significantly reduced at longer times after defeat and this effect was reversed by fluoxetine (main effect of drug [F1,20 = 19.6, p = 0.0003] and an interaction between stress and drug [F1,20 = 10, p = 0.0049]) (Fig. 1B). Transient changes in acH3K14 were also observed in amygdala, with a main effect for stress (F1,30 = 41.3, p = 0.0001), time point after the last defeat (F2,30 = 10.3, p = 0.0004), and an interaction between stress and time point (F2,30 = 10.3, p = 0.0004). In amygdala, levels of acH3K14 are significantly increased an hour after repeated stress and remain elevated for 24 hours before returning to control levels within 10 days (Fig. 1B). No change in acH3K14 was seen in amygdala after a longer interval, and there was no effect of fluoxetine.
Figure 1.
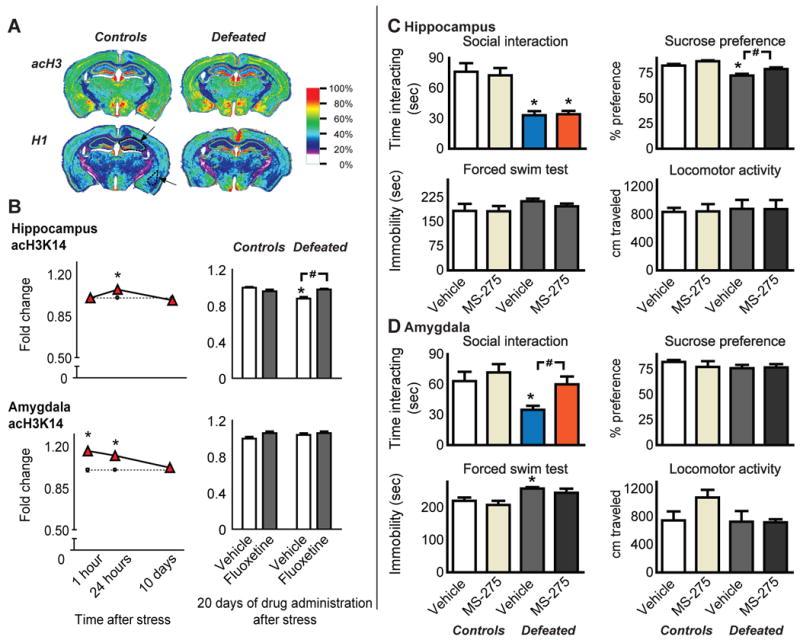
(A, B)1 hour, 24 hours, or 10 days after chronic social defeat, acH3K14, and total levels of histone H1 as a control, were quantified for hippocampus and amygdala (arrows) using immunohistochemistry. acH3K14 (normalized to total H1) was increased in hippocampus 24 hours after the last defeat, returned to normal within 10 days, and dropped below normal at 20 days after stress. This latter effect was normalized by 20 days of fluoxetine administrations. In contrast, acH3K14 was rapidly induced in amygdala and remained elevated for up to 24 hours before returning to normal. (C) In dorsal hippocampus, HDAC inhibitors have selective antidepressant-like effects. During a test for social interaction on experimental day 15, previously defeated mice spent significantly less time engaged in social interaction compared to controls under vehicle infusion conditions. Infusion of MS-275 (100 μM) had no effects on social avoidance. When assessed for sucrose preference, defeated mice exhibited significantly reduced preference for sucrose compared to controls, and this effect was reversed by HDAC inhibitor infusions. (D) When infused into the amygdala, MS-275 selectively attenuates stress-induced social avoidance. No significant effects of stress, or drug infusion, were observed during the sucrose preference test. When assessed with the forced swim test, defeated mice had significantly greater immobility, an effect no longer significant after MS-275. Differences between non-stressed controls and repeatedly defeated mice at each time point are denoted by * to indicate significance at p < 0.05. Isolated comparisons between bars are indicated by #, p < 0.05.
HDAC inhibitors, acting at the level of hippocampus or amygdala, have been reported to facilitate learning and the selective recall of memories [10,15,16]. acH3 at the bdnf gene promoter in hippocampus is induced by the antidepressant imipramine and implicated in the antidepressant-like effects of the drug [2]. The current experiments were thus conducted to examine if HDAC inhibitors delivered directly into hippocampus or amygdala are capable of reversing repeated social defeat stress-induced depressive behaviors. As expected, chronically stressed mice spent significantly less time engaged in social interaction, and exhibited reduced sucrose preference, when infused with vehicle as compared to controls (Figs. 1C). Antidepressant-like effects of intra-hippocampal MS-275 were specific to a reversal of the stress-induced deficit in sucrose preference, with significant main effects for stress (F1,36 = 30.7, p = 0.0001) and infusion (F1,36 = 11.6, p = 0.002) (Fig. 1C); no effect was seen on social interaction, nor in the forced swim test or on general locomotor activity (Fig. 1C).
Parallel studies with intra-amygdala infusions of MS-275 revealed an antidepressant-like effect in the social interaction test, with a main effect of infusion (F1,36 = 4.9, p = 0.03) and stress (F1,36 = 7.01, p = 0.01) and a-posteriori two tailed t-test comparing defeated mice infused with vehicle to those infused with MS-275 (t18 = 2.85, p = 0.01) (Fig. 1D). No effect of MS-275 was seen in the sucrose preference, forced swim, or locomotor activity tests.
Social housing with a male or female partner had no effect on social interaction behavior in controls or defeated mice, as indicated by a main effect for stress (F1,54 = 64.17, p = 0.0001), a main effect for housing condition (F1,54 = 5.13, p = 0.001), and no interaction between the two factors (Fig. 2A). However, intra-hippocampal MS-275, which had no effect on social interaction by itself (Fig. 1C), partially reversed defeat-induced social avoidance when paired with social housing, as indicated by a lack of a main effect for stress (Fig. 2B). In this experiment, a main effect for MS-275 (F1,27 = 11.82, p = 0.001), and a significant interaction between stress and drug (F1,27 = 5.62, p = 0.05), were observed.
Figure 2.
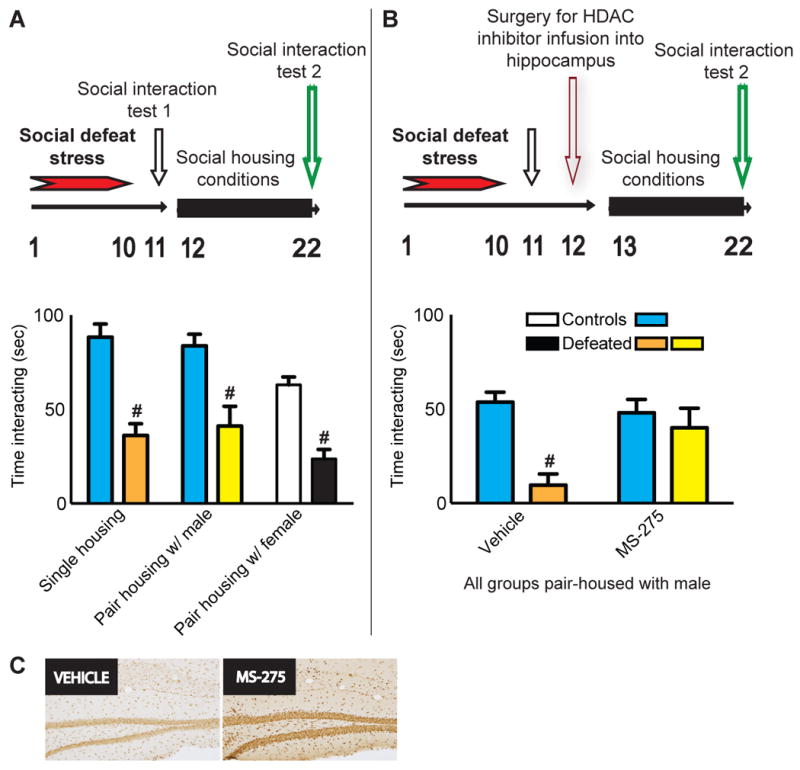
(A) Previously defeated mice spent significantly less time engaged in social interaction compared to controls. Ten days of social housing with another male, or female, had no impact on this social avoidance. (B) During social interaction testing, previously defeated mice spent significantly less time engaged in social interaction compared to controls when infused with vehicle. However, infusion of MS-275 (100 μM) into the dorsal hippocampus while pair-housed with another male reduces defeat-induced social avoidance. Differences between non-stressed controls and corresponding defeats are denoted by # to indicate significance at p < 0.05. (C) Continuous infusion of MS-275 (100 μM) into the hippocampus increases acH3 in vivo when examined by immunohistochemistry.
To confirm that MS-275 was reliably delivered into the brain via osmotic minipump infusion, and to verify that this drug is biochemically active at the site of infusion, levels of acH3K14 were assessed under the infusion site after 10 continuous days of treatment. Immunohistochemistry confirmed that acH3K14 levels are robustly elevated in hippocampus of mice that received continuous infusion of MS-275 (100 μM) as compared to vehicle treated controls (Fig. 2C).
Discussion
The current study reveals that acH3 is a promising target for novel treatments of depression and demonstrates a synergistic effect when combined with an enriched social environment. Importantly, we show that repeated social defeat in mice causes a slowly developing and prolonged reduction in acH3 in hippocampus. This change is likely pathological, since it is reversed by chronic fluoxetine and since intra-hippocampal infusion of an HDAC inhibitor, which increases acH3, exerts antidepressant-like effects. Specifically, HDAC inhibitor delivery into hippocampus increases sucrose preference following repeated stress, although it does not reverse stress-induced social avoidance or affect forced swim behavior. However, when combined with social enrichment, intra-hippocampal HDAC inhibition corrects the deficit in social interaction. These findings suggest that reversal learning in addition to HDAC inhibitor treatment is required to attenuate this deficit [17], and may explain in part why combinations of antidepressants with psychotherapy are the most effective treatments, including normalization of avoidance behavior, in humans [18]. In contrast to hippocampus, acH3 in amygdala was only transiently affected by social defeat stress. This adaptation may serve to reduce depression-like behaviors during these early time points, since MS-275 delivered into amygdala reverses stress-induced social avoidance, consistent with the importance of this limbic region in mediating the strength of conditioned fear responses elicited by chronic stress [19]. However, no effect was seen on sucrose preference or forced swimming.
It is interesting that delivery of an HDAC inhibitor into hippocampus vs. amygdala has different effects on the alleviation of social avoidance after social defeat stress. Still different responses are observed upon HDAC inhibitor infusion into the nucleus accumbens, a manipulation which produces antidepressant-like responses in the social avoidance, sucrose preference, and forced swim tests [12]. This is consistent with the notion that different limbic brain regions contribute differently to various depression-like behaviors [20,21,22]. The observation that HDAC inhibitor infusion into each of these three regions elicits antidepressant-like responses supports the potential utility of such inhibitors, given systemically, in treating depression.
Conditioned social avoidance, unlike other unconditioned behavioral probes for affective-like behavior [23], is stable across numerous experiments as it incorporates several brain circuits [11,24,25]. However, when social enrichment and hippocampal infusions were combined in the current study, social interaction behavior was uniformly decreased across all groups of mice. This suppression of social behavior is probably related to the combination of surgical and housing manipulations used, although it is known to be sensitive to fluctuations in other environmental and handling conditions as well.
Depression involves the persistent expression of diverse symptoms, suggesting the involvement of stable molecular adaptations across many brain areas. The current findings support the utility of targeting chromatin in depression, and provide novel insight regarding molecular mechanisms required to occur in conjunction with environmental stimuli to facilitate antidepressant responses.
Acknowledgments
Supported by grants from NIMH (EJN) and NARSAD (HEC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol. 2006;13:563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2007;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolaños CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;9:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine S. The influence of social factors on the response to stress, Psychother. Psychosom. 1993;60:33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;2:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 11.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 12.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- 15.Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 18.Ninan PT, Berger J. Symptomatic and syndromal anxiety and depression. Depress Anxiety. 2001;14:79–85. doi: 10.1002/da.1049. [DOI] [PubMed] [Google Scholar]
- 19.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;10:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Covington HE, III, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, Deisseroth K, Nestler EJ. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;48:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolaños-Guzmán CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;22:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 24.Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology (Berl) 2010;211:69–77. doi: 10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]


