Abstract
Objective
As the initiation and acceleration of alcohol use commonly occurs during adolescence, the etiological basis for this phenomenon is of critical importance. Using the diathesis-stress model as a framework, this review will evaluate the emerging evidence implicating the limbic-hypothalamic-pituitary-adrenal (LHPA) axis in the development of alcohol use disorder (AUD).
Method
Searches were conducted of the PubMed/Medline, PsycInfo, PsycBooks, Cochrane and ISI Web of Science databases, using a specified set of search terms.
Results
Genetic liabilities, antenatal stress/anxiety or exposure to addictive substances, exposure to maltreatment or other traumatic events in childhood and psychiatric illness in childhood/adolescence can all increase the risk, or diathesis, for AUD. Greater LHPA dysfunction may serve as a marker for higher diathesis levels in youth. When exposed to stressors in adolescence, high-risk youth (or those with greater LHPA dysfunction) may use alcohol and/or other substances to cope with stressors and, in turn, become more vulnerable to AUD.
Conclusion
Evidence suggests that LHPA dysfunction and stress play an important role in the development of AUD. Genetic liabilities, antenatal insults, maltreatment and psychiatric illness appear to increase LHPA dysfunction, raising risk for AUD. Further research is needed to clarify the complex interplay among adverse developmental experiences, LHPA dysfunction and the development of AUD in adolescents.
Keywords: Stress, Child, Adolescent, Alcohol, Addiction, Hypothalamic-Pituitary-Adrenal Axis
1. Introduction
Adolescent alcohol use is associated with a host of negative consequences, including psychiatric illness, risky behavior, neurobiological disruptions, and increased likelihood of having an alcohol use disorder (AUD) in adulthood (Hingson et al., 2006; Hingson et al., 2003; Rao and Chen, 2008; Raveis and Kandel, 1987). As such, effective prevention and treatment options are needed in youth. This goal can be advanced by a clear understanding of the etiology of AUD in adolescence.
A common thread throughout the course of AUD among adults is the role of environmental stressors (Zimmermann et al., 2007) and disruptions in the physiological stress response, particularly that of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis. Acute and chronic alcohol use affects LHPA functioning (Adinoff et al., 1998), as does the process of alcohol withdrawal (Adinoff et al., 2003; Clarke et al., 2008). Many of the risk factors (e.g., genetic liabilities or maltreatment) associated with later problematic alcohol use exert effects on LHPA functioning that are similar to those of long-term alcohol use in producing initial hyperactivity followed by long-term hyporesponsivity (Gunnar and Quevedo, 2008; Rao et al., 2008; Treutlein et al., 2006). While the evidence is preliminary, it points towards a close association between LHPA functioning and alcohol use. This will be the focus of this review.
1.1 Outline of the Theoretical Model
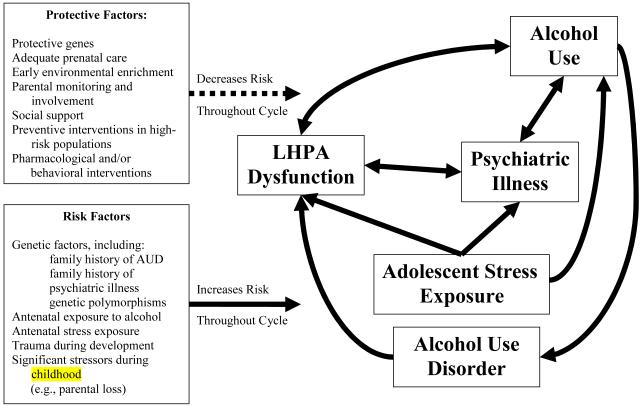
The theoretical model underlying this review is outlined in Figure 1. This model utilizes a diathesis-stress model (Rao et al., 2009a) for the role of environmental stressors and LHPA dysfunction in the development of AUD. A diathesis-stress model for AUD posits that individuals have a premorbid liability (diathesis) for AUD that is increased by risk factors (in the box at the bottom left) and decreased by protective factors (in the box at the top left). If the cumulative diathesis-stress level surpasses a certain threshold, the individual has an increased risk of developing AUD. We propose that LHPA functioning may be an indicator of the cumulative effects of environmental stressors/developmental adversities, indicating premorbid risk (or diathesis level) for AUD. Greater instability in LHPA functioning, including hyperresponsivity or hyporesponsivity, conveys greater risk. Near the threshold level of the LHPA diathesis-stress curve, even common stressors may lead to the escalation of alcohol use and AUD symptoms.
Figure 1.
Outline of the Etiological Model
Notes: Solid lines represent stimulating or amplifying influences and dashed lines represent inhibitory influences.
This review will proceed first by providing an overview of the LHPA axis structure, physiology and pathophysiology. Following this, the review will proceed developmentally to examine events that raise the diathesis level (as evidenced by the level of LHPA dysfunction), focusing on genetic liabilities, antenatal insults, maltreatment, psychiatric illness and alcohol use. These factors will then be integrated in a theoretical model proposing that the interaction of these events or experiences that increase LHPA dysfunction (i.e., the diathesis) with stressors in the adolescent period may increase the likelihood of alcohol use and entrench the behavior, leading to AUD.
2. Methods
Searches of the PubMed/Medline, PsycInfo, Cochrane, and ISI Web of Science databases were conducted, with emphasis on results published in the past 15 years. All searches utilized the following terms: stress, cortisol, ACTH, CRF/CRH, HPA axis. These were combined with terms relevant to the subsections of the review: development, antenatal stress, antenatal anxiety, antenatal alcohol exposure, antenatal substance exposure, maltreatment, trauma, abuse, neglect, alcohol use, substance use, family history of alcoholism, family history of substance use, anxiety disorder, depressive disorder, externalizing behavior, conduct disorder, and oppositional defiant disorder. The terms “child” and “adolescent” were also used to narrow the search, when applicable. Results in adults were retained if: (a) a focus of the study was the effect of a childhood event (e.g., maltreatment) on adult LHPA functioning and/or substance use, or (b) the literature in children and adolescents was limited.
3. LHPA Axis Dysfunction and Development in Adolescence
As the structure and functioning of the LHPA axis has been well described elsewhere, this review will refer readers to these sources for background information on the axis (Chrousos et al., 2009; de Kloet et al., 2005; Kronenberg and Williams, 2008; Miller and O’Callaghan, 2002; Smith and Vale, 2006; Sullivan and Gratton, 2002). The LHPA circadian rhythm can be altered by severe and/or chronic stressors and substances of abuse (Sinha, 2008; Tsigos and Chrousos, 2002). Chronic LHPA stimulation leads to hypercortisolemia, potentially resulting in hippocampal damage and psychiatric illness (Tsigos and Chrousos, 2002). Furthermore, addictive processes are linked to LHPA dysregulation (Goeders, 2002; Piazza and Le Moal, 1996; Piazza et al., 1996). Cortisol has permissive effects on mesolimbic dopamine release (Oswald et al., 2005; Saal et al., 2003; Wand et al., 2007), which is critical for reinforcement, and evidence suggests that high levels of glucocorticoids may be reinforcing and/or increase the likelihood of self-administration of substances (Piazza and Le Moal, 1997; Piazza and Le Moal, 1996; Piazza et al., 1996). While it is outside the scope of this review to fully cover the role of mesolimbic dopamine system in the development of SUD, mesolimbic dopamine signals incentive salience (that a stimulus is important for survival). Release of mesolimbic dopamine increases in response to the acute administration of most drugs of abuse (Adinoff, 2004).
3.1 Developmental Changes in LHPA Functioning during Adolescence
Prepubertal rats evidence greater, but delayed, stress-related corticosterone and ACTH release than adults (Romeo and McEwen, 2006). Potentiated responses to repeated stressors, as opposed to the typical habituation seen in adults, are observed (McCormick and Mathews, 2007). Recent reviews by McCormick and colleagues (McCormick and Mathews, 2010; McCormick et al., 2010) highlight that while aspects of LHPA function in rat models are similar to adults at the onset of adolescence (e.g., receptor expression, paraventricular nucleus morphology), many aspects of adolescent function differ from that of adults. Glucocorticoid responses to stressors appear to be greater and more prolonged in prepubertal rats than adults (Cruz et al., 2008; Romeo et al., 2004; Vazquez and Akil, 1993), but these differences are smaller when post-pubertal rats are used (Goldman et al., 1973; McCormick et al., 2008). More limited evidence indicates adolescent-adult differences in habituation to stress (Viau et al., 2005), with suggestions of decreased habituation in adolescents (Romeo et al., 2006). In all, evidence from animal studies suggests that adolescents show more HPA activation with respect to strength, duration and adaptation in response to repeated stress exposure than adults (McCormick and Mathews, 2010; McCormick et al., 2010).
In humans, adolescents exhibit higher basal cortisol levels and greater stress-induced cortisol levels than children (Stroud et al., 2009). Basal cortisol levels increase through adolescence (Kiess et al., 1995; Legro et al., 2003; Walker et al., 2001) and post-menarche females showed a later morning peak and higher cumulative levels of cortisol than pre-menarche females (Oskis et al., 2009). Stroud and colleagues (2009) exposed children and adolescents to either a performance (i.e., public speaking, mental arithmetic and mirror tracing) stressor or a peer rejection (i.e., exclusion from an activity) stressor and found that adolescents (aged 13-17) had greater post-stressor cortisol responses than children (aged 7-12), particularly in response to performance stressors.
4. Genetic Risk Factors Linking LHPA Dysfunction to Alcohol use
4.1 Family History of Alcoholism Status
Investigations of premorbid LHPA functioning in healthy young adults with a family history of alcoholism suggest a conflicting set of findings. Young adults with a family history reveal no alterations to their diurnal cortisol profile, as compared to those without a family history (Gianoulakis et al., 2005; Wand et al., 1999). That said, adult males with a family history had lower plasma ACTH levels and lower 24-hour levels of ACTH secretion (Gianoulakis et al., 2005). In some investigations, ACTH (Dai et al., 2002) and cortisol release (Sorocco et al., 2006) appear blunted in adults with a family history, as compared to those without a family history, with slower recovery of ACTH and cortisol levels to baseline in those with a family history (Dai et al., 2002). In others, however, both performance stressors and dosing of opioid antagonists are associated with exaggerated cortisol responses in those with a family history (Uhart et al., 2006; Wand et al., 1998; Zimmermann et al., 2004a). This effect may be attenuated after alcohol consumption (Zimmermann et al., 2004b).
In two published reports, one longitudinal investigation explored the interaction of family history of a substance use disorder and LHPA functioning in adolescents (Moss et al., 1999; Moss et al., 1995). Moss and collaborators (1995) found that prepubertal youngsters with a paternal history of a substance use disorder had blunted cortisol release in anticipation of taking part in a novel, potentially stressful task. Furthermore, blunted pre-task cortisol response in the family history positive youth was associated with regular tobacco or marijuana use during longitudinal follow-up (Moss et al., 1999). Evidence from adults consistently indicates that those with a family history of alcoholism have abnormal cortisol responses, as compared to family history negative individuals, albeit not always in the same direction. In adolescents, however, there are no published reports specifically examining adolescents with and without a family history of alcoholism.
4.2 Family History of Antisocial Behavior
Given the associations between antisocial behavior, alcohol use and a dysfunctional LHPA profile (Hawes et al., 2009; Jary and Stewart, 1985; Robins and Price, 1991; Susman, 2006), and the high heritability of antisocial traits (Cadoret, 1978; Slutske, 2001), another genetic liability for adolescent LHPA dysfunction and alcohol use may be familial antisocial behavior. Early investigations indicated that 10- to 12-year old offspring of fathers with conduct disorder (CD) and antisocial personality disorder (ASPD) had lower resting 9 AM cortisol levels than did children of fathers with past CD but not ASPD or children of fathers with neither CD nor ASPD (Vanyukov et al., 1993). Two later studies (Brotman et al., 2007; O’Neal et al., 2010) examining a group of young children (mean age was 3.9 years) with older siblings (mean age was 15.6 years) who had been adjudicated for committing a crime. This work indicated that the young children who did not receive an intervention aimed at preventing antisocial behavior (i.e., control participants) did not evidence increases in salivary cortisol levels prior to exposure to a novel peer group; in contrast, children in the prevention group evidenced expected increases in cortisol in anticipation of the stressor (Brotman et al., 2007). Later work linked normalization of anticipatory cortisol response to lower levels of physical aggression at 24-month follow-up assessment (O’Neal et al., 2010). As will be discussed below (section 7.3), externalizing and antisocial behavior in adolescence is associated with blunted LHPA profiles, with implications for potential alcohol use.
4.3 CRHR1 Polymorphisms
CRHR1 appears to be the main CRH receptor involved in the stress response and it is distributed in frontal cortical areas, the hippocampus, amygdala and the pituitary (Bale and Vale, 2004). Stress exerts complex effects on CRHR1 expression, including hippocampal and cortical up-regulation and pituitary down-regulation in animal models (Greetfeld et al., 2009; Klenerova et al., 2008). In adulthood, individuals who suffered moderate to severe physical, sexual or emotional abuse as children and had one of two common CRHR1 SNPs (rs110402 or rs7209436) had higher depressive symptom scores (Bradley et al., 2008). Rarer polymorphisms (a TAT haplotype including rs110402, rs 7209436 and rs 242924) conferred protective status for depressive symptoms (Bradley et al., 2008), but the protective status of the TAT haplotype was only replicated in one of two samples (Polanczyk et al., 2009). This finding may be due to methodological differences in trauma assessment. The polymorphisms associated with higher depressive symptom scores were also associated with greater release of cortisol in response to dexamethasone and CRH dosing in those with a history of moderate to severe childhood maltreatment (Tyrka et al., 2009), with evidence that the effect may be specific to males for one of the SNPs (rs110402; Heim et al., 2009).
Treutlein and colleagues (2006) examined the relationship between past 6-month alcohol use and 2 CRHR1 SNPs in 296 adolescents participating in a longitudinal study. Possession of the GG genotype for one SNP (rs242938), compared to the GA or AA genotypes, was protective for lifetime binge drinking and intoxication. The CC genotype for the other SNP (rs1876831), compared to the CT or TT genotypes, increased risk for lifetime alcohol use, binge drinking and intoxication (Treutlein et al., 2006). Blomeyer and collaborators (2008) examined lifetime and past-month alcohol use, stressful life events over the past 3 years and 2 CRHR1 SNPs in 280 adolescents. Adolescents who were homozygous for the C genotype at SNP rs1876831 had a positive relationship between negative life events and lifetime binge drinking and maximum alcohol use per occasion. In contrast, individuals homozygous for the T genotype at SNP rs1876831 were protected from problematic alcohol use even with exposure to negative life events (Blomeyer et al., 2008).
Further investigation examining the rs1876831 gene and alcohol use in the same sample used by Treutlein et al. (2006) and Blomeyer et al. (2008) indicated that number of stressful life events experienced by participants prior to alcohol use were positively associated with a younger age of initiation only among those homozygous for the C genotype at rs1876831 (Schmid et al., 2010). Earlier age of initiation was then associated with higher levels of alcohol use at 19 years of age. Furthermore, Schmid and colleagues (2010) found that those homozygous for the C genotype at rs1876831 or those homozygous for the A genotype at rs242938 were more likely to use alcohol heavily when exposed to major life stressors. Finally, results from adults provided evidence that the H2 haplotype of the CRHR1 gene, which contains rs1876831, moderated the relationship between alcohol use and childhood sexual abuse. Those with the H2 haplotype who were exposed to childhood sexual abuse were at no greater risk for heavy alcohol use, unlike those with the H1 haplotype (Nelson et al., 2010).
While preliminary, family history status and CRHR1 polymorphisms may confer greater risk for psychopathology or alcohol use. The research of Tyrka et al. (2009) and Heim et al. (2009) suggests the mechanism of risk for AUD: individuals with specific SNPs who are exposed to severe stressors and/or maltreatment may be at particular risk for LHPA dysfunction. This increased dysfunction (or elevated diathesis) may then interact with further stressors to promote alcohol use and AUD progression. Four reports (Blomeyer et al., 2008; Nelson et al., 2010; Schmid et al., 2010; Treutlein et al., 2006) examining adolescents provide evidence for the role of a specific genotype (CC) at the rs 1876831 SNP for CRHR1 in promoting problematic alcohol use. Thus, this may be a promising avenue of continued investigation, particularly for preventive or pharmacological treatment options. Future work should investigate the LHPA profiles of children with the high risk genotype longitudinally, potentially linking genotype to LHPA profile to adolescent alcohol use level.
5. Antenatal Insults: Effects on LHPA Functioning, Alcohol Use and Other Outcomes
The normal developmental progression of the LHPA axis is a plastic process that can be disrupted by antenatal insults. The increased risk for substance use could be moderated by the effects on LHPA functioning of two particular antenatal insults: stress/anxiety exposure and exposure to addictive substances.
5.1 Antenatal Stress/Anxiety
Antenatal stress (often obtained from self-report of negative life events or perceived stress in the mother) and/or anxiety levels in the parent are associated with both immediate and long-term LHPA alterations in the children. Studies in rodents and non-human primates have demonstrated that antenatal stress exposure is associated with elevated basal and post-stressor ACTH and corticosterone levels as well as postnatal alterations in the expression of hippocampal glucocorticoid receptors (Meaney et al., 2007). The timing of the antenatal stress exposure may be an influential factor of these effects, with mid-gestational exposure associated with the strongest effects (Kapoor et al., 2006).
In humans, maternal anxiety levels at 32 weeks of gestation have been associated with elevated early morning cortisol levels in 10-year-old offspring (O’Connor et al., 2005). Furthermore, adolescents exposed to higher levels of anxiety during gestational weeks 12-22 had a smaller diurnal decrease in cortisol levels than those exposed to lower levels of anxiety (Van den Bergh et al., 2008b). Exposure to severe food shortages while in utero, serving as a form of stressor, appears to increase the risk for a variety of disorders, including substance use problems. Males exposed to the Dutch “hunger winter” of 1944-45 (Lumey and Van Poppel, 1994) were more likely to seek addiction treatment than non-exposed individuals, but only if exposure was in the first trimester (Franzek et al., 2008). Females did not evidence any increased risk due to food shortage. Stress-related alterations in LHPA functioning may persist into adulthood, with exposure to antenatal stressors associated with increased stress-related ACTH and cortisol release in adulthood compared to age-matched controls (Entringer et al., 2009).
5.2 Antenatal Exposure to Alcohol
Individuals exposed to alcohol in utero often experience adverse outcomes in adolescence, including psychopathology, academic difficulties, behavior problems, and substance use (Baer et al., 1998; Hannigan et al., 2009; O’Callaghan et al., 2007; Williams and Ross, 2007). Heavier levels of exposure, producing fetal alcohol syndrome, are associated with more severe alterations and deficits, including impaired intelligence and executive functioning, psychiatric illness and significant neurobiological and neuroendocrine alterations (Guerri et al., 2009; O’Connor and Paley, 2009; Taylor et al., 1981). Huizink and Mulder (2006) and Hellemans and collaborators (2010) suggest that a potential mediator of this relationship between alcohol exposure and problematic outcome is the developing LHPA axis. Antenatal alcohol exposure in rats and primates is associated with elevated post-stressor corticosterone levels (Huizink and Mulder, 2006; Schneider et al., 2002) that may persist into adulthood (Hellemans et al., 2008; Weinberg et al., 2008). Repeated restraint stress in alcohol exposed rats did not lead to habituation of ACTH release, as is normally evidenced after repeated exposure to a specific stressor (Weinberg et al., 1996).
In humans, the findings are less consistent. Antenatal alcohol exposure was associated with elevated pre-stressor cortisol levels and a blunted cortisol release to a lab-based blood draw at both 2 (Ramsay et al., 1996) and 13 months of age (Jacobson et al., 1999). In contrast, Haley and collaborators (2006) did not find elevated basal cortisol levels (either in the home environment or in the lab) in 5- to-7- month-old infants with prenatal alcohol exposure. However, they did find greater post-stressor increases following exposure to a social stress paradigm (Haley et al., 2006). The inconsistencies in findings may be due to methodological differences in the research setting or the type of stressor. Both Ramsay et al. (1996) and Jacobson et al. (1999) conducted the research in the clinic, using a blood draw or inoculation as the stressor; in contrast, many infants in the Haley et al. (2006) study were seen in their homes and the stressor was a modified still-face procedure.
6. The Effects of Childhood Maltreatment on LHPA Functioning
Exposure to traumatic events in childhood, such as natural disasters (Goenjian et al., 1996), loss of a parent (Meinlschmidt and Heim, 2005) or exposure to marital violence (Saltzman et al., 2005), appears to lead to dysfunctional changes in LHPA functioning. However, given the relatively sparse literature and heterogeneity of these events, this review will focus on maltreatment. The experience of abuse or neglect in childhood is associated with robust alterations in LHPA functioning (Gunnar and Quevedo, 2008), with a hyperactive LHPA profile in the short-term. Maltreated youth (generally older children and younger adolescents, aged 7-13) have abnormal morning cortisol levels (most often low, but occasionally high), and elevations in 24-hour urinary free cortisol levels, ACTH levels and ACTH response to CRH administration (De Bellis, 2002; De Bellis et al., 1999; Kaufman et al., 1997). The inconsistencies in morning cortisol levels may be due to the variety (e.g., type of maltreatment), severity and duration of maltreatment to which these youth were exposed. Years after the cessation of maltreatment, however, adolescent LHPA functioning appears to be hyporesponsive, perhaps due to negative-feedback-driven alterations secondary to persistently high CRH levels (Gunnar and Quevedo, 2008). Lower basal ACTH and blunted CRH-induced ACTH release are observed despite elevated central CRH levels (De Bellis et al., 1994). Evidence from adult females indicates that this profile can persist into adulthood (Heim et al., 2001; Heim et al., 2000).
Furthermore, decreases in hippocampal volume (Bremner et al., 1997; Rao et al., 2010) and altered PFC function (Carrion et al., 2001) were found in adolescents or adults who experienced physical or sexual abuse or other trauma consistent with a PTSD diagnosis during childhood. Hippocampal volume may be directly related to circulating levels of cortisol (Pruessner et al., 2009), though this has not been found consistently (Vythilingam et al., 2004). That said, caution is warranted in interpreting these results, as youth who suffered maltreatment often had psychiatric diagnoses (e.g., major depression), which could also alter LHPA functioning (De Bellis, 2002). Moreover, maltreated youth may have been exposed to antenatal insults and also had a higher prevalence of alcohol abuse; the effects of these confounding influences on LHPA functioning in maltreated adolescents are unknown. Finally, adult (and even adolescent) reports of maltreatment must be interpreted in light of retrospective bias, with evidence that reports of maltreatment made years later are often somewhat unreliable (Widom et al., 2004) and prospective evidence is less consistent in linking maltreatment to substance use problems (Widom et al., 1999).
7. LHPA Functioning in Psychiatric Illness: Commonalities with Alcohol Use Disorders
Epidemiological evidence indicates that psychiatric illness and substance use are strongly related in adolescents, with some evidence of bidirectional causality and a synergistic relationship after symptoms are manifested (Rao, 2006; Schepis and Rao, 2009; Wilens, 2007). LHPA dysfunction co-occurs with psychiatric illness in youth and may be one of many potential underlying mechanisms for the co-occurrence of psychiatric and substance use disorders (SUD) or the risk imparted by some early psychopathology (Rao, 2006).
7. 1 LHPA Functioning in Depressive Disorders
Depressed adolescents appear to have elevated serum cortisol levels near sleep onset (Forbes et al., 2006). Among never depressed adolescents at high-risk for depression by virtue of parental depression history, elevations in nocturnal urinary cortisol was associated with higher risk for depression at follow-up (Rao et al., 2009b). While adolescents with dysthymia had blunted cortisol secretion in response to a physical stressor (Jansen et al., 1999), adolescents with major depression had an exaggerated cortisol response to a performance stressor (Lopez-Duran et al., 2009; Rao et al., 2008). Methodological differences in stressor type may explain this discrepancy, given that physical challenges and performance stressors appear to activate the LHPA axis and other stress systems somewhat differently (Linden et al., 1998; Stroud et al., 2009).
Early adversity and subsequent depressive disorder may be linked through LHPA dysfunction. Rao and collaborators (2010) found that hippocampal volume in adolescents mediated the relationship between early-life adversity and depressive disorder at follow-up. Rao and colleagues (2009a) also investigated the interaction of nocturnal urinary cortisol levels and recent stressful experiences on the development of SUD among three groups of adolescents: those with depression at baseline, those at high-risk for depression by virtue of parental history and low-risk controls. Experience of life stressors over the 5-year follow-up period moderated the effect of nocturnal urinary free cortisol levels on SUD vulnerability: adolescents with high cortisol levels were at greater risk for SUD, particularly when experiencing significant stressors during the follow-up (Rao et al., 2009a). Rao et al. (2009a) interpreted these results to suggest that underlying vulnerabilities in LHPA functioning and stressor exposure in adolescence were additive risk factors for the development of SUD in depressed adolescents. Finally, Sher (2007) has theorized that problem alcohol use or AUD status may interact with depressive and suicidal symptoms through LHPA hyperactivity and LHPA interactions with serotonergic pathways.
7. 2 LHPA Functioning in Anxiety Disorders
As with depressive disorders, anxiety disorders in youth may be associated with an altered LHPA profile (Van den Bergh et al., 2008a). In children and adolescents, elevated post-trauma levels of cortisol are predictive of later PTSD diagnosis (Pervanidou, 2008), and acute PTSD is associated with elevated cortisol levels in the evening, as compared to healthy controls (Carrion et al., 2002). In response to a modified Trier Social Stress Task (a public speaking stressor), a heightened cortisol response was observed following a performance stressor in socially phobic prepubertal children (van West et al., 2008) but not older adolescent girls (Martel et al., 1999).
7. 3 LHPA Functioning in Externalizing Behavioral Disorders
Externalizing behavioral disorders, which include the disruptive behavioral diagnoses oppositional-defiant disorder and conduct disorder, also appear to be marked by LHPA abnormalities. Disruptive behavioral diagnosis is the larger determinant of LHPA dysfunction in boys with both a disruptive behavioral diagnosis and ADHD (Hastings et al., 2009). The presence of a disruptive behavioral diagnosis is associated with a blunted cortisol response to stress exposure (Fairchild et al., 2008; van Goozen and Fairchild, 2006), though stress-induced hyperactivity has been seen (Susman et al., 1997), and the mechanisms behind this remain unclear (Dickerson and Kemeny, 2004). More consistently, those with disruptive behavioral diagnoses have lower morning cortisol levels, lower basal levels and a blunted decrease in cortisol over the first hour after awakening (McBurnett et al., 1991; Popma et al., 2007; van Goozen et al., 1998; Vanyukov et al., 1993). Sex and pubertal timing are important moderating factors (Susman et al., 2010), and may explain some of the inconsistent findings. Finally, lower morning levels of cortisol, coupled with higher levels of conduct disorder symptoms, predicted higher risk for marijuana use before 13 years of age (Huizink et al., 2006a).
7.4 Summary: LHPA Functioning in Adolescents with Psychiatric Illness
Depressive and anxiety disorders appear to be associated with elevated LHPA activity, while disruptive behavioral diagnoses appear to be associated with lower tone. Low tonic LHPA activity in those with externalizing disorders (e.g., conduct disorder) or LHPA hyperactivity in those with internalizing conditions (e.g., depressive disorders) may confer the greatest risk for substance use and SUD (Rao et al., 2009a; Schepis et al., 2008). Furthermore, adolescents may have both externalizing and internalizing disorders, complicating the associated LHPA dysfunction.
As reported by Rao et al. (2009a), the combination of underlying LHPA vulnerabilities due to psychiatric illness and the experience of major life stressors may identify those adolescents at greatest risk for the development of alcohol or other substance use problems. Adolescents with psychopathology may be using substances to counteract maladaptive LHPA alterations that result from psychopathology. In other words, they may be engaging in self-medication. Those with internalizing disorders may be most vulnerable early in the disorder, when LHPA hyperactivity is most acute. With greater exposure to stressors, which commonly co-occur with psychiatric illness, these youth may evidence LHPA hypoactivity. In those with externalizing illness, there is no evidence of LHPA hyperactivity. These individuals may use to temporarily raise tone, again implying some form of self-medication.
8. LHPA Functioning and the Effects of Alcohol Use
While virtually unstudied in adolescents (in part due to legal and ethical issues), LHPA functioning appears to be affected throughout the cycle of alcohol use in adults. Both acute alcohol use and withdrawal result in increased CRH, ACTH and cortisol levels, mirroring post-stressor LHPA response (Adinoff et al., 1996; Adinoff et al., 1991; Clarke and Schumann, 2009; Waltman et al., 1993). In contrast, chronic use down-regulates the LHPA axis and blunts ACTH and cortisol release to both pharmacological and psychological challenges, presumably through negative feedback-based modulation secondary to chronically high CRH concentrations and/or allostatic changes in adrenal responsivity (Adinoff et al., 2005a; Clarke and Schumann, 2009; Rohleder and Kirschbaum, 2006; Wand and Dobs, 1991).
Resolution of the withdrawal syndrome is associated with normalization of diurnal cortisol secretion, though pharmacological challenges indicate that LHPA responsivity may be blunted through months of sustained abstinence, as measured through serum cortisol and ACTH (Adinoff et al., 2005b; Adinoff et al., 2005c). Emerging evidence indicates that elevated cortisol levels in the cerebrospinal fluid (Walter et al., 2006) and blunted serum cortisol and ACTH responses to a physical stressor (Brady et al., 2006) may partially mediate the relationship between stress and relapse (Adinoff et al., 1998; Adinoff et al., 2005a). In adolescent animal models, adolescent rats evidenced smaller alcohol-related increases in corticosterone than adults, regardless of whether administration was of equal doses or to achieve equivalent effects (Prendergast and Little, 2007). This limited evidence indicates the probability that adolescent LHPA responses differ from adult responses, but due to limited investigation, this is unclear (Prendergast and Little, 2007).
8.1 Adolescent Studies: Predicting Substance Use from LHPA Axis Variables
While no published work has used LHPA indices to predict the initiation of alcohol use in children and adolescents, Huizink and collaborators (2006b) investigated the role of LHPA functioning on the initiation of marijuana use. They examined 10 to 12-year-old participants, using measures of diurnal salivary cortisol at three time points: immediately upon awakening, 30 minutes after awakening and at 8:00 p.m.. Participants who initiated marijuana use (assessed at follow-up) prior to age 14 had higher levels of evening cortisol and a trend towards lower cortisol levels 30 minutes after awakening than never users (Huizink et al., 2006b). This flattened diurnal pattern of cortisol release has been associated with adolescent psychopathology (Shirtcliff and Essex, 2008) and may mark general risk for psychopathology and/or substance use. Early initiators (i.e., prior to age 13) may have begun use prior to the LHPA assessment, potentially confounding the findings.
9. An Integrative Model of LHPA Dysfunction for Vulnerability to AUD
Trauma, severe stress and repeated cycles of alcohol use appear to create a pattern of acute LHPA hyperactivity followed by blunted functioning in the longer term. The contributions of psychiatric illness appear to be more complex, with some types of psychopathology associated with LHPA hyperresponsiveness and some associated with LHPA hyporesponsiveness. Regardless, psychiatric illness in childhood or adolescence appears to create a less stable LHPA environment. It is likely that such developmental liabilities combine in an additive fashion, with each risk factor increasing the diathesis for AUD through LHPA dysfunction. Increases in LHPA dysfunction through the developmental period then combine with the effects of stress exposure to contribute to the development of problematic alcohol use in the adolescent. Such use, then, continues to dysfunctional cycle of LHPA alterations, increasing the chance of AUD diagnosis and poor prognosis.
Youth with altered LHPA profiles secondary to the risk factors outlined here may be engaging in self-medication. Acute alcohol use affects LHPA functioning, and these effects may work differentially depending on whether the adolescent has LHPA hyper- or hypoactivity. In youth with hypoactive profiles, due to externalizing behavior or long-term downregulation after initial hyperactivity, alcohol use may serve the purpose of increasing LHPA activity and normalizing tone. In contrast, those with LHPA hyperactivity, due to internalizing psychopathology or trauma exposure, may use primarily to dampen stress-related LHPA activity (Funk et al., 2006; Rao et al., 2009a). In these youth, stressors induce a particularly strong LHPA response, which alcohol may reduce.
In either case, alcohol becomes associated with relief from an aversive state. Craving then occurs via an incentive salience model (Robinson and Berridge, 1993), where alcohol use is aimed at regulating LHPA function and provides negative reinforcement for continued use. While maladaptive in the short-term, chronic alcohol use becomes particularly maladaptive coping, as the LHPA modulating effects of alcohol change due to neuroplastic modulations. Early treatment that makes LHPA functioning more adaptive, then, would potentially stop this chain of events.
10. Future Directions
While LHPA dysfunction is associated with the development of AUD in youth, significant gaps remain in our understanding of their association, not least because of the need to control for many relevant variables. One notable difficulty in establishing the role of each risk factor examined above is that individuals who are exposed to one type of event that alters LHPA functioning are often exposed to multiple types of events; in other words, adolescents who are maltreated often also have psychiatric illness or genetic risk factors, or some combination. Furthermore, “dose-response” relationships need to be evaluated. It is likely that exposure to one instance of maltreatment is not equivalent to exposure to multiple instances in terms of LHPA functioning. The timing of the stressor also might be important.
In addition, premorbid (and potentially causal) LHPA dysfunction must be clearly delineated from alterations secondary to alcohol use in adolescents. Further investigations into the complex interplay of alcohol use, psychiatric illness and LHPA abnormalities are warranted, as are investigations that examine the role of potential protective factors. While this review has focused on adverse events thought to raise the diathesis level (or, increase LHPA dysfunction), protective factors are an extremely important piece of the clinical picture, both to understand the etiological factors in LHPA dysfunction and AUD that may result and the development of effective prevention and treatment options. Finally, further research is needed into the best LHPA biomarker for use in humans, balancing cost and validity. Research studies have employed a variety of markers, creating difficulties in comparing and applying the findings of the research. Establishment and consistent use of a standardized biomarker (or set of comparable biomarkers) would represent an important step towards our understanding of the relationships between AUD and LHPA functioning. Together, the evidence summarized here is only suggestive of a relationship between LHPA functioning and AUD, with many factors contributing to LHPA dysfunction and AUD risk. Future research is clearly needed to establish and clarify the nature of any associations. Such an understanding could be an important step in reducing the consequences of adolescent alcohol use and AUD.
Acknowledgements
This work was supported in part by National Institutes of Health grants DA14037 (UR), DA15131 (UR), DA17804 (UR), DA17805 (UR), MH62464 (UR), MH68391 (UR), U01AA016668 (BA) and funding from the Department of Veterans Affairs (BA).
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12(6):305–20. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology. 1996;15(3):288–95. doi: 10.1016/0893-133X(95)00212-V. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22(1):67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005a;29(7):1351–5. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005b;29(4):517–27. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. Alcohol Clin Exp Res. 2005c;29(4):528–37. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148(8):1023–5. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003;27(9):1420–7. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59(5):533–43. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63(2):146–51. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res. 2006;30(6):938–46. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Arch Gen Psychiatry. 2007;64(10):1172–9. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ. Psychopathology in adopted-away offspring of biologic parents with antisocial behavior. Arch Gen Psychiatry. 1978;35(2):176–84. doi: 10.1001/archpsyc.1978.01770260054005. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):943–51. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry. 2002;51(7):575–82. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Kino T, Charmandari E. Evaluation of the hypothalamic-pituitary-adrenal axis function in childhood and adolescence. Neuroimmunomodulation. 2009;16(5):272–83. doi: 10.1159/000216185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Schumann G. Gene-environment interactions resulting in risk alcohol drinking behaviour are mediated by CRF and CRF1. Pharmacol Biochem Behav. 2009;93(3):230–6. doi: 10.1016/j.pbb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addict Biol. 2008;13(1):1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Cruz FC, DeLucia R, Planeta CS. Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addict Biol. 2008;13(1):63–9. doi: 10.1111/j.1369-1600.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 2002;27(3):442–52. doi: 10.1016/S0893-133X(02)00308-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27(1-2):155–70. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 1999;45(10):1259–70. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78(2):249–55. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55(2):292–8. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol Psychiatry. 2008;64(7):599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry. 2006;59(1):24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzek EJ, Sprangers N, Janssens AC, Van Duijn CM, Van De Wetering BJ. Prenatal exposure to the 1944-45 Dutch ‘hunger winter’ and addiction later in life. Addiction. 2008;103(3):433–8. doi: 10.1111/j.1360-0443.2007.02084.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26(44):11324–32. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Thavundayil J, Brown T. Levels and circadian rhythmicity of plasma ACTH, cortisol, and beta-endorphin as a function of family history of alcoholism. Psychopharmacology (Berl) 2005;181(3):437–44. doi: 10.1007/s00213-005-0129-x. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27(1-2):13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry. 1996;153(7):929–34. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12(3):199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Greetfeld M, Schmidt MV, Ganea K, Sterlemann V, Liebl C, Muller MB. A single episode of restraint stress regulates central CRH receptor expression and binding in specific areas of the mouse brain. J Neuroendocrinol. 2009 doi: 10.1111/j.1365-2826.2009.01865.x. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44(2):108–14. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog Brain Res. 2008;167:137–49. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30(12):2055–64. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, James J, Ager JW, Greenwald MK, Delaney-Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2009 doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Fortier I, Utendale WT, Simard LR, Robaey P. Adrenocortical functioning in boys with attention-deficit/hyperactivity disorder: examining subtypes of ADHD and associated comorbid conditions. J Abnorm Child Psychol. 2009;37(4):565–78. doi: 10.1007/s10802-008-9292-y. [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr Opin Psychiatry. 2009;22(4):357–62. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158(4):575–81. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34(6):791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–75. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160(7):739–46. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter MR, Wechsler H. Early age of first drunkenness as a factor in college students’ unplanned and unprotected sex attributable to drinking. Pediatrics. 2003;111(1):34–41. doi: 10.1542/peds.111.1.34. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Ferdinand RF, Ormel J, Verhulst FC. HPA axis activity: A response to comments by Gunter Schumann. Addiction. 2006a;101(12):1833–4. doi: 10.1111/j.1360-0443.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Ferdinand RF, Ormel J, Verhulst FC. Hypothalamic-pituitary-adrenal axis activity and early onset of cannabis use. Addiction. 2006b;101(11):1581–8. doi: 10.1111/j.1360-0443.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999;11(2):195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Jansen MA, van der Gaag RJ, Matthys W, van Engeland H. Pituitary-adrenal reactivity in a child psychiatric population: salivary cortisol response to stressors. Eur Neuropsychopharmacol. 1999;9(1-2):67–75. doi: 10.1016/s0924-977x(98)00003-0. [DOI] [PubMed] [Google Scholar]
- Jary ML, Stewart MA. Psychiatric disorder in the parents of adopted children with aggressive conduct disorder. Neuropsychobiology. 1985;13(1-2):7–11. doi: 10.1159/000118154. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(Pt 1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol Psychiatry. 1997;42(8):669–79. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502–6. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Klenerova V, Sery O, Hynie S. Corticotropin-releasing hormone receptor subtypes in the rat anterior pituitary after two types of restraint stress. Ann N Y Acad Sci. 2008;1148:415–20. doi: 10.1196/annals.1410.043. [DOI] [PubMed] [Google Scholar]
- Kronenberg H, Williams RH. Williams textbook of endocrinology. 11th ed. Saunders/Elsevier; Philadelphia, PA: 2008. [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T. Urinary free cortisol increases in adolescent caucasian females during perimenarche. J Clin Endocrinol Metab. 2003;88(1):215–9. doi: 10.1210/jc.2002-020256. [DOI] [PubMed] [Google Scholar]
- Linden W, Rutledge T, Con A. A case for the usefulness of laboratory social stressors. Ann Behav Med. 1998;20(4):310–6. doi: 10.1007/BF02886380. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34(9):1272–83. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumey LH, Van Poppel FW. The Dutch famine of 1944-45: mortality and morbidity in past and present generations. Soc Hist Med. 1994;7(2):229–46. doi: 10.1093/shm/7.2.229. [DOI] [PubMed] [Google Scholar]
- Martel FL, Hayward C, Lyons DM, Sanborn K, Varady S, Schatzberg AF. Salivary cortisol levels in socially phobic adolescent girls. Depress Anxiety. 1999;10(1):25–7. doi: 10.1002/(sici)1520-6394(1999)10:1<25::aid-da4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, Christ MA, Hanson KS. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. J Am Acad Child Adolesc Psychiatry. 1991;30(2):192–6. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):756–65. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72(1):73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187(2):228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13(7):269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology. 2005;30(6):568–76. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51(6 Suppl 1):5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao JK, Kirillova GP. Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biol Psychiatry. 1999;45(10):1293–9. doi: 10.1016/s0006-3223(98)00216-9. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biol Psychiatry. 1995;38(8):547–55. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2010;15(1):1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W. Prenatal alcohol exposure and attention, learning and intellectual ability at 14 years: a prospective longitudinal study. Early Hum Dev. 2007;83(2):115–23. doi: 10.1016/j.earlhumdev.2006.05.011. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225–34. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58(3):211–7. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- O’Neal CR, Brotman LM, Huang KY, Gouley KK, Kamboukos D, Calzada EJ, Pine DS. Understanding relations among early family environment, cortisol response, and child aggression via a prevention experiment. Child Dev. 2010;81(1):290–305. doi: 10.1111/j.1467-8624.2009.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34(3):307–16. doi: 10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30(4):821–32. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. J Neuroendocrinol. 2008;20(5):632–8. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25(3):359–72. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–78. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Marinelli M, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Stress, glucocorticoids, and mesencephalic dopaminergic neurons: a pathophysiological chain determining vulnerability to psychostimulant abuse. NIDA Res Monogr. 1996;163:277–99. [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66(9):978–85. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popma A, Doreleijers TA, Jansen LM, Van Goozen SH, Van Engeland H, Vermeiren R. The diurnal cortisol cycle in delinquent male adolescents and normal controls. Neuropsychopharmacology. 2007;32(7):1622–8. doi: 10.1038/sj.npp.1301289. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Little HJ. Adolescence, glucocorticoids and alcohol. Pharmacol Biochem Behav. 2007;86(2):234–45. doi: 10.1016/j.pbb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. J Pediatr Psychol. 1996;21(6):833–40. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U. Links between depression and substance abuse in adolescents: neurobiological mechanisms. Am J Prev Med. 2006;31(6 Suppl 1):S161–74. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Rao U, Chen LA. Neurobiological and psychosocial processes associated with depressive and substance-related disorders in adolescents. Curr Drug Abuse Rev. 2008;1(1):68–80. doi: 10.2174/1874473710801010068. [DOI] [PubMed] [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67(4):357–64. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64(6):521–6. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE. Mechanisms underlying the comorbidity between depressive and addictive disorders in adolescents: interactions between stress and HPA activity. Am J Psychiatry. 2009a;166(3):361–9. doi: 10.1176/appi.ajp.2008.08030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE. Risk markers for depression in adolescents: Sleep and HPA measures. Neuropsychopharmacology. 2009b;34(8):1936–45. doi: 10.1038/npp.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveis VH, Kandel DB. Changes in drug behavior from the middle to the late twenties: initiation, persistence, and cessation of use. Am J Public Health. 1987;77(5):607–11. doi: 10.2105/ajph.77.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Price RK. Adult disorders predicted by childhood conduct problems: results from the NIMH Epidemiologic Catchment Area project. Psychiatry. 1991;54(2):116–32. doi: 10.1080/00332747.1991.11024540. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59(3):236–43. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147(4):1664–74. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80(6):387–93. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:14. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37(4):577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saltzman KM, Holden GW, Holahan CJ. The psychobiology of children exposed to marital violence. J Clin Child Adolesc Psychol. 2005;34(1):129–39. doi: 10.1207/s15374424jccp3401_12. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Adinoff B, Rao U. Neurobiological processes in adolescent addictive disorders. Am J Addict. 2008;17(1):6–23. doi: 10.1080/10550490701756146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Rao U. Adolescence: The Emergence of Alcohol Use and Depressive Disorders. In: Sher L, editor. Comorbidity of Depression and Alcohol Use Disorders. Nova Biomedical; New York: 2009. [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13(6):703–14. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27(1-2):285–98. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Sher L. The role of the hypothalamic-pituitary-adrenal axis dysfunction in the pathophysiology of alcohol misuse and suicidal behavior in adolescents. Int J Adolesc Med Health. 2007;19(1):3–9. doi: 10.1515/ijamh.2007.19.1.3. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158–62. doi: 10.1007/s11920-001-0014-1. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59(3):210–7. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1-2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30(3):376–89. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, Dorn LD. Cortisol and alpha amylase reactivity and timing of puberty: vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology. 2010;35(4):557–69. doi: 10.1016/j.psyneuen.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behaviour, behaviour problems, and emotionality in young adolescents: a longitudinal perspective. J Res Adolesc. 1997;7:81–105. [Google Scholar]
- Taylor AN, Branch BJ, Kokka N. Neuroendocrine effects of fetal alcohol exposure. Prog Biochem Pharmacol. 1981;18:99–110. [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11(6):594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66(7):681–5. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31(10):2255–63. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Pinna Puissant S, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Horm Behav. 2008a;54(2):253–7. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008b;33(3):536–45. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G. Neuroendocrine and neurotransmitter correlates in children with antisocial behavior. Horm Behav. 2006;50(4):647–54. doi: 10.1016/j.yhbeh.2006.06.021. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol Psychiatry. 1998;43(7):531–9. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- van West D, Claes S, Sulon J, Deboutte D. Hypothalamic-pituitary-adrenal reactivity in prepubertal children with social phobia. J Affect Disord. 2008;111(2-3):281–90. doi: 10.1016/j.jad.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: associations with cortisol. Psychiatry Res. 1993;46(1):9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res. 1993;34(5):646–53. doi: 10.1203/00006450-199311000-00017. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–46. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56(2):101–12. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13(3):721–32. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Gerlach M, Weijers HG, Boening J, Wiesbeck GA. Cortisol concentrations, stress-coping styles after withdrawal and long-term abstinence in alcohol dependence. Addict Biol. 2006;11(2):157–62. doi: 10.1111/j.1369-1600.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- Waltman C, Blevins LS, Jr., Boyd G, Wand GS. The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. J Clin Endocrinol Metab. 1993;77(2):518–22. doi: 10.1210/jcem.77.2.8393888. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72(6):1290–5. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M, Giggey P. Adrenocortical responses and family history of alcoholism. Alcohol Clin Exp Res. 1999;23(7):1185–90. [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55(12):1114–9. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32(11):2310–20. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20(4):470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Taylor AN, Gianoulakis C. Fetal ethanol exposure: hypothalamic-pituitary-adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res. 1996;20(1):122–31. doi: 10.1111/j.1530-0277.1996.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Widom CS, Raphael KG, DuMont KA. The case for prospective longitudinal studies in child maltreatment research: commentary on Dube, Williamson, Thompson, Felitti, and Anda (2004) Child Abuse Negl. 2004;28(7):715–22. doi: 10.1016/j.chiabu.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Widom CS, Weiler BL, Cottler LB. Childhood victimization and drug abuse: a comparison of prospective and retrospective findings. J Consult Clin Psychol. 1999;67(6):867–80. doi: 10.1037//0022-006x.67.6.867. [DOI] [PubMed] [Google Scholar]
- Wilens TE. The nature of the relationship between attention-deficit/hyperactivity disorder and substance use. J Clin Psychiatry. 2007;68(Suppl 11):4–8. [PubMed] [Google Scholar]
- Williams JH, Ross L. Consequences of prenatal toxin exposure for mental health in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2007;16(4):243–53. doi: 10.1007/s00787-006-0596-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Kunz-Ebrecht SR, Uhr M, Wittchen HU, Holsboer F. Effect of ethanol on hypothalamic-pituitary-adrenal system response to psychosocial stress in sons of alcohol-dependent fathers. Neuropsychopharmacology. 2004a;29(6):1156–65. doi: 10.1038/sj.npp.1300395. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Wittchen HU, Himmerich H, Landgraf R, Uhr M, Holsboer F. Arginine vasopressin and adrenocorticotropin secretion in response to psychosocial stress is attenuated by ethanol in sons of alcohol-dependent fathers. Journal of Psychiatric Research. 2004b;38(4):385–393. doi: 10.1016/j.jpsychires.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Blomeyer D, Laucht M, Mann KF. How gene-stress-behavior interactions can promote adolescent alcohol use: the roles of predrinking allostatic load and childhood behavior disorders. Pharmacol Biochem Behav. 2007;86(2):246–62. doi: 10.1016/j.pbb.2006.09.024. [DOI] [PubMed] [Google Scholar]