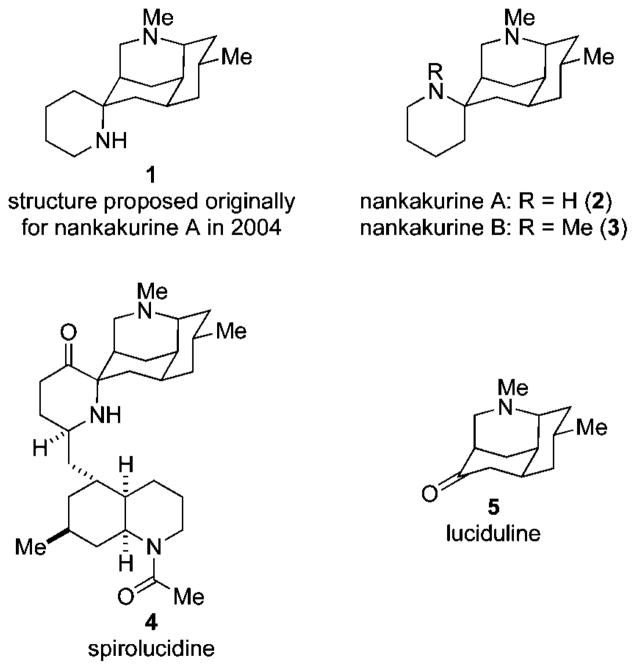
The Lycopodium alkaloids1 have attracted synthetic interest for many years, as a result of their diverse architectures and to a lesser extent their biological activities. In 2004, Kobayashi and co-workers reported the isolation of nankakurine A,2 a minor component of the club moss Lycopodium hamiltonii. Utilizing NMR and mass spectrometric analyses, structure 1 was proposed (Figure 1).2 The relative configuration at the spiro stereocenter of nankakurine A was assigned on the basis of 1H NMR NOE experiments and was in accord with the structure of spirolucidine (4), whose relative configuration had been secured by single-crystal X-ray analysis of a derivative.3 Further purification of this club moss extract led to the isolation of a related, slightly less abundant, alkaloid nankakurine B, for which structure 3 was proposed.4 In this case, 1H NMR NOE data were interpreted to support a configuration at the spiro stereocenter opposite to that found in spirolucidine (4) and that proposed originally for nankakurine A (1). Since nankakurine A was converted to nankakurine B upon reductive methylation, the structure of nankakurine A was revised to 2 in 2006.4
Figure 1.
Nankakurines A and B and two structurally related Lycopodium alkaloids.
Preliminary biological studies of nankakurine A (2) suggested its ability to induce secretion of neurotrophic factors and promote neuronal differentiation of rat adrenal PC-12 cells.4 Neurotrophic factors play an important role in mediating neuronal growth and survival; consequently, they have long been recognized for their potential in combating neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases.5 Recently, there has been interest in small molecules that exhibit neurotrophic effects6 because of the poor pharmacokinetics of naturally occurring polypeptidyl neurotrophic factors.7
The scarcity of the nankakurines (2 and 3 were isolated in 0.0003 and 0.0002%, respectively),4 contributed to the uncertainty regarding their relative configuration, and has prevented further evaluation of the purported neurotrophic properties of nankakurine A. We report herein total syntheses of (+)-nankakurine A, (+)-nankakurine B, and the originally purported structure 1 of nankakurine A, which rigorously establish the relative and absolute configuration of these rare alkaloids as 2 and 3, respectively.
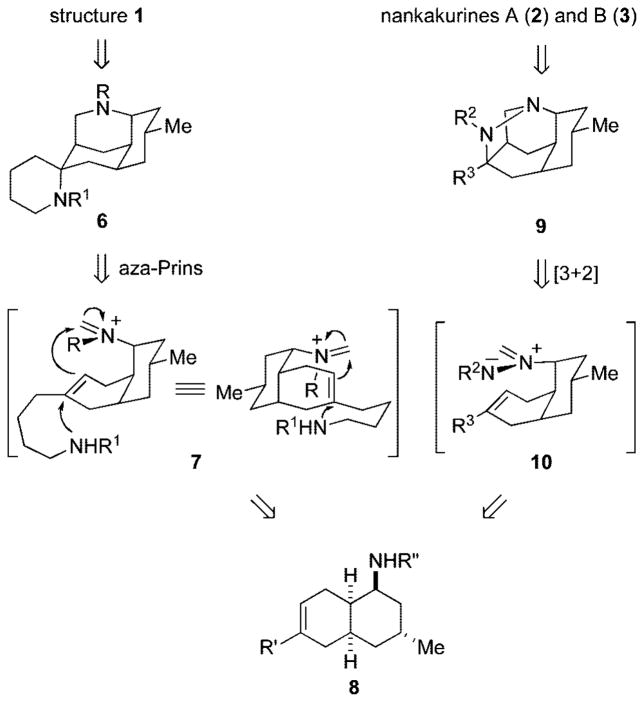
Although nankakurines A and B might well be assembled from luciduline (5), we were intrigued by the opportunity to evaluate an amino-terminated aza-Prins cyclization for directly assembling the diazatetracyclic ring system of structure 1 (Scheme 1, 7 → 6). Although aza-Prins cyclizations are often used to assemble azacyclic ring systems,8 few examples of terminating such cyclizations by tethered heteroatom nucleophiles have been described and none to our knowledge wherein this nucleophile is nitrogen.9cis-Decalin amine 8, the precursor of formaldiminium ion 7, would be available by a Diels–Alder construction that draws direct precedent from the first total synthesis of (+)-luciduline (5) by Oppolzer and Petrzilka.10
Scheme 1.
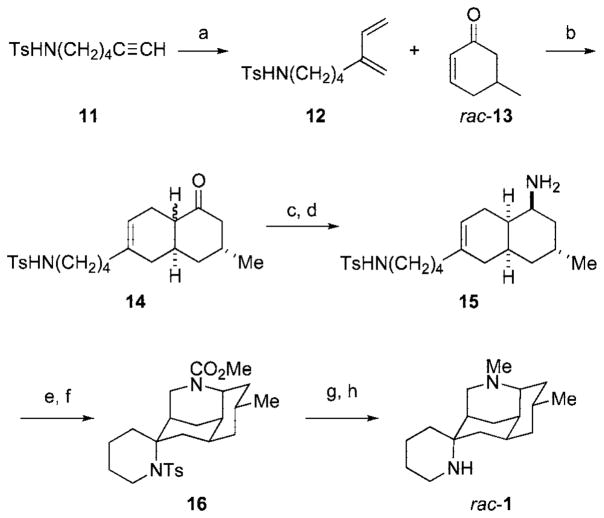
An unoptimized sequence that led to diazatetracycle 1, the structure proposed originally for nankakurine A, is summarized in Scheme 2. Enyne metathesis of N-tosylaminoalkyne 1111 and ethylene in the presence of 2.5 mol % of Grubbs’ second-generation catalyst12 provided 1,3-diene 12 in 90% yield.13 The Diels–Alder reaction of diene 12 and racemic cyclohexenone rac-13 took place at room temperature in the presence of 50 mol % of EtAlCl2 to generate decalone 14 in 74% yield as a mixture of cis and trans epimers. Conversion of the cis epimer to the oxime under conditions designed to equilibrate the decalone epimers,10 and subsequent reduction with NaBH4 and MoO314 provided cis-decalin amine 15 in 55% yield. Attempts to cyclize formaldiminium ions derived from 15, or its N-Me congener, yielded only tricyclic aza-Prins products. However, the desired nitrogen-terminated aza-Prins biscyclization could be realized by reaction of the methyl carbamate derived from amine 15 with 1 equiv of paraformaldehyde and 20 equiv of TFA at room temperature in CHCl3. Although the yield of this conversion was low, it did provide sufficient amounts of tetracyclic diamine 16 to allow rac-1 to be accessed in two additional reductive steps. 1H and 13C NMR spectra of this product are quite different from those reported for nankakurine A,2 confirming that the initial structural assignment for this alkaloid was incorrect.
Scheme 2.
a Reagents: (a) 2.5 mol % Grubbs’ second-generation catalyst, ethylene (300 psi), CH2Cl2, 25 °C (90%); (b) 50 mol % of EtAlCl2, CH2Cl2, PhMe, 25 °C (74%, 1:1–1:3 cis:trans); (c) HONH2 · HCl, MeOH, KOH, 25 °C (56%); (d) MoO3, NaBH4, MeOH, 25 °C (98%); (e) ClCO2Me, Et3N, CH2Cl2, 25 °C (56%); (f) (CH2O)n, TFA, CHCl3, 25 °C (20%); (g) Na, NH3, THF, −78 °C (98%); (h) LiAlH4, THF, 25 °C (65%).
At this point, it seemed likely that nankakurine A was indeed structure 24 having the relative configuration of the spiropiperidine unit that orients both nitrogen atoms on the concave face of the bridged tricyclic moiety. Connecting these atoms provides potential precursor 9, which we hoped could be accessed by intramolecular dipolar cycloaddition of azomethine imine intermediate 10 (Scheme 1).15
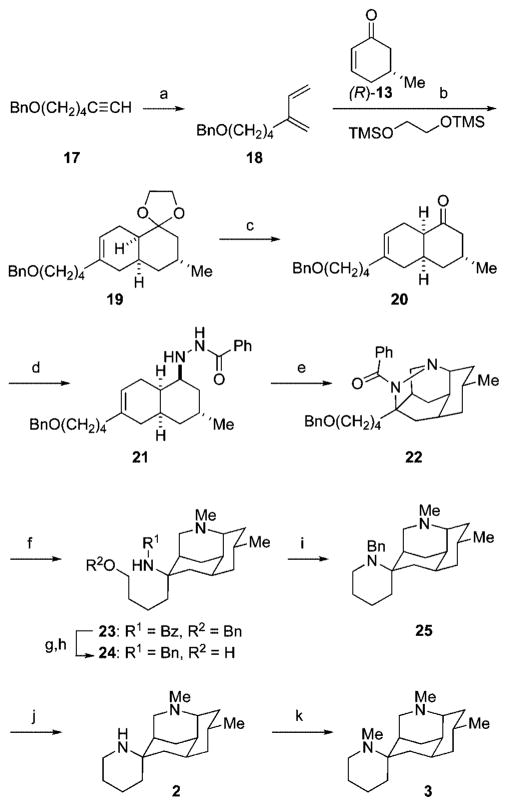
The successful asymmetric total syntheses of (+)-nankakurines A (2) and B (3) commenced with the preparation of diene 18 in 90% yield by ruthenium-catalyzed cross metathesis of benzyloxyalkyne 1716 and ethylene (Scheme 3).12,13 To circumvent epimerization of the cis-decalone product during the Diels–Alder reaction, the cycloaddition of diene 18 and (R)-5-methylcyclohex-2-en-1-one [(R)-13]17 was carried out at low temperature on multigram scale by a modification of the method of Gassman.18 Allowing diene 18, cyclohexenone (R)-13, and 1,2-bis(trimethylsiloxy)ethane to react in CH2Cl2 at −78 °C in the presence of 10 mol % of TMSOTf provided cis-decalone acetal 19 in 64% yield, as a single stereoisomer. Cleavage of the dioxolane with FeCl3 adsorbed on silica gel19 gave cis-decalone 20 in 99% yield. Condensation of this product with benzoic hydrazide, followed by a stereoselective reduction of the hydrazone intermediate with NaCNBH3 delivered benzoic hydrazide 21 in 80% yield.
Scheme 3.
a Reagents: (a) 5 mol % of Grubbs’ second-generation catalyst, ethylene (300 psi), CH2Cl2, 25 °C (90%); (b) 10 mol % of TMSOTf, CH2Cl2, −78 °C (64%); (c) FeCl3/SiO2, acetone, 25 °C (99%); (d) i. H2NNHCOPh, MeOH, ii. NaCNBH3, MeOH, HCl, 25 °C (80%); (e) (CH2O)n, 4 Å molecular sieves powder, (i-Pr)2NEt, PhMe, 115 °C (82%); (f) i. SmI2, 9:1 THF–MeOH, ii. 37% aq formaldehyde, NaCNBH3, MeOH, HCl, 25 °C (80%); (g) H2, 10 mol % of Pd(OH)2, HCl, MeOH, 25 °C (97%); (h) AlH3, THF, 25 °C (74%); (i) MsCl, Et3N, CH2Cl2, −40 °C (96%); (j) H2, 10 mol % of Pd/C, HCl, MeOH, 25 °C (99%); (k) 37% aq formaldehyde, NaCNBH3, MeOH, HCl, 25 °C (80%).
With hydrazide 21 in hand, we turned to the pivotal intramolecular azomethine imine cycloaddition reaction. Early survey experiments revealed that addition of base was required in order to obtain tetracyclic pyrazolidine 22 in high yield; in the absence of base, tricyclic aza-Prins products predominated.20,21 Under optimized conditions, pyrazolidine 22 was isolated in 82% yield when hydrazide 21 was heated with excess paraformaldehyde, 1 equiv of N,N-diisopropylethylamine, and powdered 4 Å molecular sieves in toluene at 115 °C. Single-crystal X-ray analysis of product 2222 confirmed that the dipolar cycloaddition had taken place with the expected regioselectivity.10,15
The conversion of tetracyclic pyrazolidine 22 to (+)-nankakurines A (2) and B (3) commenced with cleavage of the N–N bond with SmI223 and selective in situ reductive methylation of the secondary amine to generate diamine 23 in 80% yield. Hydrogenolytic cleavage of the O-benzyl protecting group, followed by reduction of the amide with AlH3,24 gave diamine alcohol 24 in 72% yield.25 Selective O-mesylation of this intermediate at −40 °C, followed by warming the primary mesylate to ambient temperature to form the spiropiperidine ring, provided N-benzylnankakurine A (25) in 96% yield. Hydrogenolysis of this intermediate in acidic methanol gave (+)-nankakurine A (2), [α]24D +13 (c 0.4, MeOH),26a in 99% yield. Standard reductive methylation of nankakurine A (2) delivered (+)-nankakurine B (3), [α]24D +12 (c 1.5, MeOH),26b in 80% yield.27
In summary, the first total syntheses of (+)-nankakurine A (2) and (+)-nankakurine B (3) were accomplished, respectively, in 13 steps and 20% overall yield and 14 steps and 16% overall yield. These syntheses, together with the synthesis of the originally purported structure 1 of nankakurine A, rigorously establish the relative and absolute configuration of (+)-nankakurines A (2) and B (3). These enantioselective total syntheses are sufficiently concise that gram quantities of (+)-nankakurine A (2) and (+)-nankakurine B (3) will be available for further biological studies.
Supplementary Material
Acknowledgments
This research was supported by the NIH Neurological Disorders & Stroke Institute (NS-12389), and by unrestricted grants from Amgen and Merck. J.R.A. thanks the University of California President’s Postdoctoral Fellowship Program for funding. NMR and mass spectra were obtained at UC Irvine using instrumentation acquired with the assistance of NSF and NIH Shared Instrumentation grants. We thank Dr. Hiroshi Morita, Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Hoshi University, Japan, for copies of 1H and 13C NMR spectra of natural nankakurine B, and Dr. Joe Ziller, X-Ray Crystallography Facility Director, UC Irvine, for X-ray analyses.
Footnotes
Supporting Information Available: Experimental details and copies of 1H and 13C NMR spectra of new compounds; CIF file for compound 22. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews of the Lycopodium alkaloids, see: Kobayashi J, Morita H. In: The Alkaloids. Cordell GA, editor. Vol. 61. Academic Press; New York: 2005. p. 1.Ma X, Gang DR. Nat Prod Rep. 2004;21:752–772. doi: 10.1039/b409720n.Ayer WA, Trifonov LS. In: The Alkaloids. Cordell GA, Brossi A, editors. Vol. 45. Academic Press; New York: 1994. p. 233.Ayer WA. Nat Prod Rep. 1991;8:455–463. doi: 10.1039/np9910800455.MacLean DB. In: The Alkaloids. Brossi A, editor. Vol. 26. Academic Press; New York: 1985. p. 241.MacLean DB. In: The Alkaloids. Manske RHF, editor. Vol. 14. Academic Press; New York: 1973. p. 348.MacLean DB. In: The Alkaloids. Manske RHF, editor. Vol. 10. Academic Press; New York: 1968. p. 305.
- 2.Hirasawa Y, Morita H, Kobayashi J. Org Lett. 2004;6:3389–3391. doi: 10.1021/ol048621a. [DOI] [PubMed] [Google Scholar]
- 3.Ayer WA, Ball LF, Browne LM, Tori M, Delbaere LTJ, Silverberg A. Can J Chem. 1984;62:298–302. [Google Scholar]
- 4.Hirasawa Y, Kobayashi J, Obara Y, Nakahata N, Kawahara N, Goda Y, Morita H. Heterocycles. 2006;68:2357–2364. [Google Scholar]
- 5.(a) Dawbarn D, Allen SJ. Neuropath Appl Neurobiol. 2003;29:211–230. doi: 10.1046/j.1365-2990.2003.00487.x. [DOI] [PubMed] [Google Scholar]; (b) Dauer W. Science. 2007;411:60–62. doi: 10.1126/stke.4112007pe60. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hefti F. Annu Rev Pharmacol Toxicol. 1997;37:239–267. doi: 10.1146/annurev.pharmtox.37.1.239. [DOI] [PubMed] [Google Scholar]; (b) Luu B, González de Aguilar J-L, Girlanda-Junges C. Molecules. 2000;5:1439–1460. [Google Scholar]; (c) Water SP, Tian Y, Li YM, Danishefsky SJ. J Am Chem Soc. 2005;127:13514–13515. doi: 10.1021/ja055220x. and references therein. [DOI] [PubMed] [Google Scholar]
- 7.Kirik D, Georgievska B, Björklund A. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 8.For a review, see: Overman LE, Ricca DJ. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 2. Pergamon Press; Oxford: 1991. pp. 1007–1046.An intramoleular Mannich reaction was a key element of the first total synthesis of (±)-luciduline; see: Scott WL, Evans DA. J Am Chem Soc. 1972;94:4779–4780. doi: 10.1021/ja00768a083.
- 9.For examples of carboxylate-terminated aza-Prins biscyclization reactions, see: Heathcock CH, Ruggeri RB, McClure KF. J Org Chem. 1992;57:2585–2594.Lögers M, Overman LE, Welmaker GS. J Am Chem Soc. 1995;117:9139–9150.
- 10.(a) Oppolzer W, Petrzilka M. J Am Chem Soc. 1976;98:6722–6723. [Google Scholar]; (b) Oppolzer W, Petrzilka M. Helv Chim Acta. 1978;61:2755–2762. [Google Scholar]
- 11.Luo FT, Wang RT. Tetrahedron Lett. 1992;33:6835–6838.(b) See the Supporting Information for details.
- 12.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 13.For selected examples of enyne metathesis, see: Hansen EC, Lee D. J Am Chem Soc. 2004;126:15074–15080. doi: 10.1021/ja045422d.Smulik JA, Diver ST. Org Lett. 2000;2:2271–2274. doi: 10.1021/ol006035l.
- 14.Ipaktschi J. Chem Ber. 1984;117:856–858. [Google Scholar]
- 15.For a recent review, see: Schantl JG. Science of Synthesis. Vol. 27. Georg Thieme Verlag; Stuttgart: 2004. Azomethine Imines; p. 731.For select examples, see: Oppolzer W. Tetrahedron Lett. 1970;11:3091–3094.Oppolzer W. Tetrahedron Lett. 1972;13:1707–1710.Oppolzer W. Angew Chem, Int Ed Engl. 1977;16:10–23.Jacobi PA, Martinelli MJ, Polanc S. J Am Chem Soc. 1984;106:5594–5598.Katz JD, Overman LE. Tetrahedron. 2004;60:9559–9568.Gergely J, Morgan JB, Overman LE. J Org Chem. 2006;71:9144–9152. doi: 10.1021/jo061537j.
- 16.Takami K, Mikami S, Yorimitsu H, Shinokubo H, Oshima K. J Org Chem. 2003;68:6627–6631. doi: 10.1021/jo0344790. [DOI] [PubMed] [Google Scholar]
- 17.Fleming I, Maiti P, Ramarao C. Org Biomol Chem. 2003;1:3989–4004. doi: 10.1039/b305880h. and references therein. [DOI] [PubMed] [Google Scholar]
- 18.(a) Gassman PG, Singleton DA, Wilwerding JJ, Chavan SP. J Am Chem Soc. 1987;109:2182–2184. [Google Scholar]; (b) Grieco PA, Collins JL, Handy ST. Synlett. 1995:1155–1158. [Google Scholar]
- 19.(a) Anderson JC, Blake AJ, Graham JP, Wilson C. Org Biomol Chem. 2003;1:2877–2885. doi: 10.1039/b305116a. [DOI] [PubMed] [Google Scholar]; (b) Kim KS, Song YH, Lee BH, Hahn CS. J Org Chem. 1986;51:404–407. [Google Scholar]
- 20.In the absence of base, cycloadduct 22 was produced in 25–50% yield, with the remaining material being a mixture of tricyclic alkene isomers. The use of triethylamine resulted in 80% yield of 22; however, the reaction was much slower (24 vs 5 h, 50 mg scale).21
- 21.Kanemasa S, Tomoshige N, Wada E, Tsuge O. Bull Chem Soc Jpn. 1989;62:3944–3949. [Google Scholar]
- 22.CCDC 690534. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre. via www.ccdc.cam.ac.uk/data_request/cif.
- 23.Freshly prepared SmI2 was required to obtain reproducible yields for this reaction; see the Supporting Information for details.
- 24.Brown HC, Yoon NM. J Am Chem Soc. 1966;88:1464–1472. [Google Scholar]
- 25.To facilitate monitoring reactions and purifying subsequent intermediates, the N-benzyl protecting group was retained until the last step.
- 26.Reported optical rotations for the natural products are: (a) nankakurine A: [α]21D +16 (c 0.4, MeOH).2 (b) nankakurine B: [α]19D +12 (c 1.0, MeOH)4
- 27.Because they are strong bases and readily pick up protons (and potentially also CO2), we could reproducibly obtain 1H and 13C NMR spectra of the free-base forms of nankakurines A (2) and B (3) only in CD3OD containing a trace amount of NaOCD3. These spectra were not identical to those reported for natural 2 and 3 in CD3OD.2,4 However, by adding successive amounts of CF3CO2H to samples of synthetic 2 and 3, 1H and 13C NMR spectra identical to those of the natural products were obtained, consistent with the notion that the natural samples contained an undetermined amount of the conjugate acids. See the Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.