Abstract
The mechanisms underlying alterations in muscle lipid metabolism in obesity are poorly understood. The primary aim of this study was to compare the abundance and/or activities of key proteins that regulate intramyocellular triglyceride (IMTG) concentration in the skeletal muscle obtained from obese (OB; n = 8, BMI 38 ± 1 kg/m2) and nonobese (NOB; n = 9, BMI 23 ± 1 kg/m2) women. IMTG concentration was nearly twofold greater in OB vs. NOB subjects (75 ± 15 vs. 40 ± 8 μmol/g dry wt, P < 0.05). In contrast, the activity and protein abundance of key enzymes that regulate the esterification of IMTG (i.e., glycerol-3-phosphate acyltransferase and diacylglycerol acyltransferase) were not elevated. We also found no differences between groups in muscle adipose triglyceride lipase and hormone-sensitive lipase (HSL) protein abundance and no differences in phosphorylation of specific sites known to affect HSL activity. However, we did find the elevated IMTG in obesity to be accompanied by a greater abundance of the fatty acid transporter FAT/CD36 in the membrane fraction of muscle from OB vs. NOB subjects (P < 0.05), suggestive of an elevated fatty acid transport capacity. Additionally, protein abundance of the lipid-trafficking protein perilipin 3 was lower (P < 0.05) in muscle from OB vs. NOB when expressed relative to IMTG content. Our findings indicate that the elevated IMTG content found in obese women was not due to an upregulation of key lipogenic proteins or to the suppression of lipolytic proteins. The impact of a low perilipin protein abundance relative to the amount of IMTG in obesity remains to be clarified.
Keywords: intramyocellular lipid, tail-interacting protein of 47 kDa, intramuscular triglyceride, fatty acid transport
alterations in lipid metabolism underlie much of the metabolic complications associated with obesity (9). The excessive systemic availability of fatty acids and the ultimate metabolic fate of these fatty acids within highly metabolic tissues such as liver and muscle are key mediators of insulin resistance in obesity. Several studies have reported the association between accumulation of intramyocellular triglycerides (IMTG) and insulin resistance (11, 35). However, it is now well recognized that, rather than direct effects stemming from a high IMTG concentration, the accumulation of alternative lipid metabolites in muscle, like ceramide and diacylglyceride (DAG), is directly linked with impairing the intracellular insulin signal (11, 21, 44). Understanding the regulation of IMTG metabolism still remains of high importance because we (37) and others (30) have suggested that increasing the esterification of fatty acids entering the myocyte may protect against insulin resistance by reducing the substrate available for formation and accumulation of more harmful lipid intermediates. Moreover, it has been proposed that, after entering muscle, fatty acids are primarily esterified and enter the IMTG pool (22, 26). If correct, IMTG may be the precursor for all subsequent metabolic fates of fatty acid in skeletal muscle (e.g., oxidation, lipid intermediate formation), and therefore, a better understanding of the metabolic regulation of IMTG is of great importance.
The accumulation of IMTG is regulated by several factors, including the uptake of fatty acids into the myocyte, the activity of the enzymes catalyzing the esterification of fatty acids into triglycerides, and the enzymes that hydrolyze triglycerides (i.e., lipolysis). Fatty acid transport into skeletal muscle is largely a protein-mediated process (16, 38). Several putative fatty acid transporter proteins have been identified, and among them, fatty acid translocase (FAT/CD36) is a major fatty acid transporter in human muscle (1, 4). The abundance of FAT/CD36 on the muscle plasma membrane has been found to be directly proportional to the rate of fatty acid uptake (5), and therefore, it helps dictate the availability of substrate for IMTG synthesis. In turn, a series of esterification reactions synthesize IMTG from these fatty acids that have entered the myocyte. The first committed step of this esterification pathway is catalyzed by the enzyme glycerol-3-phosphate acyltransferase (GPAT), and the final step in this pathway leading to the formation of IMTG is catalyzed by the enzyme diacylglyceride acyltransferase (DGAT). GPAT and DGAT are commonly considered the key regulatory enzymes in this esterification pathway, yet the role of these enzymes in the elevated accumulation of IMTG in obesity is poorly understood. Similarly, the role that the key lipolytic enzymes in skeletal muscle [i.e., adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL)] may play in determining the elevated concentration of IMTG in obesity is also not well described.
IMTG are stored mainly in droplets encased in an emulsifying coat that partitions fat out of the cytosol and into thermodynamically stable fat droplets. One important family of proteins surrounding lipid is referred to as perilipins (27). Mounting evidence suggests that these perilipin proteins help regulate the metabolic fate of the triglycerides they enclose (3, 6). Perlipin 1 [formerly known simply as “perilipin” (27)] is the best-described member of the perilipin family. Perilipin 1 is highly expressed in adipose tissue (not expressed in skeletal muscle) and is associated with the regulation of lipolysis (18, 32). The other known perilipin proteins [i.e., perilipins 2, 3, 4, and 5, formerly known as adipophilin, tail-interacting protein of 47 kDa (TIP47), S3–12, and OXPAT, respectively (27)] have been found in skeletal muscle (12, 31) and have been suggested to be involved in trafficking lipid toward specific sites and/or signaling pathways within cells. Unlike perilipins 1 and 2, which are stable only when bound to the lipid droplet, perilipins 3–5 are stable both when bound to lipid as well as when in the cytosol (48). These perilipins appear to migrate from the cytosol to the surface of the lipid droplet during periods of lipid storage (48). Therefore, the abundance of these perilipins in the cytosol may provide a readily available source of lipid-coating proteins for use when muscle lipid storage is elevated. However, the roles of these perilipin proteins in skeletal muscle are not well known.
The mechanisms underlying the accumulation of IMTG in obesity are poorly understood. Therefore, we obtained skeletal muscle samples from healthy nonobese and obese volunteers, with the primary aim to compare factors that mediate 1) fatty acid transport capacity, 2) triglyceride esterification, 3) triglyceride lipolysis, and 4) the abundance of perilipin proteins, which may help regulate the metabolic fate of the IMTG. We hypothesized that, compared with skeletal muscle from nonobese volunteers, muscle from obese subjects would have a greater abundance of FAT/CD36 associated with the plasma membrane, and a resultant increase in fatty acid transport capacity in muscle would lead to an upregulation in the abundance and activity of key enzymes within the triglyceride synthesis pathway (i.e., GPAT and DGAT) without factors that regulate triglyceride lipolysis being affected (i.e., ATGL and HSL). We also anticipated that muscle perilipin protein abundance will increase in parallel with the increased accumulation of IMTG.
METHODS
Subjects
Nine nonobese and eight obese women participated in this study (Table 1). All subjects were premenopausal and were considered to be in good health after a comprehensive medical examination. No subject was taking regular medications. All subjects were nonsmokers and weight stable (±2 kg) for 4–6 mo before the beginning of the study, and all were sedentary (i.e., no structured exercise program and <2 h/wk of moderate or vigorous physical activity). Subjects with type 2 diabetes, coronary heart disease, clinically significant hyperglyceridemia (i.e., plasma triglycerides >150 mg/dl), or hypertension were excluded. The experiments in this study were all performed while the women were in the follicular phase of their menstrual cycle. All subjects were fully informed of the possible risks associated with the study, which was approved by the University of Michigan Institutional Review Board, and signed an informed consent document.
Table 1.
Subject characteristics
| Nonobese | Obese | |
|---|---|---|
| Age, yr | 25 ± 2 | 31 ± 3 |
| Body weight, kg | 62.6 ± 1.8 | 109.3 ± 3.8* |
| BMI, kg/m2 | 23 ± 1 | 38 ± 1* |
| Body fat, % | 32.4 ± 2.4 | 51.4 ± 1.0* |
| Fat mass, kg | 20.3 ± 2.1 | 56.3 ± 3.0* |
| Fat-free mass, kg | 42.0 ± 1.1 | 52.9 ± 1.3* |
Values are means ± SE. BMI, body mass index.
P < 0.001, obese vs. nonobese.
General Study Design
Subjects were admitted to the Michigan Clinical Research Unit of the University of Michigan Medical Center the night before the study. Subjects were provided a standardized meal and stayed in their hospital room overnight. The next morning, after an overnight fast, we measured basal oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) for 20 min using a Vmax Metabolic Cart (Sensor Medics, Yorba Linda, CA) to assess whole body fatty acid oxidation. After this measurement we obtained a fasting blood sample, and a muscle biopsy was obtained from the vastus lateralis using percutaneous biopsy technique (2). Muscle samples were frozen immediately in liquid nitrogen and stored at −80°C until analysis.
Analytic Procedures
Plasma substrate and insulin concentrations.
Standard commercial kits were used to measure plasma concentrations of glucose (glucose oxidase method; Thermo Scientific, Middletown, VA), fatty acid (Wako Chemicals USA, Richmond, VA), and triglyceride (Sigma, St. Louis, MO). Plasma insulin concentration was measured by radioimmunoassay (human insulin-specific RIA; Millipore, Billerica, MA).
Muscle IMTG concentration.
Muscle biopsy samples (∼15 mg wet wt) were lyophilized at −60°C for 48 h. Dried muscle samples were weighed to the nearest 0.1 mg, and ∼3–5 mg dry muscle was homogenized in chloroform-methanol (2:1, vol/vol) mixture. After extraction at 4°C for ∼16 h, the organic phase containing muscle lipids was dried down under vacuum (SpeedVac; ThermoSavant, Holbrook, NY). The lipids were then saponified in 500 μl of 4% ethanoic KOH for 25 min at 75°C, and the glycerol released was measured fluorometrically (14).
Muscle GPAT enzyme activity.
GPAT enzyme activity was assessed in partially purified membrane fraction from skeletal muscle samples, as reported previously (49). Briefly, 20–30 mg wet wt of each muscle sample was homogenized in buffer containing 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM DTT, 250 mM sucrose, and a mixture of protease inhibitors. Thirty minutes after homogenization, the homogenates were centrifuged at 1,500 g for 10 min at 4°C. The pellets were discarded, and the supernatants were centrifuged for 2 h at 38,000 rpm at 4°C. The pellets were manually homogenized and redissolved in the homogenization buffer. Protein content of the resultant solution was measured using Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). Ten micrograms of protein from the sample was incubated in the presence or absence of 2 mM N-ethylmaleimide (NEM; an inhibitor of GPAT2, GPAT3, and GPAT4) on ice for 15 min before the start of the reaction. The reaction was carried out in a 200-μl reaction mixture containing 75 mM Tris, pH 7.5, 1 mg/ml BSA (fatty acid free), 4 mM MgCl2, 1 mM DTT, 8 mM NaF, 80 μM palmitoyl-CoA, and 414 mM [14C]glycerol 3-phosphate [specific activity (SA) >20,000 dpm/nmol] for 20 min in a 37°C H2O bath with agitation (45). The organic phase containing 14C-labeled lysophosphatidic acid was dried, and the radioactivity was measured in scintillation fluid using a Tri-Carb 2800TR scintillation counter (PerkinElmer, Waltham, MA).
Muscle DGAT enzyme activity.
DGAT enzyme activity was assessed in partially purified membrane fractions, as reported by Liu et al. (30) and Yu et al. (50) with minor modifications. Briefly, 20–30 mg wet wt of each muscle sample was homogenized in buffer containing 20 mM HEPES, pH 7.4, 1 mM CaCl2, 1 mM DTT, 250 μM sucrose, and a mixture of protease inhibitors. Thirty minutes after homogenization, the homogenates were centrifuged at 1,500 g for 10 min at 4°C. The pellets were discarded, and the supernatants were centrifuged for 2 h at 38,000 rpm (>150,000 g) at 4°C. Supernatants were saved for immunoblot analysis of cytosolic proteins (see below). The pellets were manually homogenized and redissolved in homogenization buffer. Ten micrograms of protein was used for DGAT activities in a 200-μl reaction mixture containing 100 mM Tris, pH 7.5, 250 mM sucrose, 1 mg/ml BSA (fatty acid free), 150 mM MgCl2, 0.8 mM EDTA, 0.25 mM DAG, and 25 μM palmitoyl-CoA with 0.1 μCi [14C]palmitoyl-CoA (SA >30,000 dpm/nmol). The reaction was carried out at 37°C for 20 min in a water bath with agitation. The reaction was stopped with 0.75 ml chloroform-methanol (2:1, vol/vol). The lipid was extracted at room temperature for 2 h; 0.375 ml of a 1 mM H2SO4-17 mM NaCl solution was added to break the organic and aqueous phases. The organic phase was dried and redissolved in 30 μl of chloroform and spotted on a Whatman Silica Gel thin-layer chromatography plate. The lipids were separated in chloroform-acetic acid (96:4, vol/vol). Triglyceride spots were visualized with iodine vapor and scraped off and counted in scintillation fluid.
Immunoblot analysis.
Cytosolic and crude membrane fractions of muscle homogenates from DGAT activity preparation (see above) were used for electrophoresis analysis of protein contents in muscle. Supernatants containing cytosolic proteins from DGAT activity preparation were concentrated using Amicon Ultra Centrifugal Filters (MWCO 3KD; Millipore) and used for electrophoresis analysis of cytosolic proteins. Thirty micrograms of cytosolic proteins or 10 μg of membrane proteins was separated by SDS-PAGE and transferred to nitrocellulose membrane. These membranes were incubated with antibodies for the proteins of interest (see below), followed by a 60-min incubation with the appropriate secondary antibody. The blots were developed with enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ), and the bands for the proteins of interest were quantified using AlphaEaseFC (AlphaInnotech, San Leandro, CA). Blots were probed with the following antibodies: α-CD36 (cat. no. ab-17044; Abcam, Cambridge, MA), α-HSL (cat. no. 4107; Cell Signaling Technology, Danvers, MA), α-p-HSL Ser660 (cat. no. 4126; Cell Signaling Technology), α-p-HSL Ser563 (cat. no. 4139; Cell Signaling Technology), α-p-HSL Ser565 (cat. no. 4137; Cell Signaling Technology), α-ATGL (cat. no. 2138; Cell Signaling Technology), α-GPAT1 (a gift from Dr. Rosalind Coleman), α-DGAT1 (cat. no. NB110-41487; Novus Biologicals, Littleton, CO), α-DGAT2 (cat. no. sc-66859; Santa Cruz Biotechnology), NADH-ubiquinol oxidoreductase [cytochrome oxidase I (COX-I)] (cat. no. A21344; Molecular Probes, Carlsbad, CA), α-perilipin 2 (adipophilin) (19), α-perilipin 3 (carboxyl terminus) (TIP47) (40), and α-perilipin 5 (OXPAT) (cat. no. 03-GP31; American Research Products). Polyclonal antibodies to human perilipin 1 (perilipin) and human perilipin 4 (S3–12) were generated by Proteintech Group with their standard rabbit protocol, using keyhole limpet hemocyanin-conjugated peptide CVEYLLPADKEESAPAPGH (perilipin 1) or CASAEDAAVQEERDAGV (perilipin 4) as the immunogen. The perilipin 1 and 4 antibodies were affinity purified using the immunizing peptide. The names of the perilipin proteins measured in this study are listed above, using the unified nomenclature that was recently adopted (27), followed in parentheses by alternative names that have been commonly used to identify these proteins in previous studies. These perilipin proteins will be referred to using the new nomenclature throughout the remainder of this article.
Calculations
The homeostasis model assessment of insulin resistance (HOMA-IR) was used to assess insulin sensitivity of our subjects. HOMA-IR was calculated using the subjects' fasting plasma glucose (FG) and fasting plasma insulin (FI) concentrations: HOMA-IR = FG (mM) × FI (μU/ml)/22.5. Whole body triglyceride oxidation (g/min) was calculated from V̇o2 and V̇co2 measurements using the equations of Frayn (13). In turn, whole body fatty acid oxidation (μmol/min) was calculated by dividing triglyceride oxidation by an estimated molecular weight of triglyceride (860 g/mol) and multiplying by 3. Resting energy expenditure was calculated from resting V̇o2 and V̇co2 measurements using the Weir equation (47).
GPAT enzyme activity was calculated as
The conversion factor was calculated by dividing the counts of 1 μl of [14C]glycerol 3-phosphate (G-3-P) (dpm) by [14C]G-3-P concentration (pmol/ul). The fraction of [14C]G-3-P refers to the ratio of [14C]G-3-P to total G-3-P (mol) in the reaction mixture. GPAT activity measured in reactions containing NEM (i.e., “NEM-resistant GPAT activity”) was representative of GPAT1 activity because NEM is a known inhibitor of GPAT2, GPAT3, and GPAT4. GPAT activity calculated from reactions without NEM was representative of total GPAT activity. Therefore, the difference between the total GPAT activity and the NEM-resistant GPAT activity (i.e., GPAT1 activity) results in the summed activity of GPAT2, GPAT3, and GPAT4, which we refer to collectively as “other GPATs.”
DGAT activity (pmol·min−1·mg−1) was calculated as
The conversion factor was calculated by dividing counts of 1 μl of [14C]palmitoyl-CoA (dpm) by [14C]palmitoyl-CoA concentration (pmol/ul). The fraction of [14C]palmitoyl-CoA was the percentage of [14C]palmitoyl-CoA in total palmitoyl-CoA (mol) in the reaction mixture.
Statistical Analysis
All data are presented as means ± SE. Unless otherwise indicated, n = 9 for nonobese group and n = 8 for obese group. We had specific a priori evidence-based hypotheses about the direction of the difference (e.g., obese > lean, or obese < lean) for HOMA-IR, IMTG concentration, DGAT and GPAT abundance and activities, and FAT/CD36 abundance. Therefore, we used one-tailed Student's t-test to compare differences between groups for these variables. For all other comparisons, we used a two-tailed Student's t-test to compare differences between the groups. Statistical significance was accepted for P < 0.05.
RESULTS
Plasma Substrate and Insulin Concentrations
Although fasting glucose concentration was not different in obese and nonobese subjects (Table 2), insulin sensitivity was suppressed in our obese subjects, as indicated by a nearly twofold higher fasting plasma insulin concentration and HOMA-IR in obese compared with nonobese subjects (P < 0.05 for insulin and P < 0.01 for HOMA-IR; Table 2). Plasma triglyceride concentration tended to be greater in obese compared with nonobese subjects (P = 0.055), but plasma fatty acid concentration was not different between groups (Table 2).
Table 2.
Fasting plasma substrate and insulin concentrations and HOMA-IR
| Nonobese | Obese | |
|---|---|---|
| Glucose concentration, mM | 4.5 ± 0.1 | 4.9 ± 0.2 |
| Fatty acid concentration, mM | 0.50 ± 0.07 | 0.44 ± 0.05 |
| Triglyceride concentration, mM | 0.63 ± 0.07 | 0.86 ± 0.08 |
| Insulin concentration, μU/ml | 10.5 ± 1.2 | 19.6 ± 3.8* |
| HOMA-IR | 2.1 ± 0.2 | 3.9 ± 0.6** |
Values are means ± SE. HOMA-IR, homeostasis model assessment of insulin resistance. Plasma samples collected after an overnight fast.
P < 0.05;
P < 0.01, obese vs. nonobese.
IMTG Synthesis
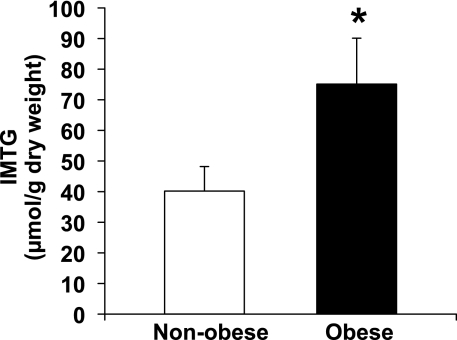
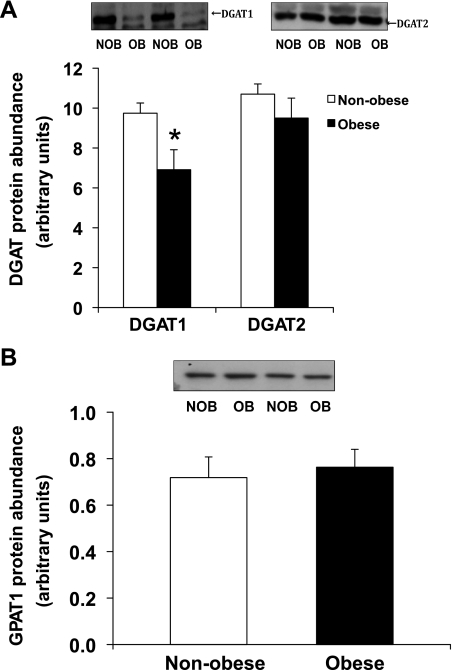
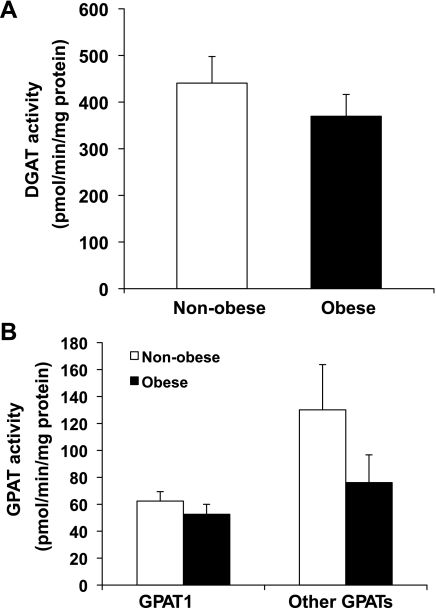
Obese women had a nearly twofold greater IMTG concentration compared with nonobese women (P < 0.05; Fig. 1). Despite the elevated IMTG concentration in our obese subjects, we found the protein abundance of DGAT1 to be significantly lower in our obese compared with nonobese women (P = 0.02; Fig. 2A). We also found a trend for a lower GPAT enzyme activity in the NEM-sensitive fraction (i.e., GPAT2, GPAT3, and GPAT4) of skeletal muscle, but this difference did not reach statistical significance (P = 0.1; Fig. 3B). Total GPAT protein abundance (Fig. 2B), DGAT enzyme activity (Fig. 3A), and GPAT1 enzyme activity (Fig. 3B) were not different between groups.
Fig. 1.
Muscle intramyocellular triglyceride (IMTG) concentrations in skeletal muscle from nonobese and obese women. *Significantly different from nonobese, P < 0.05.
Fig. 2.
A: diacylglyceride acyltransferase (DGAT)1 and DGAT2 protein abundance in skeletal muscle. B: glycerol-3-phosphate acyltransferase (GPAT)1 protein abundance in skeletal muscle in nonobese and obese women. A and B, top, show Western blots from 2 representative subjects for each protein, respectively. Data are means ± SE. NOB, nonobese subjects; OB, obese subjects. *Significantly different from nonobese, P < 0.05.
Fig. 3.
A: total DGAT activity in skeletal muscle from NOB and OB women. B: GPAT enzyme activities in skeletal muscle from nonobese and obese women. Ten-micrgoram membrane proteins were used for the in vitro enzyme activity assays.
IMTG Lipolysis
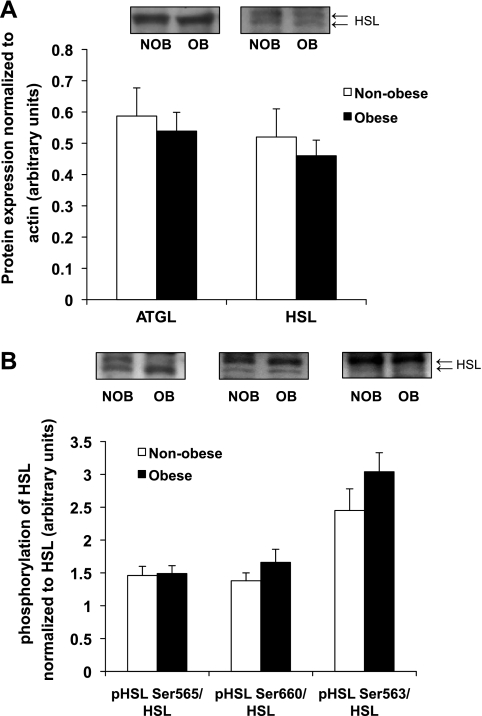
Protein abundance of the lipolytic enzymes ATGL and HSL were also not different between our obese and nonobese women (Fig. 4A), and the phosphorylation state of HSL associated with augmented lipolytic activity (p-HSL Ser563 and Ser660) and suppression of lipolytic activity (p-HSL Ser565) were not different between groups (Fig. 4B). Together with our findings for the esterification proteins outlined above, these data indicate that the marked increased in IMTG concentration in our obese women was not a consequence of an elevated capacity for triglyceride synthesis or alterations in lipolytic capacity.
Fig. 4.
A: adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) protein abundance in skeletal muscle from NOB and OB women. Data are normalized to levels of actin. B: HSL serine phosphorylation in skeletal muscle from NOB and OB women after being normalized to total HSL level. A and B, top: Western blots from 2 representative subjects for each protein. All HSL antibodies pick up 2 bands, and both bands were scanned for data shown in the bar graph.
Lipid Droplet-Associated Proteins
Importantly, perilipin 1 was not detectable in any of our muscle samples (Fig. 5), indicating that our muscle samples were free of adipose tissue contamination. Total protein abundance of perilipin 3 was similar in our obese and nonobese subjects [0.34 ± 0.04 vs. 0.48 ± 0.08 arbitrary units (AU), respectively; see Supplemental Fig. S1 for validation experiment of perilipin 3 antibody in skeletal muscle; Supplemental Material for this article can be found online at the AJP-Endocrinology and Metabolism web site]. However, when normalized to the amount of lipid in the muscle (i.e., normalized to IMTG concentration), perilipin 3 was significantly lower in obese compared with nonobese women (4.4 ± 0.9 vs. 11.4 ± 3.0 AU, respectively, P < 0.05). We could not confirm detectable levels of perilipin 2, 4, or 5 in our skeletal muscle samples.
Fig. 5.
Western blot probing with anti-perilipin 1, demonstrating no contamination of adipose tissues in skeletal muscle samples. FAT, human adipose tissue.
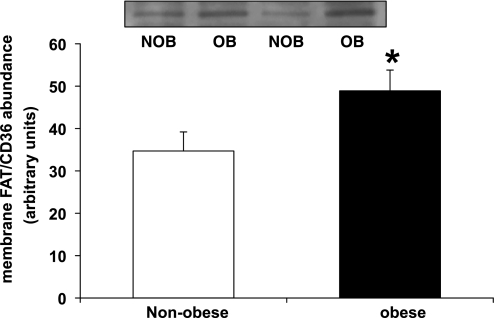
Fatty Acid Transporter Protein (FAT/CD36)
Protein abundance of FAT/CD36 was greater in the membrane fraction of skeletal muscle obtained from obese compared with nonobese subjects (P < 0.05; Fig. 6). However, protein abundance of FAT/CD36 in the cytosolic fraction was not different between groups (data not shown).
Fig. 6.
Membrane-associated fatty acid translocase (FAT/CD36) abundance in skeletal muscle from NOB and OB women; n = 6 for NOB and n = 7 for OB group. Top: Western blots from 2 representative subjects. *Significantly different from nonobese, P < 0.05.
Whole Body Fatty Acid Oxidation
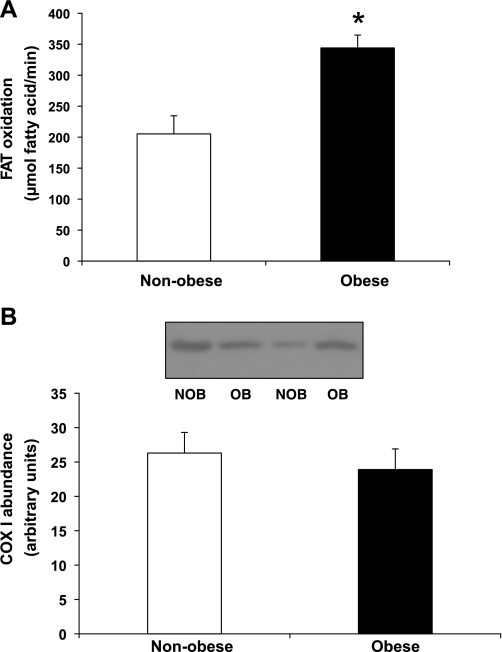
Whole body fatty acid oxidation rate was significantly greater in obese vs. nonobese subjects (P < 0.05; Fig. 7A). Even when expressed relative to fat-free mass (FFM), fatty acid oxidation tended to be greater in obese vs. lean subjects (6.5 ± 0.5 vs. 4.8 ± 0.7 mmol·kg FFM−1·min−1, P = 0.059). The greater rate of fatty acid oxidation in obese compared with nonobese subjects was a consequence of both a significantly greater relative contribution of fat to total energy expenditure in obese vs. nonobese subjects (RER: 0.75 ± 0.01 vs. 0.81 ± 0.02; P = 0.03) as well as a significantly greater rate of resting energy expenditure (1.18 ± 0.04 vs. 0.89 ± 0.03 kcal/min, P < 0.0001). We measured COX-I protein abundance in skeletal muscle as a marker for mitochondrial fatty acid oxidative capacity. Despite the elevated rate of fatty acid oxidation in our obese vs. nonobese subjects, COX-I protein content in muscle was similar between groups (Fig. 7B), suggesting that the difference in fat oxidation between groups was not a consequence of differences in mitochondrial density.
Fig. 7.
A: Whole body fatty acid oxidation in NOB and OB subjects. B: cytochrome oxidase I (COX-I) expression in skeletal muscle from NOB and OB women. B, top: Western blots from 2 representative subjects. *Significantly different from nonobese, P < 0.05.
DISCUSSION
The major findings of this study indicate that the increased accumulation of IMTG in muscle from obese compared with nonobese women was not due to an upregulation of key enzymes of the triglyceride synthesis pathway in muscle. The elevated IMTG concentration in obesity was also not accompanied by evidence of a lower lipolytic capacity in muscle from obese compared with nonobese women. In contrast, the greater accumulation of IMTG in obesity was accompanied by an elevated FAT/CD36 transporter abundance in the muscle membrane fraction, suggesting that a greater capacity for fatty acid flux into the myocyte may be an important contributor to the high IMTG content in obesity without the esterification pathway being augmented. Furthermore, we were able to detect the lipid droplet-associated protein perilipin 3 in skeletal muscle from both nonobese and obese women. Interestingly, the abundance of perlilpin 3 was significantly lower in muscle from obese compared with nonobese women when expressed relative to IMTG content. It is important to note that insulin resistance in our obese subjects may be an important contributor to the differences we observed between groups.
Our results confirmed several previous studies (17, 20) reporting elevated concentrations of IMTG in obese compared with sedentary nonobese adults. Delivery of fatty acids into muscle to serve as the substrate for triglyceride synthesis a is key factor regulating the formation of IMTG. Clearly, systemic mobilization of fatty acids from adipose tissue (i.e., lipolysis) is far greater in obese compared with nonobese adults (24). It is important to note that this elevated lipolytic rate in obesity does not always result in a marked elevation in plasma fatty acid concentration (24, 25), indicating that a high rate of fatty acid uptake often matches the high rate of fatty acid release from adipose tissue. Along these lines, Jocken et al. (25) reported that, despite similar fasting plasma fatty acid concentrations between nonobese and obese subjects, the net uptake of fatty acid into forearm muscle was profoundly greater in their obese subjects. Similarly, in vitro findings indicate that fatty acid transport into skeletal muscle obtained from obese adults was severalfold greater than that measured in muscle obtained from their nonobese counterparts (5). Fatty acid uptake into muscle is regulated largely by facilitated transport (39), and our current findings agree with previous data (4, 5) indicating that the key fatty acid transporter FAT/CD36 is greater in the membrane fraction of skeletal muscle taken from obese compared with nonobese adults. Moreover, Holloway et al. (22) suggest that the augmented fatty acid uptake due to the increased plasmalemmal FAT/CD36 content in muscle from obese compared with nonobese animals is directed largely toward the triglyceride synthesis pathway for esterification. However, to date it was unclear whether the elevated fatty acid availability in skeletal muscle affected the abundance or activity of lipogenic enzymes.
GPAT is the initial and rate-limiting step of the triglyceride synthesis pathway. At least four isoforms of GPAT (GPAT1-4) have been identified so far. GPAT1 and -2 are mitochondrial bound (mtGPAT), whereas GPAT3 and -4 are present in the microsomal fraction (15). The mitochondrial, NEM-resistant GPAT (GPAT1) was the first identified GPAT and has been the most studied isoform of GPAT so far. Overexpression of GPAT1 in liver causes a significant increase in hepatic triglyceride content in rats (33), whereas knocking down expression of hepatic GPAT1 in ob/ob mice resulted in reduced hepatic triglyceride accumulation (49). Studies based on gain/loss-of-function have also suggested that GPAT1 may play an important role in directing fatty acyl-CoA toward triglyceride biosynthesis (28, 29). Although there is a large body of literature about GPAT1 regulation in liver and adipose tissue, much less is known about GPAT1 regulation in skeletal muscle from either animal or human studies. Our findings indicate that the augmented IMTG accumulation in obesity was not due to enhanced GPAT enzyme activity. Therefore, the apparent high flux of fatty acids into skeletal muscle of our obese volunteers did not augment expression or activity of this first regulating step of triglyceride synthesis. Alternatively, it has been reported previously that reducing fatty acid availability (via administration of the lipolytic inhibitor nicotinic acid) also did not change GPAT1 activity in skeletal muscle of young men (45), supporting the notion that muscle GPAT1 activity is not affected by substrate availability.
Similar to GPAT, our findings indicate that elevated IMTG accumulation in obese women was also not due to an upregulation of the protein abundance or enzyme activity of the final step of the triglyceride synthesis pathway (i.e., DGAT). Both DGAT1 and -2 are bound to the membrane of the endoplasmic reticulum, and both have acyltransferase activity. However, data from knockout mouse models suggest that DGAT1 and -2 play fundamentally different roles in mammalian triglyceride metabolism (10, 41). Interestingly, we found that DGAT1 protein abundance was actually lower in muscle from our obese compared with our nonobese subjects, but total DGAT enzyme activity was identical between groups. Although it may be attractive to conclude that DGAT2 activity may be greater in obesity to compensate for the lower abundance of DGAT1, as suggested by data from mouse models (42), the DGAT activity measured in this study is believed to be mainly DGAT1 activity because DGAT2 activity has been found to be suppressed at magnesium concentrations used in our assay (150 mM MgCl2) (8). This suggests that the intrinsic activity of DGAT1 may be greater in the muscle samples from our obese compared with our nonobese women. However, we cannot rule out the possibility that DGAT2 or other proteins with acyltransferase activity may have contributed to compensate for the lower DGAT1 protein content in our obese women. In contrast to our findings, Thrush et al. (43) reported recently that the protein abundance of DGAT1 was similar in their nonobese and obese women. The reason for this discrepancy is unclear, but importantly, our measurement of DGAT activity confirms the notion that the functional capacity of this enzyme in skeletal muscle was similar in nonobese and obese women, and clearly, the accumulation of IMTG in obesity is not a consequence of an enhanced abundance or activity of DGAT. Together, our GPAT and DGAT enzyme activity data suggest that key steps of the triglyceride synthesis pathway are not augmented in obesity. These data also indicate that the capacity of the triglyceride synthesis pathway in muscle is in excess of substrate supply, and when intramyocellular fatty acid availability is high (as in obesity), triglyceride synthesis can increase without enhancing the abundance or activity of key enzymes within the esterification pathway.
Our findings also suggest that differences in factors regulating IMTG hydrolysis do not appear to provide a mechanistic explanation for the elevated muscle lipid concentrations in our obese compared with nonobese women. IMTG lipolysis is mediated by sequential hydrolysis reactions regulated by different lipases. ATGL is reported to mainly catalyze the hydrolysis of triglyceride to DAG, whereas HSL is required for hydrolysis of DAG to monoglyceride, which is then hydrolyzed by monoglyceride lipase (46). In contrast to findings in rat muscle (46), we found that ATGL protein expression was not different in nonobese and obese humans. Although species differences could explain these discrepant findings, this discrepancy may also be explained by the length of fast before the muscle samples were excised, because the duration of fasting has been found to influence ATGL expression (34). Watt et al. (46) removed the muscles from their rats 4 h after eating, whereas our muscle biopsies were performed after an overnight (∼12 h) fast. Similar to ATGL data, we also found no difference between our obese and nonobese subjects in the total abundance of HSL protein in skeletal muscle. More important than simply the total abundance of the lipolytic enzyme, HSL activity is regulated via a phosphorylation-dependent mechanism (7, 23), and there are multiple serine phosphorylation sites on HSL (23). Among them, phosphorylation at either Ser563 or Ser660 is known to increase HSL activity (7), whereas phosphorylation HSL at Ser565 is known to inhibit HSL activity (7). Our finding that the phosphorylation states of HSL at serine residues 563, 565, and 660 were the same in muscle samples from nonobese and obese subjects supports the notion that difference in muscle lipolytic regulation does not account for the greater accumulation of IMTG in obesity. In contrast to our findings, Jocken et al. (25) reported that total HSL protein abundance as well as the phosphorylation of HSL at Ser563 and Ser565 were slightly yet significantly lower in muscle from obese compared with nonobese men. Sex-related differences in HSL expression and phosphorylation have been reported (36) and may help explain this discrepancy. Importantly, however, when normalized to total HSL protein content, our data and those of Jocken et al. (25) agree that phosphorylation state of HSL at Ser565 and Ser563 was not different between nonobese and obese groups.
Most mammalian cells contain at least a small amount of triglycerides and other very hydrophobic lipids emulsified in a membrane leaflet. Amphipathic compounds span the neutral lipid/cytosol interface with the hydrophobic region embedding into the triglyceride and hydrophilic regions facing the aqueous cytosol. The most abundant proteins coating mammalian cytosolic triglycerides are the five perilipin family members (4, 27). Perilipins are a component of the partition between insoluble triglycerides and the aqueous cytosol. Therefore, these proteins connect cellular fat storage and cytosolic “machinery.” Perilipin interactions with triglycerides and DAGs as well as cytoskeleton elements, lipases, and lipase coactivators have been reported (3, 40). Perilipin 3 has been implicated in the process of assembling nascent lipid droplets. We hypothesize that diminished perilipin content relative to the amount of IMTG we found in our obese compared with our nonobese subjects may result in IMTG being poorly integrated into muscle fiber cytosol.
In summary, our findings indicate that the high IMTG concentration found in obesity was not a consequence of an upregulation of key steps of the triglyceride esterification pathway or a downregulation of lipolytic enzymes in muscle. Instead, we found that the elevated IMTG concentrations in obesity were simply accompanied by a greater capacity for fatty acid flux into the myocyte via an increased abundance of the key fatty acid transporter FAT/CD36 on the muscle membrane. Together, these findings suggest that the capacity of the triglyceride synthesis pathway in muscle exceeds substrate supply such that augmented intramyocellular fatty acid availability (as in obesity) can increase triglyceride synthesis without altering the abundance or activity of key enzymes within the esterification pathway. Additionally, our novel finding that total perilipin 3 protein abundance was similar in obese and nonobese subjects indicates that content of this important lipid-coating protein was not elevated in parallel with the greater accumulation of IMTG. It remains to be determined whether this represents a dysregulation in the synthesis or integration of perilipin proteins in parallel with the growing lipid droplet in obesity.
GRANTS
N. E. Wolins was supported by and antibodies to the perilipins were generated through the Washington University School of Medicine's Nutrition Obesity Research Center via a pilot and feasibility grant (P30 DK-056341). This project was supported in part by a grant from the National Institutes of Health (R01-DK-071955; principal investigator: J. F. Horowitz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the Michigan Clinical Research Unit (NIH-UL1-RR-024986) and the Michigan Nutrition and Obesity Research Center (P30-DK-089503) for help in conducting the experimental protocols, Lisa Michael for recruiting, screening, and enrolling subjects, Dr. Simon Schenk, Dr. Matthew Harber, Dr. Nicolas Knuth, Andrea Cornford, and Sean Newsom for their assistance with sample collection, the staff at the Michigan Diabetes Research and Training Center (NIH-NIDDK 5P60-DK-20572) chemistry core laboratory for processing the insulin assay, and the subjects for their participation.
REFERENCES
- 1. Aguer C, Mercier J, Man CY, Metz L, Bordenave S, Lambert K, Jean E, Lantier L, Bounoua L, Brun JF, Raynaud de Mauverger E, Andreelli F, Foretz M, Kitzmann M. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia 53: 1151–1163, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35: 609–616, 1975 [PubMed] [Google Scholar]
- 3. Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791: 419–440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonen A, Holloway GP, Tandon NN, Han XX, McFarlan J, Glatz JF, Luiken JJ. Cardiac and skeletal muscle fatty acid transport and transporters and triacylglycerol and fatty acid oxidation in lean and Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol 297: R1202–R1212, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48: 2547–2559, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal 18: 401–408, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem 276: 38870–38876, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta 1801: 252–265, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV., Jr Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest 109: 1049–1055, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coen PM, Dube JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, Goodpaster BH. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59: 80–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Wilde J, Smit E, Snepvangers FJ, de Wit NW, Mohren R, Hulshof MF, Mariman EC. Adipophilin protein expression in muscle—a possible protective role against insulin resistance. FEBS J 277: 761–773, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Frayn KN. Calculation of substrate oxidation rates in vivo from gas exchange. J Appl Physiol 55: 628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 14. Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res 21: 139–144, 1980 [PubMed] [Google Scholar]
- 15. Gimeno RE, Cao J. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res 49: 2079–2088, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 90: 367–417, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49: 467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J Biol Chem 284: 34538–34544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gropler MC, Harris TE, Hall AM, Wolins NE, Gross RW, Han X, Chen Z, Finck BN. Lipin 2 is a liver-enriched phosphatidate phosphohydrolase enzyme that is dynamically regulated by fasting and obesity in mice. J Biol Chem 284: 6763–6772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haugaard SB, Mu H, Vaag A, Madsbad S. Intramyocellular triglyceride content in man, influence of sex, obesity and glycaemic control. Eur J Endocrinol 161: 57–64, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Holloway GP, Benton CR, Mullen KL, Yoshida Y, Snook LA, Han XX, Glatz JF, Luiken JJ, Lally J, Dyck DJ, Bonen A. In obese rat muscle transport of palmitate is increased and is channeled to triacylglycerol storage despite an increase in mitochondrial palmitate oxidation. Am J Physiol Endocrinol Metab 296: E738–E747, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31: 1120–1124, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol Endocrinol Metab 276: E278–E284, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Jocken JW, Roepstorff C, Goossens GH, van der Baan P, van Baak M, Saris WH, Kiens B, Blaak EE. Hormone-sensitive lipase serine phosphorylation and glycerol exchange across skeletal muscle in lean and obese subjects: effect of beta-adrenergic stimulation. Diabetes 57: 1834–1841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 587: 5939–5950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51: 468–471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewin TM, Wang S, Nagle CA, Van Horn CG, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 directs the metabolic fate of exogenous fatty acids in hepatocytes. Am J Physiol Endocrinol Metab 288: E835–E844, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Linden D, William-Olsson L, Rhedin M, Asztely AK, Clapham JC, Schreyer S. Overexpression of mitochondrial GPAT in rat hepatocytes leads to decreased fatty acid oxidation and increased glycerolipid biosynthesis. J Lipid Res 45: 1279–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117: 1679–1689, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minnaard R, Schrauwen P, Schaart G, Jorgensen JA, Lenaers E, Mensink M, Hesselink MK. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J Clin Endocrinol Metab 94: 4077–4085, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem 280: 43109–43120, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nagle CA, An J, Shiota M, Torres TP, Cline GW, Liu ZX, Wang S, Catlin RL, Shulman GI, Newgard CB, Coleman RA. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem 282: 14807–14815, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palou M, Priego T, Sánchez J, Villegas E, Rodríguez AM, Palou A, Picó C. Sequential changes in the expression of genes involved in lipid metabolism in adipose tissue and liver in response to fasting. Pflugers Arch 456: 825–836, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Roepstorff C, Donsmark M, Thiele M, Vistisen B, Stewart G, Vissing K, Schjerling P, Hardie DG, Galbo H, Kiens B. Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am J Physiol Endocrinol Metab 291: E1106–E1114, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids 82: 149–154, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Shirreffs SM. Markers of hydration status. Eur J Clin Nutr 57: S6–S9, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Skinner JR, Shew TM, Schwartz DM, Tzekov A, Lepus CM, Abumrad NA, Wolins NE. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J Biol Chem 284: 30941–30948, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25: 87–90, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Suzuki R, Tobe K, Aoyama M, Sakamoto K, Ohsugi M, Kamei N, Nemoto S, Inoue A, Ito Y, Uchida S, Hara K, Yamauchi T, Kubota N, Terauchi Y, Kadowaki T. Expression of DGAT2 in white adipose tissue is regulated by central leptin action. J Biol Chem 280: 3331–3337, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Thrush AB, Brindley DN, Chabowski A, Heigenhauser GJ, Dyck DJ. Skeletal muscle lipogenic protein expression is not different between lean and obese individuals: a potential factor in ceramide accumulation. J Clin Endocrinol Metab 94: 5053–5061, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav 94: 242–251, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab 287: E120–E127, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Watt MJ, van Denderen BJ, Castelli LA, Bruce CR, Hoy AJ, Kraegen EW, Macaulay L, Kemp BE. Adipose triglyceride lipase regulation of skeletal muscle lipid metabolism and insulin responsiveness. Mol Endocrinol 22: 1200–1212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett 580: 5484–5491, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Xu H, Wilcox D, Nguyen P, Voorbach M, Suhar T, Morgan SJ, An WF, Ge L, Green J, Wu Z, Gimeno RE, Reilly R, Jacobson PB, Collins CA, Landschulz K, Surowy T. Hepatic knockdown of mitochondrial GPAT1 in ob/ob mice improves metabolic profile. Biochem Biophys Res Commun 349: 439–448, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Yu YH, Zhang Y, Oelkers P, Sturley SL, Rader DJ, Ginsberg HN. Posttranscriptional control of the expression and function of diacylglycerol acyltransferase-1 in mouse adipocytes. J Biol Chem 277: 50876–50884, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.