Abstract
Genome stability is essential for maintaining cellular and organismal homeostasis, but it is subject to many threats. One ubiquitous threat is from a class of compounds known as reactive oxygen species (ROS), which can indiscriminately react with many cellular biomolecules including proteins, lipids, and DNA to produce a variety of oxidative lesions. These DNA oxidation products are a direct risk to genome stability, and of particular importance are oxidative clustered DNA lesions (OCDLs), defined as two or more oxidative lesions present within 10 bp of each other. ROS can be produced by exposure of cells to exogenous environmental agents including ionizing radiation, light, chemicals, and metals. In addition, they are produced by cellular metabolism including mitochondrial ATP generation. However, ROS also serve a variety of critical cellular functions and optimal ROS levels are maintained by multiple cellular antioxidant defenses. Oxidative DNA lesions can be efficiently repaired by base excision repair or nucleotide excision repair. If ROS levels increase beyond the capacity of its antioxidant defenses, the cell's DNA repair capacity can become overwhelmed, leading to the accumulation of oxidative DNA damage products including OCDLs, which are more difficult to repair than individual isolated DNA damage products. Here we focus on the induction and repair of OCDLs and other oxidatively induced DNA lesions. If unrepaired, these lesions can lead to the formation of mutations, DNA DSBs, and chromosome abnormalities. We discuss the roles of these lesions in human pathologies including aging and cancer, and in bystander effects.
Keywords: Reactive oxygen species, DNA damage, DNA double-strand breaks, Aging, Cancer, Bystander effect
1. Induction and processing of oxidative DNA lesions in human cells and tissues
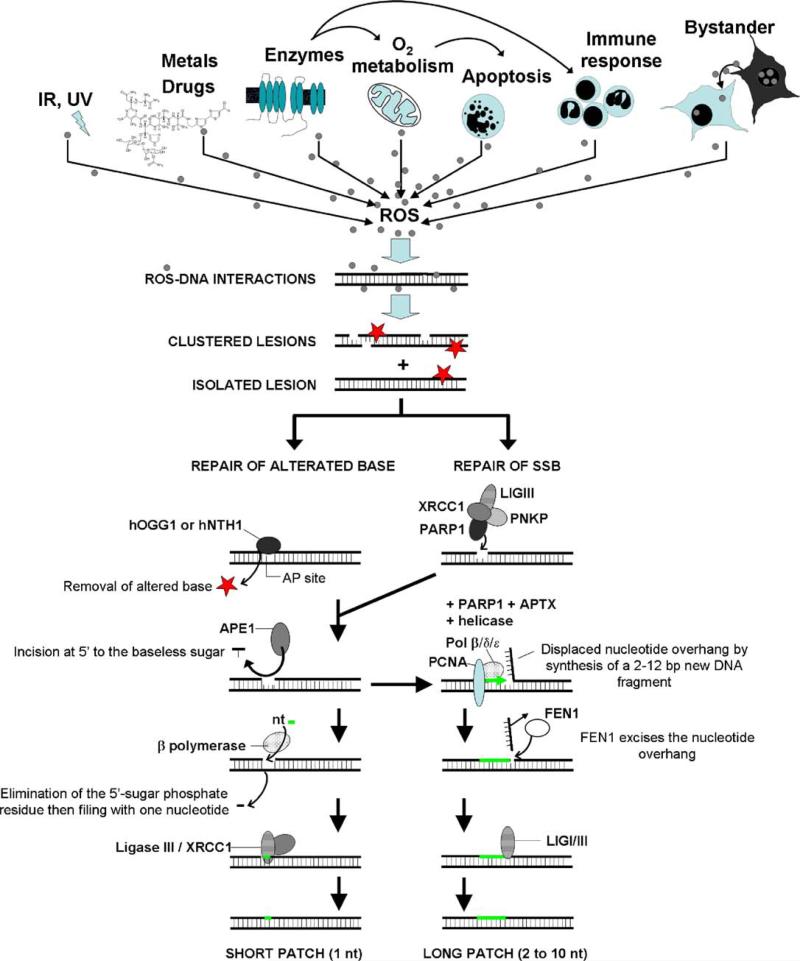
Elevated ROS levels can create oxidative stress in a cell and chronic exposure to this stress can result in permanent changes in the genome [1,2]. It is generally accepted that the accumulation of oxidative DNA lesions may promote mutagenesis, human pathogenesis and loss of homeostasis. These oxidative lesions can be induced not only by ROS generated by exposure to exogenous agents including ionizing or non-ionizing radiation (IR), drugs, and other chemicals such as metals [3–7] but also from endogenous sources including oxygen metabolism, apoptosis, and inflammatory responses involving the immune system [2,8–13] (Fig. 1).
Fig. 1.
ROS have different origins. ROS can arise following exposure to ionizing radiation or light (IR, UV), drugs and other chemicals such as metals. Enzymes, oxygen metabolism and apoptosis also account for ROS production. Finally, the inflammatory responses involving the immune system and bystander signaling also utilize ROS. When ROS enter the nuclear cell compartment, they interact with DNA creating lesions ranging from base or sugar modifications to abasic sites (represented by red stars) and SSBs. ROS-induced DNA lesions can appear in an isolated or clustered form and they are primarily repaired by two BER subpathways: the short-patch and the long-patch pathways. The short-patch or single-nucleotide pathway is initiated by a DNA glycosylase (hOGG1 or hNTH1) that cleaves and removes the altered base, giving an abasic site. This abasic site is then processed by an endonuclease (APE1) allowing DNA polymerase β to process the next step, catalyzing the elimination of the 5′-sugar phosphate residue and filling the gap with a nucleotide. Finally, the nick is sealed by the ligase III/XRCC1 complex. To simplify, only the branch of the short-pathway utilizing a monofunctional glycosylase is represented. SSBs can be repaired by the long-patch pathway (replacing approximately 2–12 nucleotides). This subpathway is dependant on PCNA and FEN1. It contains many of the same factors as the short-patch pathway but in contrast to the short-patch subpathway, DNA synthesis is thought to be mediated by several DNA polymerases including polymerases β, δ and ε. nt: nucleotide.
Among these oxidative DNA lesions are abasic sites, single strand DNA breaks (SSBs), sugar moiety modifications, and deaminated and adducted bases [2,14,15]. Various studies have estimated that anywhere from 0.1 to 100 oxidative DNA lesions per Mbp may exist in normal cells and tissues [16–19]. One of the more common oxidative DNA lesions, 8-oxo-2′-deoxyguanosine (8-oxo-dG), is estimated to be present at approximately 1 per Mbp [20–22]. When two or more oxidative DNA lesions are present within 10 base pairs of each other, it is considered an oxidative clustered DNA lesion (OCDL). These OCDLs have been variously estimated to be present at levels between 0.02 and 0.8 clusters per Mbp in normal human primary cells as well as in tumor cells [23–25].
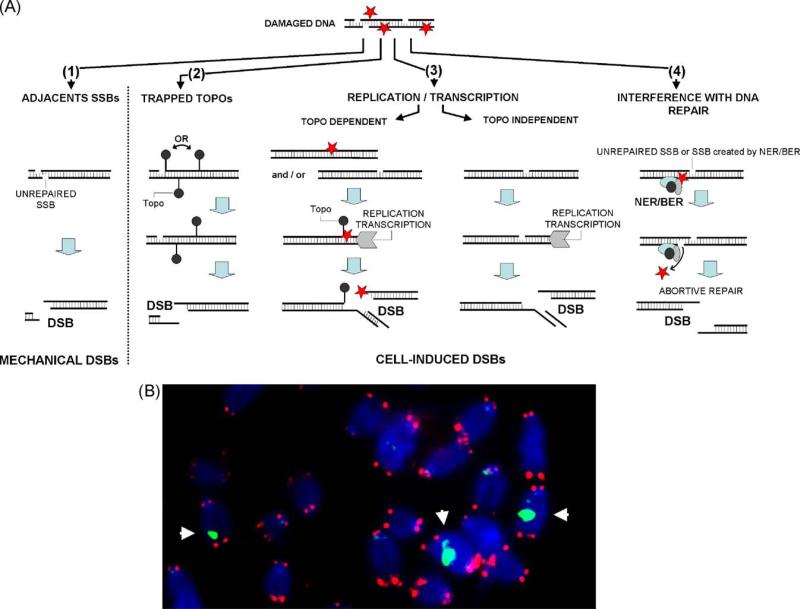
While individual DNA lesions are generally repaired efficiently, some OCDLs may be more difficult to resolve [26–31]. In some circumstances oxidative lesions can lead to DNA double-strand break (DSB) formation (Fig. 2) [32]. A DSB can arise when two SSBs form close to each other on opposite strands, when topoisomerases (Topos) cleave next to a SSB on the opposite strand, and when ROS-induced DNA damage interferes with either DNA replication or transcription [33–35]. In addition, DSBs can be generated during attempted simultaneous repair of two lesions in a cluster or when excision of a modified base takes place near an unrepaired SSB on the opposite strand [36]. DSBs are the most serious type of DNA damage because a small number of these lesions are sufficient to induce gene mutations, chromosomal aberrations, and cell transformation [37].
Fig. 2.
Oxidative stress can lead to DNA DSB formation. (A) A DSB can arise when two SSBs form close to each other (1), when topos cleave next to a SSB (2) and when ROS-induced DNA damage interferes with both DNA replication and transcription (3). A DSB is generated during DNA repair when excision of a modified base takes place near an unrepaired SSB. Oxidative DNA lesions can also interfere with reversible topo cleavage complexes during DNA replication and RNA transcription. In such cases, DNA/RNA polymerase forks run off the DNA to generate DSBs. Finally, DSBs can also appear when transcription and replication forks collide directly with SSBs or other ROS-induced lesions. Rarely, interference during DNA repair by BER also leads to DSB formation (4). (B) Representative image showing DNA DSBs induced by oxidative stress on mouse chromosomes. Blue: DNA; red: FISH signal indicating telomeres; green: DSBs visualized by γ-H2AX foci. White arrows indicate chromosomes containing a DSB.
Oxidative base lesions and abasic sites are predominantly repaired by base excision repair (BER) and to a lesser extent nucleotide excision repair [38,39]. The core BER pathways (Fig. 1) are initiated by a DNA glycosylase (mainly hOGG1 or hNTH1 in human cells) that recognizes and hydrolytically cleaves and removes the altered base, giving rise to an abasic site. The abasic site is then processed by an AP endonuclease (APE1), which incises the DNA strand 5′ to the baseless sugar. Then, DNA polymerase β catalyzes the β-elimination of the 5′-deoxyriboso-phosphate residue and fills the one-nucleotide gap (short-patch BER). Finally, the nick is sealed by the DNA ligase III/XRCC1 complex [40]. This repair process results in the removal and replacement of a single damaged nucleotide with a proper one [41]. In addition to the above pathway an alternative pathway may occur. DNA glycosylases that process oxidative DNA lesions have an intrinsic AP lyase activity that incises abasic sites 3′ to the baseless sugar leaving a 3′(2,3-didehydro-2,3-dideoxyribose) termini that is then removed by AP endonuclease. As in the main pathway, the gap is filled by DNA polymerase and the nick is sealed by DNA ligase [17,42–44]. Short-patch BER accounts for 80–90% of all BER. Long-patch BER, which replaces 2–10 nucleotides of DNA, is utilized when the oxidized lesion is refractory to the AP lyase activity of DNA polymerase β. Long-patch BER is dependant on PCNA and FEN1 and DNA synthesis is thought to be mediated by several DNA polymerases including polymerases β, δ and ε. SSBs can also be repaired by the long-patch pathway after processing by the XRCC1/PARP1 complex [45] (Fig. 2A).
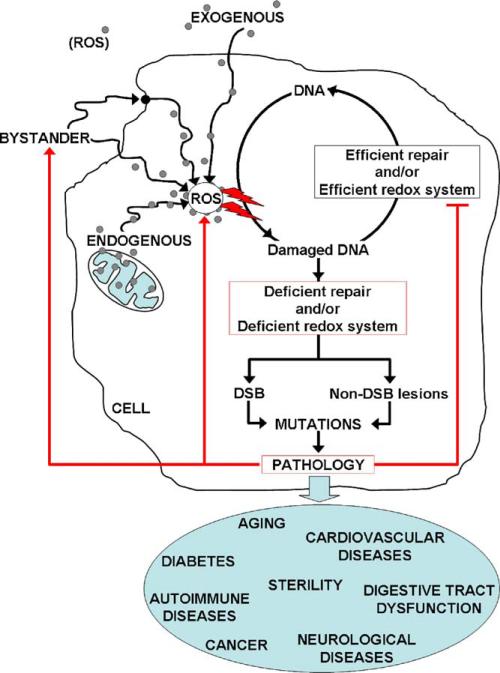
Typically, the rate of formation of ROS-induced lesions is balanced by their rate of repair. However, chronic exposure to oxidative stress due to deficiencies in cellular repair processes or changes in mitochondrial redox potential can result in persistently high levels of DNA lesions [25]. Numerous studies suggest that ROS play a role in normal and pathological aging, cancer and a wide variety of aging-related diseases such as some neurological diseases, type 2 diabetes, autoimmune, reproductive disorders and cardiovascular diseases, and others [12,46–51] (Fig. 3). In addition, ROS have been implicated in intracellular communication of stress. In the next sections we will discuss the contribution of oxidatively induced DNA damage in aging and cancer, and its role in stress signaling.
Fig. 3.
The role of oxidative stress in human pathogenesis. Many studies suggest that ROS have a role in normal aging, cancer, infertility, and a wide variety of age-related diseases such as some neurological diseases, type 2 diabetes, autoimmune and cardiovascular diseases. In healthy cells, the steady-state level of the ROS-induced lesions depends on the relative rates for their formation and repair. Chronic exposure to ROS and/or deficiencies in DNA repair processes or redox machinery can result in persistent DNA lesions. Accumulation of DNA lesions can lead to point mutations and/or chromosomal aberrations via the formation of DNA DSBs leading to the development of disease. Diseased cells, in turn, develop increased ROS production and/or decreased efficiency of DNA repair processes or redox systems. Red arrows represent the possible negative feedback of pathology on ROS production.  : increase of ROS production. T: decrease of DNA repair or redox capacity.
: increase of ROS production. T: decrease of DNA repair or redox capacity.
2. Oxidative DNA damage and aging
There is considerable evidence suggesting that oxidative stress plays a critical role in both in vitro senescence and in vivo aging [52,53]. Cells of laboratory mice were reported to reach senescence after 4–5 population doublings under standard cell culture conditions, however, the onset of senescence was substantially delayed when the O2 level was reduced from 21% to 3% [54]. The discovery that lower O2 increased plating efficiencies [55] was an important milestone in development of the experimental conditions for culturing bone marrow stem cells [56]. These findings may not be totally unexpected, given the natural hypoxic environment of stem cells [57], which could be interpreted as a strategy to avoid oxidative damage and senescence. In fact, the average life span of mice which are treated with antioxidant drugs increases up to 25% [58], and mice lacking the antioxidant enzyme super oxide dismutase 1 exhibit a 30% decrease in life expectancy [59]. Likewise, although mice lacking either Ogg1 or Myh, both members of the BER pathway, exhibit normal life spans, mice lacking both enzymes exhibit a 50% reduction in life expectancy [60]. Other evidence has also suggested that DNA lesions induced by the oxidative stress play an important role in mammalian aging [53,61–65]. These observations implicate oxidative stress in cellular senescence and aging, and further suggest that antioxidants and efficient repair of oxidative damage may extend life span.
Oxidative DNA lesions can be difficult to quantitate in situ. However, as mentioned above, they may lead to the formation of DSBs which can more easily be quantified by immunocytochemical detection of phosphorylated histone H2AX (γ-H2AX). When a DSB forms, many H2AX molecules become phosphorylated within a few minutes of break formation to form a γ-H2AX focus, a highly amplified response which enables the individual DSB site to be visualized in situ [32]. The chemical nature of the DSBs marked by γ-H2AX varies; reflecting different mechanisms of generation (reviewed in [32])(Fig. 2A). For example, prompt strand breaks induced by ionizing radiation generally arise from oxidative cleavage of the deoxyribosyl moiety, generating termini of various sorts (which are later excised by repair enzymes); two such breaks, in close proximity on opposite strands, comprising the DSB. Other DSBs arise solely by the action of endonucleases, and others by a combination; for example when topoisomerases cleave next to a prompt SSB, and when ROS-induced DNA damage interferes with both DNA replication and transcription. A DSB can be generated during DNA repair when excision of a modified base takes place near an unrepaired SSB. Oxidative DNA lesions can also interfere with reversible topoisomerase cleavage complexes during DNA replication and RNA transcription. In such cases, DNA/RNA polymerase forks run off the DNA to generate DSBs. Finally, DSBs can also appear when transcription and replication forks collide directly with SSBs or other ROS-induced lesions. Rarely, interference during DNA repair by BER also leads to DSB formation. γ-H2AX is a key component of the DNA damage response. Upon DSB formation, optimal kinase activity is required for the phosphorylation of H2AX as well as for activation of many other DNA repair and checkpoint proteins. Following DSB induction by irradiation, cells respond by activating the ATM signal transduction pathway, while replication-induced DSBs trigger an ATR response [66]. The same amplified response occurs when telomeres become critically shortened and uncapped, exposing a DNA double-stranded end. As with frank DSBs, the γ-H2AX foci formed on these eroded telomeres include the accumulation of DNA repair proteins [52,66,67].
These two types of γ-H2AX foci can be differentiated by their position on metaphase chromosomes. Foci on chromosome arms can be classified as marking DSBs, while those on chromosome ends as marking eroded telomeres. A combination of immunocytochemical γ-H2AX detection to monitor DSB formation and fluorescent in situ hybridization (FISH) for telomere-specific probes on metaphase chromosomes permitted direct visualization of oxidative stress-induced DNA DSBs during both in vitro and in vivo aging as foci on chromosome arms [52,67] (Fig. 2B). During mouse aging and senescence in culture, γ-H2AX foci were found to accumulate predominantly on chromosome arms, suggesting that oxidative stress-induced DNA DSBs may occur during both in vitro and in vivo aging [52]. Senescence-associated γ-H2AX foci on chromosome arms decrease when cells are transferred to low oxygen (3%) or treated with antioxidants, supporting the notions that oxidative stress is involved in aging-associated DNA DSB induction and that these DSBs may be repairable [52].
Another important target of oxidative stress is telomeric DNA which in vertebrates contains multiple TTAGGG repeats [68]. Since guanine is the most easily oxidized of the four DNA nucleobases [69], especially runs of consecutive guanines [70], telomeres are particularly susceptible to ROS [71]. Telomeric DNA becomes shorter with each cell division [68,71] and below a threshold length, the telomeric proteins dissociate, resulting in uncapped telomeres, which in turn trigger cellular growth arrest. Oxidized bases destabilize telomeric DNA resulting in accelerated telomere shortening during cell proliferation and even in the absence of DNA replication [72,73]. In contrast to the oxidative stress-induced DNA DSBs localized on chromosome arms which are potentially repairable under low oxidative stress conditions, critically short telomeric DNA requires telomerase to add TTAGGG repeats [52,74]. Since somatic cells generally lack telomerase activity, telomere dysfunction is irreversible. Because the rate of telomeric DNA shortening can be slowed under conditions of low oxidative stress, reduction of ROS levels may be an effective anti-aging strategy [75].
While many external agents are capable of generating ROS in cells, there are also important internal agents of ROS generation. Mitochondria are considered to be the primary source of endogenous ROS, which are released during ATP production. Mice deficient in the synthesis of Cytochrome C Oxidase 2, which functions in the regulation of the mitochondrial Cytochrome C Oxidase complex, exhibited increased mitochondrial oxidative stress and premature aging [76]. Mitochondria may also present a target of ROS because they contain a circular DNA (mtDNA) molecule with 16,569 bp which lacks protective histones, perhaps making it more vulnerable to oxidative stress [77]. While the magnitude of the role of oxidative stress-induced mtDNA damage in aging is not clear, evidence does suggest that the accumulation of mitochondrial genomic damage by oxidative stress may be related to aging [77]. In humans, point mutations in the control/D-loop region of mtDNA accumulate during aging, and age-related large rearrangements of mtDNA have been reported [78]. In addition, numerous human diseases including premature aging, cancer, diabetes and neurodegenerative disorders, have been linked to mutations in mtDNA [79]. Thus, there is ample circumstantial evidence that oxidative stress leads to the accumulation of DNA damage during aging. Future research will clarify the roles this type of damage plays in the causes of aging.
3. Oxidative DNA damage and cancer
That oxidative stress-induced DNA lesions may contribute to carcinogenesis is suggested by the increased cancer susceptibility of persons with a variety of chronic inflammatory diseases, such as ulcerative colitis, viral hepatitis, prostatitis, Helicobacter pylori infection, parasitic diseases, and others [3]. In these diseases, cancer induction may be a pathological consequence of elevated ROS levels which lead to increased steady-state levels of oxidative DNA damage which in turn leads to a higher risk of mutations that may activate oncogenes or inactivate tumor-suppressor genes [7,80].
In addition to cancer induction, cancer progression may also be a consequence of high levels of endogenous ROS. Elevated ROS levels, DNA damage, and defective DNA repair have been reported in different malignancies [10,14,15,26,27,41,81,82]. Nearly 20 purine- and pyrimidine-derived oxidative lesions have been implicated in cancer development. One, the 8-oxo-dG lesion is an accepted biomarker of oxidative stress and DNA damage [10,83]. Elevated levels of 8-oxo-dG and other characteristic DNA base lesions like thymine glycol, 4,6-diamino-5-formamidopyrimidine, and 2,6-diamino-4-hydroxy-5-formamidopyrimidine have been found in several pre-malignant and malignant cells or tissues including acute lymphoblastic leukemia, breast (invasive ductal carcinoma), cervical, colorectal, gastric adenocarcinoma, hepatocellular carcinoma, renal cell carcinoma, and small cell lung carcinoma [10,82]. Comparison of the levels of OCDLs in surgically removed malignant tumors of different origins and normal adjacent tissues demonstrated increased oxidative DNA damage in the tumor tissues [25]. In addition, the levels of DSBs in these tissues exhibited significant increases in tumor samples compared to corresponding normal tissues [84]. Tumor cells may contain increased levels of ROS due to higher cellular metabolism rates and deficiency in redox systems [85–87], characteristics which may aid cancer progression. Since cancer cells generally already have mutations in their DNA repair machinery and cell cycle checkpoints, elevated ROS levels continue to generate elevated levels of oxidative DNA lesions which disrupt normal cellular replication and lead to DSBs and further chromosome abnormalities [88] (Fig. 3).
Elevated DNA DSB levels have been widely reported in cancer cells and pre-malignant lesions, often by measuring the levels of γ-H2AX [84,89–92]. Two characteristics of cancer cells in culture are the different average numbers of γ-H2AX foci in different tumor lines, and the heterogeneity of focal numbers in individual cells of a tumor line [91,92]. However, as described above, γ-H2AX is induced by two types of DNA double-strand damage, the DSBs and the eroded telomere, which can be distinguished by γ-H2AX/telomere-FISH staining of metaphase chromosomes. This technique reveals that the differences in the average number of γ-H2AX foci among different tumor lines and the large heterogeneity of focal numbers in cells of the same culture are both primarily due to differences in foci associated with chromosome ends. Differences in average numbers of γ-H2AX foci among tumor lines correlate inversely with their levels of telomerase. Chromosome arm-associated DNA damage was present at more constant levels in these tumor lines. These results suggest that critically short telomeres are giving rise to the differences observed in these tumor lines [93]. The origins of telomere heterogenity may be numerous. In addition to differences of telomerase levels in tumor cells and the environmental impact such as oxidative stress level, telomere length is tissue-specific and age-dependent even in normal cells [94,95], and there is considerable heterogeneity between individuals [96].
The presence of persistent DNA lesions in tumor cells has led many to propose that DNA damage markers are potential biomarkers for screening ongoing oxidative stress and for cancer diagnostics and prediction [84]. Additionally, because ROS levels are higher in cancer cells, inhibiting redox machinery may be an effective and specific way to induce cell death in tumors [97].
4. Oxidative DNA damage is induced in bystander cell populations
Intercellular communication has been well studied in relation to bystander effects in vitro and in vivo. Bystander effects are seen in cell populations neighboring or sharing media with damaged or stressed cells [98] including those under biological stresses such as aging and cancer [99]. Some examples of bystander effects include increased mutations, DNA DSB formation, and apoptosis [98,100,101].
The signaling in vitro has been shown to be reminiscent of the inflammatory response mediated by COX-2 related pathways, involving cytokines, growth factors, and membrane-permeable ROS, including nitric oxide (NO) [98,102]. There are several lines of evidence that support a role for ROS in the production of bystander DNA damage. ROS can be produced in directly damaged cells (Fig. 1) or indirectly via inflammatory process, and can pass to neighboring cells through passive diffusion, gap junctions, or active transport [103,104]. Although most ROS have a short half-life and cause damage locally, H2O2 has a relatively long half-life and can travel long distances, causing DNA damage at distant sites [105]. Once in neighboring cells, ROS can cause oxidative lesions by directly acting on the DNA or indirectly through lipid peroxidation or protein damage [3,106]. Alternatively, the damaged or stressed cells may release cytokines that bind to bystander cells, inducing the local production of ROS possibly by activating transcription of NO synthase and cyclooxygenase II [3,107]. That NO can function as an agent of the bystander effect is evidenced by the findings that NO synthase inhibitors mitigate DSB formation in bystander cells though not in directly hit cells [99,108,109] and that diethylamine NONOate (DEANO), a compound that releases NO induces a comparable increase in DNA DSBs when added to the media of otherwise undamaged cells [99,108–110]. Other reactive species including superoxide anion, hydrogen peroxide, and hydroxyl radicals have also been proposed as agents of the bystander effect, since antioxidants and DMSO have been shown to reduce bystander cellular damage [99,107,111,112]. Additionally, mitochondrial damage or dysfunction has also been implicated in bystander effects [103,113]. Though the precise mechanism of bystander-induced DSB formation has yet to be elucidated, several groups have proposed that the bystander DNA DSBs are initially oxidative lesions that are converted to DSBs upon collision with moving replication machinery [98,99,103,104,114–116] (Fig. 2).
The potentially deleterious effects of bystander signaling are obvious. Any increase in DNA DSB frequency may increase the risk of mutation and other forms of genome instability [114]. This, in turn, increases the risk for cell transformation and tumor development [3,106], especially when the DNA damage repair machinery is altered by mutation [117,118]. However, the possibility remains that ROS production and bystander signaling in response to stress might be beneficial to the organism. For instance, it has been reported that bystander signaling from nontransformed damaged cells selectively kills precancerous transformed cells in culture [119]. In a fully developed organism, most cells are not cycling and while capable of generating bystander signals, they are less susceptible to bystander effects [99,115,116]. Therefore, the susceptibility of cycling cells to bystander signaling [113,114] might result in mitigating tumor development by eliminating cells that have begun to replicate aberrantly [119]. Another possibility is that bystander signaling acts to induce an adaptive response in cycling cell populations, in which a small amount of damage permits cells to better survive larger amounts of damage that may occur in the future.
Finally, there is evidence that tumors growing in vivo, even at early stages of development, produce oxidative damage in neighboring tissues through similar bystander mechanisms [120]. Additionally, mice deficient in oxidative DNA damage repair mechanisms accumulate DSBs in bystander tissue after local direct irradiation, suggesting that ROS bystander signaling in response to other forms of stress exists in vivo [121]. This information potentially has important implications for the study of genomic instability resulting from ROS signaling from tumors and other damage.
5. Conclusions
There is an abundance of evidence implicating ROS as one source of DNA damage associated with aging, cancer, stress signaling, and other conditions. However, ROS are essential to numerous cellular processes including apoptosis [122], cell growth [123] and the activation of redox system proteins [97]. In addition, ROS play a role in acquired immunity, killing bacteria and other pathogens, when produced by macrophages and neutrophils [124]. Moreover, extreme hypoxia (less than 1% O2) also activates DNA damage responses including γ-H2AX focus formation [125,126]. Counterintuitively, a recent study demonstrated that certain types of antioxidant supplementation may increase mortality [127]. These results highlight how little is known about the roles played by ROS in cellular metabolism. Nevertheless, maintenance of the balance of ROS levels appears to be an important consideration for the prevention of human disease.
Understanding ROS roles in cellular homeostasis could help improve human health. Clinical data suggests that in some cases of human pathology, monitoring oxidatively induced DNA lesions can be used for optimizing disease treatment including targeted radio-or chemotherapy [32,128,129]. These lesions could also be used to detect exposures to various toxic chemicals and metals [4]. The presence of persistent DNA lesions in tumor cells has led many to propose that DNA damage markers are potential biomarkers for screening ongoing oxidative stress and for cancer diagnostics and prediction [84]. Additionally, because ROS levels are higher in cancer cells, it has been suggested that inhibiting redox machinery may be an effective and specific way to induce cell death in tumors [97]. There are many opportunities for elucidating the role of oxidatively induced DNA lesions in human health and disease. Better understanding of the mechanism of ROS-induced human pathology will help to develop effective strategies to prevent and/or treat human disease.
Acknowledgements
This work has been partially supported by funds provided to A.G. by the Biology Department of East Carolina University and a Research/Creative Activity Grant to A.G. This work was also supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Abbreviations
- 8-oxo-dG
8-oxo-2′-deoxyguanosine
- BER
base excision repair
- DEANO
diethylamine NONOate
- DSB
double-strand break
- FISH
fluorescent in situ hybridization
- Mbp
mega base pair
- mtDNA
mitochondrial DNA
- NO
nitric oxide
- OCDL
oxidative clustered DNA lesions
- ROS
reactive oxygen species
- SSB
single-strand break
- Topo
topoisomerase
Footnotes
Conflict of interest statement
The authors declare that there is no conflict of interest.
References
- 1.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 2.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neill P, Wardman P. Radiation chemistry comes before radiation biology. Int. J. Radiat. Biol. 2009;85:9–25. doi: 10.1080/09553000802640401. [DOI] [PubMed] [Google Scholar]
- 6.McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J. Pharm. Pharmacol. 2008;60:969–976. doi: 10.1211/jpp.60.8.0004. [DOI] [PubMed] [Google Scholar]
- 7.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 8.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 9.Georgakilas AG, Aziz K, Ziech D, Georgakila S, Panayiotidis MI. BRCA1 involvement in toxicological responses and human cancer etiology. Toxicol. Lett. 2009;188:77–83. doi: 10.1016/j.toxlet.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat. Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A. Oxidative stress and reactive oxygen species. Contrib. Nephrol. 2005;149:240–260. doi: 10.1159/000085686. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Kohchi C, Inagawa H, Nishizawa T, Soma G. ROS and innate immunity. Anticancer Res. 2009;29:817–821. [PubMed] [Google Scholar]
- 14.Ward JF. The complexity of DNA damage: relevance to biological consequences. Int. J. Radiat. Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland BM, Bennett PV, Sidorkina O, Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura J, Swenberg JA. Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer Res. 1999;59:2522–2526. [PubMed] [Google Scholar]
- 17.Klungland A, Hoss M, Gunz D, Constantinou A, Clarkson SG, Doetsch PW, Bolton PH, Wood RD, Lindahl T. Base excision repair of oxidative DNA damage activated by XPG protein. Mol. Cell. 1999;3:33–42. doi: 10.1016/s1097-2765(00)80172-0. [DOI] [PubMed] [Google Scholar]
- 18.Atamna H, Cheung I, Ames BN. A method for detecting abasic sites in living cells: age-dependent changes in base excision repair. Proc. Natl. Acad. Sci. U.S.A. 2000;97:686–691. doi: 10.1073/pnas.97.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 20.Cadet J, Douki T, Gasparutto D, Ravanat JL. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat. Res. 2003;531:5–23. doi: 10.1016/j.mrfmmm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Poulsen HE. Oxidative DNA modifications. Exp. Toxicol. Pathol. 2005;57(Suppl. 1):161–169. doi: 10.1016/j.etp.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Gedik CM, Collins A. Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J. 2005;19:82–84. doi: 10.1096/fj.04-1767fje. [DOI] [PubMed] [Google Scholar]
- 23.Bennett PV, Cintron NS, Gros L, Laval J, Sutherland BM. Are endogenous clustered DNA damages induced in human cells? Free Radic. Biol. Med. 2004;37:488–499. doi: 10.1016/j.freeradbiomed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Bennett PV, Cuomo NL, Paul S, Tafrov ST, Sutherland BM. Endogenous DNA damage clusters in human skin, 3-D model, and cultured skin cells. Free Radic. Biol. Med. 2005;39:832–839. doi: 10.1016/j.freeradbiomed.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowsheen S, Wukovich RL, Aziz K, Kalogerinis PT, Richardson CC, Panayiotidis MI, Bonner WM, Sedelnikova OA, Georgakilas AG. Accumulation of oxidatively induced clustered DNA lesions in human tumor tissues. Mutat. Res. 2009;674:131–136. doi: 10.1016/j.mrgentox.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward JF. Some biochemical consequences of the spatial distribution of ionizing radiation-produced free radicals. Radiat. Res. 1981;86:185–195. [PubMed] [Google Scholar]
- 27.Harrison L, Hatahet Z, Wallace SS. In vitro repair of synthetic ionizing radiation-induced multiply damaged DNA sites. J. Mol. Biol. 1999;290:667–684. doi: 10.1006/jmbi.1999.2892. [DOI] [PubMed] [Google Scholar]
- 28.David-Cordonnier MH, Cunniffe SM, Hickson ID, O'Neill P. Efficiency of incision of an AP site within clustered DNA damage by the major human AP endonuclease. Biochemistry. 2002;41:634–642. doi: 10.1021/bi011682l. [DOI] [PubMed] [Google Scholar]
- 29.Georgakilas AG, Bennett PV, Sutherland BM. High efficiency detection of bi-stranded abasic clusters in gamma-irradiated DNA by putrescine. Nucleic Acids Res. 2002;30:2800–2808. doi: 10.1093/nar/gkf393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eot-Houllier G, Eon-Marchais S, Gasparutto D, Sage E. Processing of a complex multiply damaged DNA site by human cell extracts and purified repair proteins. Nucleic Acids Res. 2005;33:260–271. doi: 10.1093/nar/gki165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunniffe SM, Lomax ME, O'Neill P. An AP site can protect against the mutagenic potential of 8-oxoG when present within a tandem clustered site in E. coli. DNA Repair (Amst.) 2007;6:1839–1849. doi: 10.1016/j.dnarep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilstermann AM, Osheroff N. Base excision repair intermediates as topoisomerase II poisons. J. Biol. Chem. 2001;276:46290–46296. doi: 10.1074/jbc.M105733200. [DOI] [PubMed] [Google Scholar]
- 34.Pommier Y, Barcelo JM, Rao VA, Sordet O, Jobson AG, Thibaut L, Miao ZH, Seiler JA, Zhang H, Marchand C, Agama K, Nitiss JL, Redon C. Repair of topoisomerase I-mediated DNA damage. Prog. Nucleic Acid Res. Mol. Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sordet O, Redon CE, Guirouilh-Barbat J, Smith S, Solier S, Douarre C, Conti C, Nakamura AJ, Das BB, Nicolas E, Kohn KW, Bonner WM, Pommier Y. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 2009;10:887–893. doi: 10.1038/embor.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang N, Galick H, Wallace SS. Attempted base excision repair of ionizing radiation damage in human lymphoblastoid cells produces lethal and mutagenic double strand breaks. DNA Repair (Amst.) 2004;3:1323–1334. doi: 10.1016/j.dnarep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 38.Memisoglu A, Samson L. Base excision repair in yeast and mammals. Mutat. Res. 2000;451:39–51. doi: 10.1016/s0027-5107(00)00039-7. [DOI] [PubMed] [Google Scholar]
- 39.Wilson DM, 3rd, Sofinowski TM, McNeill DR. Repair mechanisms for oxidative DNA damage. Front. Biosci. 2003;8:d963–981. doi: 10.2741/1109. [DOI] [PubMed] [Google Scholar]
- 40.Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat. Res. 2003;531:231–251. doi: 10.1016/j.mrfmmm.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 42.Demple B, DeMott MS. Dynamics and diversions in base excision DNA repair of oxidized abasic lesions. Oncogene. 2002;21:8926–8934. doi: 10.1038/sj.onc.1206178. [DOI] [PubMed] [Google Scholar]
- 43.Ocampo MT, Chaung W, Marenstein DR, Chan MK, Altamirano A, Basu AK, Boorstein RJ, Cunningham RP, Teebor GW. Targeted deletion of mNth1 reveals a novel DNA repair enzyme activity. Mol. Cell. Biol. 2002;22:6111–6121. doi: 10.1128/MCB.22.17.6111-6121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marenstein DR, Chan MK, Altamirano A, Basu AK, Boorstein RJ, Cunningham RP, Teebor GW. Substrate specificity of human endonuclease III (hNTH1). Effect of human APE1 on hNTH1 activity. J. Biol. Chem. 2003;278:9005–9012. doi: 10.1074/jbc.M212168200. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto Y, Kim K, Bogenhagen DF. Proliferating cell nuclear antigen-dependent abasic site repair in Xenopus laevis oocytes: an alternative pathway of base excision DNA repair. Mol. Cell. Biol. 1994;14:6187–6197. doi: 10.1128/mcb.14.9.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blum A. Heart failure—new insights. Isr. Med. Assoc. J. 2009;11:105–111. [PubMed] [Google Scholar]
- 47.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer's disease. J. Alzheimers Dis. 2009;16:763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao KS. Free radical induced oxidative damage to DNA: relation to brain aging and neurological disorders. Indian J. Biochem. Biophys. 2009;46:9–15. [PubMed] [Google Scholar]
- 49.Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol. Endocrinol. 2009;25:75–81. doi: 10.1080/09513590802485012. [DOI] [PubMed] [Google Scholar]
- 50.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009;21:219–222. doi: 10.1097/gco.0b013e32832924ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura AJ, Chiang YJ, Hathcock KS, Horikawa I, Sedelnikova OA, Hodes RJ, Bonner WM. Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence. Epigenetics Chromatin. 2008;1:6. doi: 10.1186/1756-8935-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust. J. Exp. Biol. Med. Sci. 1966;44:287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 56.Bradley TR, Hodgson GS, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J. Cell Physiol. 1978;97:517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- 57.Wion D, Christen T, Barbier EL, Coles JA. PO(2) matters in stem cell culture. Cell Stem Cell. 2009;5:242–243. doi: 10.1016/j.stem.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Erker L, Schubert R, Yakushiji H, Barlow C, Larson D, Mitchell JB, Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Hum. Mol. Genet. 2005;14:1699–1708. doi: 10.1093/hmg/ddi181. [DOI] [PubMed] [Google Scholar]
- 59.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 60.Xie Y, Yang H, Cunanan C, Okamoto K, Shibata D, Pan J, Barnes DE, Lindahl T, McIlhatton M, Fishel R, Miller JH. Deficiencies in mouse Myh and Ogg1 result in tumor predisposition and G to T mutations in codon 12 of the K-ras oncogene in lung tumors. Cancer Res. 2004;64:3096–3102. doi: 10.1158/0008-5472.can-03-3834. [DOI] [PubMed] [Google Scholar]
- 61.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 62.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 63.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol. Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 64.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell. Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 65.Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 66.Riches LC, Lynch AM, Gooderham NJ. Early events in the mammalian response to DNA double-strand breaks. Mutagenesis. 2008;23:331–339. doi: 10.1093/mutage/gen039. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura A, Sedelnikova OA, Redon C, Pilch DR, Sinogeeva NI, Shroff R, Lichten M, Bonner WM. Techniques for gamma-H2AX detection. Methods Enzymol. 2006;409:236–250. doi: 10.1016/S0076-6879(05)09014-2. [DOI] [PubMed] [Google Scholar]
- 68.Klapper W, Parwaresch R, Krupp G. Telomere biology in human aging and aging syndromes. Mech. Ageing Dev. 2001;122:695–712. doi: 10.1016/s0047-6374(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 69.Steenken S, Jovanovic SV. How easily oxidizable Is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997;119:617–618. [Google Scholar]
- 70.Lewis FD, Liu X, Liu J, Hayes RT, Wasielewski MR. Dynamics and equilibria of oxidation of G, GG, and GGG sequences in DNA hairpins. J. Am. Chem. Soc. 2000;122:12037–12038. [Google Scholar]
- 71.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 72.von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 73.Vaziri H, West MD, Allsopp RC, Davison TS, Wu YS, Arrowsmith CH, Poirier GG, Benchimol S. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 1997;16:6018–6033. doi: 10.1093/emboj/16.19.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 75.Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998;63:935–948. doi: 10.1016/s0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- 76.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 77.Druzhyna NM, Wilson GL, LeDoux SP. Mitochondrial DNA repair in aging and disease. Mech. Ageing Dev. 2008;129:383–390. doi: 10.1016/j.mad.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp. Biol. Med. (Maywood) 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- 79.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ellsworth DL, Ellsworth RE, Liebman MN, Hooke JA, Shriver CD. Genomic instability in histologically normal breast tissues: implications for carcinogen-esis. Lancet Oncol. 2004;5:753–758. doi: 10.1016/S1470-2045(04)01653-5. [DOI] [PubMed] [Google Scholar]
- 82.Gackowski D, Speina E, Zielinska M, Kowalewski J, Rozalski R, Siomek A, Paciorek T, Tudek B, Olinski R. Products of oxidative DNA damage and repair as possible biomarkers of susceptibility to lung cancer. Cancer Res. 2003;63:4899–4902. [PubMed] [Google Scholar]
- 83.Dizdaroglu M, Jaruga P, Rodriguez H. Measurement of 8-hydroxy-2′-deoxyguanosine in DNA by high-performance liquid chromatography–mass spectrometry: comparison with measurement by gas chromatography–mass spectrometry. Nucleic Acids Res. 2001;29:E12. doi: 10.1093/nar/29.3.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sedelnikova OA, Bonner WM. GammaH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle. 2006;5:2909–2913. doi: 10.4161/cc.5.24.3569. [DOI] [PubMed] [Google Scholar]
- 85.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 86.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 87.Verrax J, Pedrosa RC, Beck R, Dejeans N, Taper H, Calderon PB. In situ modulation of oxidative stress: a novel and efficient strategy to kill cancer cells. Curr. Med. Chem. 2009;16:1821–1830. doi: 10.2174/092986709788186057. [DOI] [PubMed] [Google Scholar]
- 88.Liang Y, Lin SY, Brunicardi FC, Goss J, Li K. DNA damage response pathways in tumor suppression and cancer treatment. World J. Surg. 2009;33:661–666. doi: 10.1007/s00268-008-9840-1. [DOI] [PubMed] [Google Scholar]
- 89.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 90.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr., Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 91.Banath JP, Macphail SH, Olive PL. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 2004;64:7144–7149. doi: 10.1158/0008-5472.CAN-04-1433. [DOI] [PubMed] [Google Scholar]
- 92.Yu T, MacPhail SH, Banath JP, Klokov D, Olive PL. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA Repair (Amst.) 2006;5:935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura AJ, Redon CE, Bonner WM, Sedelnikova OA. Telomere-dependent and telomere-independent origins of endogenous DNA damage in tumor cells. Aging. 2009;1:212–218. doi: 10.18632/aging.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 95.Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 96.Risques RA, Lai LA, Brentnall TA, Li L, Feng Z, Gallaher J, Mandelson MT, Potter JD, Bronner MP, Rabinovitch PS. Is ulcerative colitis a disease of accelerated colon aging? Evidence from telomere attrition and DNA damage. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dickey JS, Baird BJ, Redon CE, Sokolov MV, Sedelnikova OA, Bonner WM. Intercellular communication of cellular stress monitored by {gamma}-H2AX induction. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sowa Resat MB, Morgan WF. Radiation-induced genomic instability: a role for secreted soluble factors in communicating the radiation response to non-irradiated cells. J. Cell. Biochem. 2004;92:1013–1019. doi: 10.1002/jcb.20149. [DOI] [PubMed] [Google Scholar]
- 101.Sokolov MV, Dickey JS, Bonner WM, Sedelnikova OA. Gamma-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication. Cell Cycle. 2007;6:2210–2212. doi: 10.4161/cc.6.18.4682. [DOI] [PubMed] [Google Scholar]
- 102.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation-induced bystander effects: a unifying model. J. Pharm. Pharmacol. 2008;60:943–950. doi: 10.1211/jpp.60.8.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 105.Lu W, Ogasawara MA, Huang P. Models of reactive oxygen species in cancer. Drug Discov. Today Dis. Models. 2007;4:67–73. doi: 10.1016/j.ddmod.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 107.Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007;67:5872–5879. doi: 10.1158/0008-5472.CAN-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shao C, Prise KM, Folkard M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mutat. Res. 2008;638:139–145. doi: 10.1016/j.mrfmmm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 109.Han W, Wu L, Chen S, Bao L, Zhang L, Jiang E, Zhao Y, Xu A, Hei TK, Yu Z. Constitutive nitric oxide acting as a possible intercellular signaling molecule in the initiation of radiation-induced DNA double strand breaks in non-irradiated bystander cells. Oncogene. 2007;26:2330–2339. doi: 10.1038/sj.onc.1210024. [DOI] [PubMed] [Google Scholar]
- 110.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 111.D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 112.Kashino G, Prise KM, Suzuki K, Matsuda N, Kodama S, Suzuki M, Nagata K, Kinashi Y, Masunaga S, Ono K, Watanabe M. Effective suppression of bystander effects by DMSO treatment of irradiated CHO cells. J. Radiat. Res. (Tokyo) 2007;48:327–333. doi: 10.1269/jrr.07008. [DOI] [PubMed] [Google Scholar]
- 113.Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McKinnon PJ, Caldecott KW. DNA strand break repair and human genetic disease. Annu. Rev. Genomics Hum. Genet. 2007;8:37–55. doi: 10.1146/annurev.genom.7.080505.115648. [DOI] [PubMed] [Google Scholar]
- 115.Burdak-Rothkamm S, Rothkamm K, Prise KM. ATM acts downstream of ATR in the DNA damage response signaling of bystander cells. Cancer Res. 2008;68:7059–7065. doi: 10.1158/0008-5472.CAN-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced gamma H2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007;26:993–1002. doi: 10.1038/sj.onc.1209863. [DOI] [PubMed] [Google Scholar]
- 117.Cheadle JP, Sampson JR. Exposing the MYtH about base excision repair and human inherited disease. Hum. Mol. Genet. 2003;12(Spec No. 2):R159–165. doi: 10.1093/hmg/ddg259. [DOI] [PubMed] [Google Scholar]
- 118.Hanada K, Hickson ID. Molecular genetics of RecQ helicase disorders. Cell. Mol. Life Sci. 2007;64:2306–2322. doi: 10.1007/s00018-007-7121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Portess DI, Bauer G, Hill MA, O'Neill P. Low-dose irradiation of nontrans-formed cells stimulates the selective removal of precancerous cells via inter-cellular induction of apoptosis. Cancer Res. 2007;67:1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- 120.Jungst C, Cheng B, Gehrke R, Schmitz V, Nischalke HD, Ramakers J, Schramel P, Schirmacher P, Sauerbruch T, Caselmann WH. Oxidative damage is increased in human liver tissue adjacent to hepatocellular carcinoma. Hepatology. 2004;39:1663–1672. doi: 10.1002/hep.20241. [DOI] [PubMed] [Google Scholar]
- 121.Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, Pazzaglia S, Toni MP, Pimpinella M, Covelli V, Saran A. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 123.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 124.Knight JA. Review: free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci. 2000;30:145–158. [PubMed] [Google Scholar]
- 125.Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J. Biol. Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- 126.To KK, Sedelnikova OA, Samons M, Bonner WM, Huang LE. The phosphor-ylation status of PAS-B distinguishes HIF-1alpha from HIF-2alpha in NBS1 repression. EMBO J. 2006;25:4784–4794. doi: 10.1038/sj.emboj.7601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 128.Kopjar N, Miocic S, Ramic S, Milic M, Viculin T. Assessment of the radio-protective effects of amifostine and melatonin on human lymphocytes irradiated with gamma-rays in vitro. Arh. Hig. Rada. Toksikol. 2006;57:155–163. [PubMed] [Google Scholar]
- 129.Zhao H, Shen J, Deininger P, Hunt JD. Abasic sites and survival in resected patients with non-small cell lung cancer. Cancer Lett. 2007;246:47–53. doi: 10.1016/j.canlet.2006.01.031. [DOI] [PubMed] [Google Scholar]