Abstract
Background.
The net metabolic cost of walking (Cw) as well as the level of neural activation of agonist and antagonist leg muscles are higher in healthy old compared with young adults. This study examined the association between Cw and agonist muscle activity and antagonist coactivity in young and old adults.
Methods.
Young and old adults walked at 0.98 m/s on a treadmill set at 6% decline, level, and 6% incline, while Cw and neural activation of leg muscles were measured.
Results.
Cw was 7.0% (incline), 19.2% (level), and 47.3% (decline) higher in old adults (overall 18.3%). Old (67.1%) versus young (40.1%) adults activated their leg muscles 67.3% more during the gait tasks and had 152.8% higher antagonist muscle coactivation (old: 67.1%, young: 19.9%). Agonist muscle activation was unrelated to Cw on incline, but it explained up to 42% (level), 48% (decline), and 70% (three tasks combined) of variance in Cw. Antagonist coactivation accounted for up to 41% (incline), 45% (level), 59% (decline), 39% (three tasks combined) of variance in Cw.
Conclusions.
Age-related adaptations in the recruitment pattern of leg muscles during gait significantly contribute to the high Cw in old adults. Clinical interventions optimizing the neural control of leg muscles during gait could reduce Cw consequently the relative effort needed for exercise and activities of daily living in old adults.
Keywords: Biomechanics, Gait, Coactivation, Activities of daily living, Fatigue
ADVANCING age brings about profound changes in human locomotion. Old adults’ gait is characterized by slow speed, short steps, reduced joint range of motion, and mechanical plasticity, reflecting the age-related changes in the neuromuscular system (1–4). For an unknown reason, however, and despite these adaptations, the net metabolic cost of walking (Cw) increases with age (5–11). Although directly not examined yet, such an increase in Cw could be clinically significant because it can increase the sense of effort, fatigue, and the potential for accidents and reduces the ability for physical activity. Because increased Cw in old adults is not related to gender, level of physical fitness, and internal mechanical limb work, we hypothesized (12) that neural mechanisms, an increase in agonist muscle activation and antagonist muscle coactivation (reviewed in (13)) during the gait cycle, mediate the age-related increase in Cw. Although an increase in muscle activation and coactivation would increase cost, it could also have benefits by increasing stability of joints involved in gait. The hypothesized neural mechanism would increase the amount of active muscle involved in gait without an increase in mechanical work, increasing Cw. Previous studies reported low-to-moderate associations between coactivity and Cw (14,15) but did not examine the possibility that an age-related increase in muscle activity per se (12,16–18) contributes to Cw and whether increased Cw is also present in other locomotor tasks such as incline and decline gaits routinely prescribed exercise modalities (10). Incline and decline gaits comprise, respectively, mostly shortening and lengthening contractions with shortening contractions requiring higher metabolic cost (19). Because old adults tend to activate sooner and more strongly their leg muscles in descending gait tasks (16,20–24), we expected to observe an interaction between age and gait tasks due to the especially high Cw in old adults in decline gait. The purpose of the present study was to compare Cw during incline, level, and decline gaits between healthy young and old adults and determine if agonist and antagonist coactivity of leg muscles is associated with Cw.
METHODS
Participants
We recruited six healthy young male and six young female and five old male and seven old female adults (n = 24) through newspaper advertisements and word of mouth. Based on a physical exam by each participant's family physician and an extensive telephone interview conducted by an experienced laboratory staff, old adults were extremely healthy and free of orthopedic, neurological, and behavioral conditions, and all were able to execute the walking tasks without complications or fatigue. Table 1 shows that young and old adults were similar in height, mass, body mass index, preferred walking speed, standing oxygen uptake, and had similar mobility scores in the Short Physical Performance Battery test (25). Thus, the old participants in the present study were highly mobile, functional, and healthy and were thus limited to old adults with excellent motor function. All participants gave written informed consent before participating in the study.
Table 1.
Participant Characteristics
| Young (n = 12) | Old (n = 12) | |
| Age, y | 20.8 ± 2.2 | 77.4 ± 4.8 |
| Height, m | 1.75 ± 8.5 | 1.69 ± 8.5 |
| Mass, kg | 68.4 ± 6.9 | 72.2 ± 13.5 |
| Body mass index, kg/m2 | 22.3 ± 1.5 | 24.9 ± 3.5 |
| SPPB (1–12) | 12.0 ± 0.0 | 11.7 ± 0.5 |
| Standing VO2, mL·kg−1·min−1 | 4.4 ± 0.94 | 4.1 ± 0.62 |
| Preferred walking speed, m/s | 1.30 ± 0.14 | 1.21 ± 0.13 |
Notes: Values are mean ± SD. SPPB = Short Physical Performance Battery in which a score of 12 suggests no mobility impairments.
Experimental Protocol
Participants reported to the laboratory for one 2-hour session and wore a heart rate monitor, shorts, sneakers, and a foot switch in the shoe under the right heel. Participants completed the Short Physical Performance Battery test to assess mobility (25). The skin over the right fibula head, vastus lateralis (VL), biceps femoris (BF), tibialis anterior (TA), and gastrocnemius lateralis (GL) was shaved, alcohol washed, and abraded with LemonPrep abrasive skin prep. Two single-use silver–silver chloride electromyographic (EMG) electrodes with 20-mm interelectrode distance (Noraxon USA, Inc., Scottsdale, AZ) were taped to each muscle belly. The ground electrode was affixed to the skin over the right fibula head. The EMG signals were sampled at 960 Hz, preamplified 500× 15 cm from the electrode site, and amplified 1000× in a bandwidth of 10–1000 Hz (Bortec, Alberta, Canada; Common mode rejection ratio = 115 dB at 60 Hz, input impedance 10 GΩ). Participants were then equipped with a mask connected to a computerized portable metabolic system (Cosmed K4b2, Rome, Italy), worn on the back, to measure breath-by-breath oxygen consumption (VO2) and carbon dioxide production (VCO2). Gas analyzers were calibrated using standard calibration gases. Flow meter was calibrated using a 3-L syringe. Participant then practiced treadmill (model T9500; Vision Fitness, Cottage Grove, WI) walking at their preferred walking speed normally for 5 minutes. Next, participants performed maximal voluntary contractions against manual resistance, and the EMG activity was recorded from each of the four muscles to normalize the EMG amplitude measured during gait.
There were four 6-minute conditions. Condition 1 was rest, and the subject stood calmly for 6 minutes, and EMG and metabolic data were collected. Conditions 2, 3, and 4 were randomized and consisted of, respectively, level walking, 6% incline, and 6% decline walking at 0.98 m/s. We selected this specific speed based on preliminary work (26) showing that old adults were able to complete the 6 minute–long slope walking trials safely and comfortably without using handrails. In particular, the descending walking task was the primary limiting factor to a safe walking speed. Five minutes into each testing condition, participants rated their perceived exertion. Participants sat for 3 minutes to rest between conditions. Metabolic, heart rate, and EMG data were collected for 6 minutes. EMG was collected with Qualisys Track Manager Software (Qualisys North America, Inc., Deerfield, IL) and analyzed in MatLab (Mathworks, Natick, MA).
Data Analysis
Data analysis was done on the data collected during Minutes 5 and 6 when every participant reached aerobic metabolic steady state in the four conditions indicated by a respiratory exchange ratio less than 1.0. VO2 and VCO2 (milliliters/minute) were averaged over the final 2 minutes of each trial. We used Brockaway's method to estimate the rate of energy cost (J/s) from the VO2 and VCO2 data normalized for body mass (27). To determine net metabolic rate, resting metabolic rate measured during standing was subtracted from gross metabolic rate for each trial. Cw was computed by dividing net metabolic rate (J·kg−1·s−1) by walking speed (m/s), giving energy cost per unit distance traveled (J·kg−1·m−1).
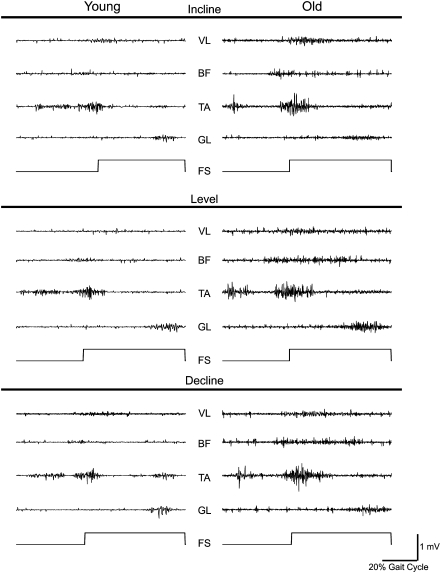
EMG analysis was done on the data collected during the entire duration of the gait cycle in Minutes 5 and 6 (∼120 steps per condition per participant). Instead of using just the stance phase, we used this approach because simulation studies show that swing phase also contributes ∼30% to the energy cost of gait (28). Raw EMG data were full wave rectified and bandpass filtered between 20 and 350 Hz. The onset and offset of each EMG burst were determined with high accuracy using the Teager–Kaiser Energy Operator detailed previously (12,29). Agonist and antagonist EMG signals were normalized to their maximal EMG values measured during maximal voluntary contractions. Activation amplitude of VL, BF, TA, and GL was determined as the mean value of the linear envelop. Coactivation of the BF was measured during the main burst of the VL, and coactivation of the VL was determined during the main burst of the BF. Coactivation of TA was measured during the main burst of the LG, and coactivation of LG was determined during the main burst of TA. Total agonist muscle activity was the sum of the normalized EMG activity of VL, BF, TA, and LG during their agonist actions, and total antagonist coactivation was the sum of normalized EMG of VL, BF, TA, and LG when these muscles acted as antagonists. Figure 1 shows a typical data in a young and old adult.
Figure 1.
Typical surface electromyographic (EMG) activity recorded during incline, level, and decline walking at 0.98 m/s in a young (age 25 years, female participant, left column) and an old (age 83 years, male participant) participant. Within each panel, the top tracing is the activity of the vastus lateralis (VL), second tracing is biceps femoris (BF), third tracing is tibialis anterior (TA), and the fourth tracing is gastrocnemius lateralis (GL) of the right leg. The lowest tracing in each panel depicts the foot switch signal, and the upward deviation denotes heel strike. Note that the level of the gross EMG activity and coactivation of muscle pairs (VL–BF and TA–GL) is higher in the old compared with the young participant. Calibration bar refers to all EMG signals.
Statistical Analyses
Each dependent variable (Cw, agonist and antagonist muscle activity) was analyzed with an age (young and old) by condition (incline, level, and decline walking) analysis of variance with repeated measures on condition followed by a Tukey's post-hoc contrast in case of a significant interaction or condition effect. Because muscle activation and Cw both can be related to age, correlations were computed between muscle activation and Cw in young participant alone, old participants alone, and in the two groups combined. Statistical significance was set at p < .05.
RESULTS
Muscle Activity and Metabolic Cost of Walking
Table 2 shows the data for metabolic variables in the two age groups and three walking conditions. Heart rate was 15 beats or 16% higher in old versus young adults across the three conditions (age main effect, p = .001), and heart rate was hierarchically highest during incline followed by level and decline walking (all different at p < .05, Tukey's post-hoc). The respiratory exchange ratios were below 1.0, and heart rates were stable (data not shown), suggesting steady state under the three conditions in both groups, and were also 5.7% lower (p = .025) in old versus young adults. Mass-normalized VO2 was highest during incline (17.1 mL·kg−1·min−1) compared with level (12.5 mL·kg−1·min−1) and decline walking (10.2 mL·kg−1·min−1, all different at p < .05), and it was 8.7% higher in old versus young adults (p = 0.041). Mass- and distance-normalized Cw was 18.4% higher in old versus young adults (p = .011). The age by condition interaction (p = .013) showed that Cw was 7.0% (incline), 19.2% (level), and 47.3% (decline) higher in old versus young adults. Old versus young adults activated their leg muscles 67.3% more during the three gait tasks (p = .001). The age by condition interaction (p = .001) showed that muscle activation was similar (incline) but 115.6% (level) and 102.7% (decline) greater in old versus young adults. Antagonist muscle coactivation was 152.8% higher in old versus young adults (p = .001).
Table 2.
Cardiovascular and Metabolic Data
| Incline | Level | Decline | Age Effect | |
| Heart rate, beats per minute | ||||
| Young | 103.7 ± 4.5 | 90.6 ± 4.1 | 85.2 ± 4.3 | 93.2 ± 4.3 |
| Old | 119.4 ± 4.6 | 105.8 ± 4.1 | 99.6 ± 4.3 | 108.3 ± 4.2* |
| RER, unitless | ||||
| Young | 0.88 ± 0.02 | 0.87 ± 0.01 | 0.91 ± 0.02 | 0.88 ± 0.02 |
| Old | 0.86 ± 0.02 | 0.81 ± 0.02 | 0.83 ± 0.02 | 0.83 ± 0.02* |
| VO2, mL·kg−1·min−1 | ||||
| Young | 16.9 ± 0.4 | 12.0 ± 0.4 | 9.3 ± 0.5 | 12.7 ± 0.3 |
| Old | 17.2 ± 0.4 | 12.9 ± 0.4 | 11.1 ± 0.5 | 13.8 ± 0.4* |
| Cw, J·kg−1·m−1 | ||||
| Young | 4.28 ± 0.15 | 2.61 ± 0.14 | 1.67 ± 0.19 | 2.86 ± 0.16 |
| Old | 4.58 ± 0.13 | 3.09 ± 0.12 | 2.46 ± 0.14 | 3.38 ± 0.13† |
| M. activation, % | ||||
| Young | 45.8 ± 4.4 | 37.2 ± 3.6 | 37.3 ± 4.9 | 40.1 ± 4.3 |
| Old | 45.8 ± 1.2 | 80.2 ± 5.1 | 75.1 ± 5.2 | 67.1 ± 3.9† |
| M. coactivation, % | ||||
| Young | 20.6 ± 2.1 | 20.8 ± 2.6 | 18.3 ± 1.4 | 19.9 ± 2.1 |
| Old | 50.9 ± 3.0 | 52.9 ± 6.4 | 46.9 ± 4.0 | 50.3 ± 4.5* |
Notes: Values are mean ± SD. Cw = metabolic cost of walking; M. activation, summed electromyographic muscle activation in percent of maximum voluntary contraction for the vastus lateralis, biceps femoris, tibialis anterior, and lateral gastrocnemius acting as agonist muscles during the gait cycle; M. coactivation, summed electromyographic muscle activation in percent of maximum voluntary contraction for the vastus lateralis, biceps femoris, tibialis anterior, and lateral gastrocnemius acting as antagonist muscles during the gait cycle; RER = respiratory exchange ratio.
Age main effect.
Age by condition interaction effect.
Correlation Analyses
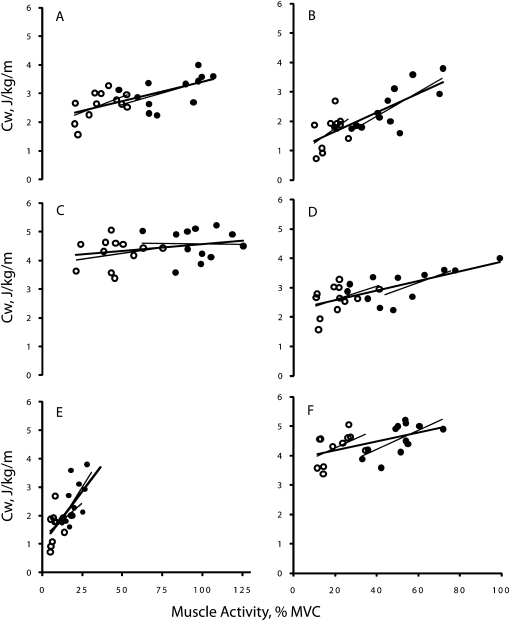
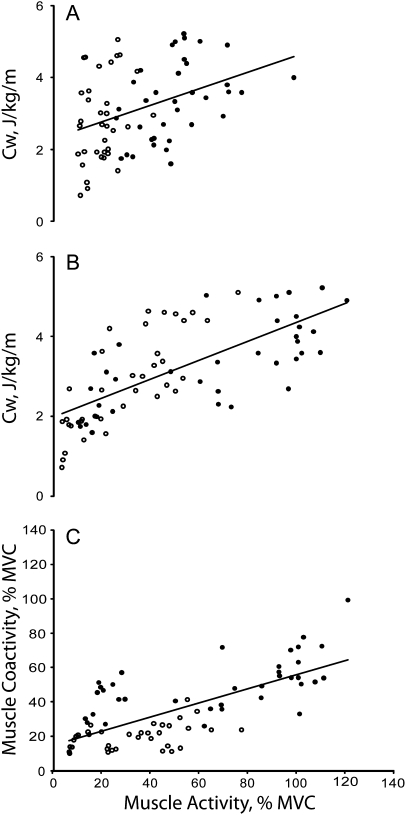
Figure 2 shows that there was no association between agonist muscle activation and Cw during incline gait. In level walking, muscle activity accounted for 30% (coefficient of determination for young), 31% (old), and 42% (both) of the variance in Cw. The corresponding values in decline gait were 13%, 39%, and 48%. Figure 2 also shows that during incline walking antagonist muscle coactivation accounted for 23% (young), 41% (old), and 26% (both) of the variation in Cw. The corresponding values during level walking were 18% (young), 43 (old), and 45% (both), and during decline walking, the values were 22% (young), 58% (old), and 59% (both). When the data were pooled across the two age groups and three walking conditions, agonist and antagonist muscle activity, respectively, accounted for 70% and 39% of the variance in Cw (Figure 3). Figure 3 also shows that agonist muscle activation accounted for 71% of the variation in antagonist muscle coactivation in the pooled data.
Figure 2.
Correlation between muscle activity and cost of walking (Cw). Panels (A), (C), and (E) are data for agonist muscle activity and panels (B), (D), and (F) are for antagonist muscle activity. Each type of muscle activity data is the sum of the activity measured in four leg muscles, expressed as a percent of maximal voluntary contraction. Panels (A) and (B) are during incline, (C) and (D) during level, and (E) and (F) during decline walking. Open symbols denote young and filled symbols refer to old adults. VL, vastus lateralis; BF, biceps femoris; TA, tibialis anterior; LG, lateral gastrocnemius. Thin lines denote regression within each age group and thick line denotes regression for both young and old adults. (A)—young: y = 0.006x + 4.0, r = .18; old: y = −0.0007x + 4.6, r = .02; both: y = 0.0048x + 4.1, r = .28. (B)—young: y = 0.0327x + 3.6, r = .48; old: y = 0.0325x + 2.9, r = .64; both: y = 0.0152x + 3.9, r = .51. (C)—young: y = 0.0215x + 1.8, r = .55; old: y = 0.0158x + 1.8, r = .56; both: y = 0.0132x + 2.1, r = .64. (D)—young: y = 0.0229x + 2.1, r = .42; old: y = 0.0164x + 2.2, r = .66; both: y = 0.0163x + 2.2, r = .67. (E)—young: y = 0.0472x + 1.2, r = .37; old: y = 0.0865x + 0.8, r = .62; both: y = 0.0755x + 1.0, r = .70. (F)—young: y = 0.0503x + 0.7, r = .46; old: y = 0.0405x + 0.6, r = .76; both: y = 0.0323x + 1.0, r = .76.
Figure 3.
Correlation between cost of walking pooled across incline, level, and decline surfaces (Cw) and agonist muscle activity (A), between Cw and antagonist muscle activity (B), and between agonist and antagonist muscle activity (C). Open symbols denote young and filled symbols refer to old adults. (A)—young: y = 0.0501x + 1.3, r = .80; old: y = 0.0212x + 2.0, r = .71; both: y = 0.0243x + 1.9, r = .70. (B)—young: y = 0.0472x + 1.9, r = .28; old = y = 0.0287x + 1.9, r = .43; both: y = 0.023x + 2.3, r = .39. (C)—young: y = 0.1703x + 14.6, r = .46; old: y = 0.2599x + 33.3, r = .59; both: y = 0.4104x + 15.3, r = .70.
DISCUSSION
The main findings of the present study were that old versus young adults expended 18.4% more metabolic energy per unit mass and distance of walking, and this increased cost of gait was associated with the magnitude of agonist and antagonist muscle coactivation. These differences were not due to gait speed because young and old participants walked at the same speed in the three gait conditions. The 19.2% higher Cw during level walking in old versus young adults is similar to Cw published previously (range: 18%–24%) in individuals walking at ∼1.0 m/s (7,8,14,15,30). Of particular interest is the study by Peterson and colleagues (15) who recently reported that coactivation is moderately associated with Cw and contributes to higher Cw in older adults. We extend these data from level gait to walking on 6% incline and decline at 0.98 m/s and confirm that the age-related differences in Cw persist for walking on sloped surfaces. The data in Table 2 are consistent with the prediction that the Cw is higher during incline versus decline walking because incline walking comprises predominantly shortening contractions of the lower extremity muscles, whereas decline walking is biased toward lengthening contractions and shortening contractions are associated with higher metabolic cost than lengthening contractions (19). It was especially notable from the age by condition interaction that old versus young adults, in line with our hypothesis, used 47.3% more oxygen per unit mass and distance in decline walking compared with the 7% difference during incline walking. The clinical implications of the age-related increase in Cw are many. Although physical activity is now widely accepted as an effective means to slow declines in cognitive and neuromuscular function in aging adults (31–33), the increased Cw raises the relative exercise intensity at a given walking speed in old adults. The increased Cw may ab ovo discourage old adults from participating in physical activity and promote inactivity due an increased sense of effort and tiredness. Therefore, Cw emerges as a mediator of mobility impairment in aging, but so far studies have produced mixed results to experimentally lower Cw (7,11,34).
Despite its clinical significance and concerted previous efforts, the cause of elevated Cw in old adults remains elusive. Reduced gait economy in old adults is not related to gender, physical fitness, amount of internal mechanical limb work, step width, step length, gait instability, and stride variability (5,6,8,9,35). To illustrate, old versus young adults spend more time in double support during walking. Thus, the two stance limbs in old adults may generate opposing forces over a greater distance and perform more opposing work during double support. However, old adults generate actually less mechanical work coupled with higher Cw (8). We thus proposed the hypothesis that instead of such mechanical variables, neural factors mediate the age-related increase in Cw (12). A recent study reported that the increased leg muscle coactivation during level walking at five speeds did indeed moderately contribute to Cw in old adults (15). Results of the present and the previous study (15) further agree that in both cases, the correlations were driven by old adults’ data.
Here, we expanded the original hypothesis by including muscle activation in predicting Cw. Old adults activated their leg muscles during ascent and level gaits substantially higher (Table 2), confirming previous data on the age-related increase of neural cost and relative effort needed to execute activities of daily living (16–18,36–38). Although many factors affect direct comparisons of absolute levels of EMG activity between different individuals, the present study (Figure 1) and a few previous studies showed higher muscle activation levels during gait and balancing tasks while standing in old compared with young individuals (12,39). Higher muscle activity is expected because there is a general reduction in the activity of motor cortical inhibitory circuits with age at rest (13,40) and, more importantly, during standard motor tasks (41–44). There is also a downregulation of spinal reciprocal inhibition during standing and walking in old compared with young adults (45). These data are, however, complicated by the finding that during incline gait, the relative muscle activation levels were similar in the two age groups, indicating perhaps that the ascent task was marginally taxing. In contrast with mechanical factors discussed earlier that accounted for up to ∼25% of variation in Cw, agonist muscle activity accounted for up to ∼50% of the variation in Cw in old adults (Figure 2). Using a sensitive method of EMG analysis and computing coactivation for four instead of the normally used two muscles (4,14–16), we found that coactivation in each gait task was over twofold greater in old versus young adults. Coactivation accounted for up to 60% of variation in Cw. Figure 2 shows that the data in old adults drove the correlations between Cw and muscle activity in all cases, suggesting a key role for neural factors in the age-related increase in Cw. This argument is underscored by the summary plots showing that agonist muscle activity and antagonist coactivity, respectively, account for ∼70% and ∼40% of the variation in Cw for the three gait tasks combined (Figure 3).
The significant role of muscle activation in Cw is expected because the age-related increase in antagonist muscle coactivation would in turn require each agonist muscle to produce additional force, recruit a greater portion of muscle mass, and consume more metabolic energy to offset the opposing force of the antagonist muscles. However, the mechanical work generated by joint torques would not increase because the opposing joint torques by agonist and antagonist muscles would act in the opposite direction without an increase in net mechanical work. Although healthy old adults normally perceive ascending more taxing than descending, the energy cost of descent was still relatively higher in old versus young adults probably due to the early and high activation and coactivation of muscles in order to stiffen and prepare the limb for impact and compensate for low leg strength in old age (24,46). Previous studies showed that old adults activate their muscles well before foot contact and also at a high intensity during downward stepping and stair descent (16,20–24), a mechanism observed in the present study through the high association between Cw and muscle activation especially in old adults (Figure 2). Although coactivation seems to be linked to increased Cw, this unfavorable adaptation is compensated by an increase in safety through increased joint stability needed especially during descending gaits during which falls occur at a high rate (47).
Although an increase in muscle activation and coactivation would increase cost, it could also have benefits by increasing stability of joints involved in gait.
One limitation of the present study is its sole focus on muscle activation's role in Cw instead of using a multifactorial approach by including neural factors combined with variables of gait mechanics to predict Cw, an approach we plan to use in the future. It is also unclear whether the results would be reproducible for gait tasks performed freely and not on a treadmill. Another limitation is that although we expressed muscle activation relative to maximal EMG activity, such a normalization was not done for oxygen uptake and underestimated Cw, but it is unclear if such a normalization would have affected the association between Cw and muscle activation and coactivation. Although manual muscle testing is used extensively to measure maximal EMG activity in old adults, there is a possibility that the EMG activities recorded in such tasks underestimated the maximal activity, yielding somewhat inflated normalized EMG values in old compared with young adults. This study also failed to examine whether some form of exercise or other intervention would reduce Cw by itself or through modifications of neural activation of leg muscles (7,11,34). Finally, we did not examine the possibility how impaired mitochondrial function contributes to the reduced Cw in old adults. Although reactive oxygen species damages mitochondrial DNA of aged muscle (48), causing reduced ATP production (49), neurodegeneration, sarcopenia (50), and perhaps reduced Cw, but this dysfunction occurs in type II muscle fibers that is counteracted by the increased efficiency of mitochondrial function of type I muscle fibers (33).
In conclusion, the present study showed that the Cw is significantly higher in old versus young adults while walking on level and sloped surfaces, especially in decline gait. The increased Cw was significantly associated with activity of the agonist and antagonist leg muscles during gait. Adopting methods from neurorehabilitation that are known to modify muscle recruitment during gait (51,52) and Cw in old adults (11) would lead to a mechanistic way to reduce the age-related increase in Cw.
FUNDING
Supported in part by National Institutes of Health AG024161.
Acknowledgments
We thank Jonathan Gomez, Charles Kemble, Dr. Kerry McIver, and Michael McNally for their technical assistance.
References
- 1.DeVita P, Hortobágyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88:1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- 2.Judge JO, Davis RB, 3rd, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Med Sci. 1996;51:M303–M312. doi: 10.1093/gerona/51a.6.m303. [DOI] [PubMed] [Google Scholar]
- 3.Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil. 1998;79:317–322. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2008;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malatesta D, Simar D, Dauvilliers Y, et al. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol. 2003;95:2248–2256. doi: 10.1152/japplphysiol.01106.2002. [DOI] [PubMed] [Google Scholar]
- 6.Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol. 1992;73:200–206. doi: 10.1152/jappl.1992.73.1.200. [DOI] [PubMed] [Google Scholar]
- 7.Mian OS, Thom JM, Ardigo LP, Morse CI, Narici MV, Minetti AE. Effect of a 12-month physical conditioning programme on the metabolic cost of walking in healthy older adults. Eur J Appl Physiol. 2007;100:499–505. doi: 10.1007/s00421-006-0141-9. [DOI] [PubMed] [Google Scholar]
- 8.Ortega JD, Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J Appl Physiol. 2007;102:2266–2273. doi: 10.1152/japplphysiol.00583.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ortega JD, Fehlman LA, Farley CT. Effects of aging and arm swing on the metabolic cost of stability in human walking. J Biomech. 2008;41:3303–3308. doi: 10.1016/j.jbiomech.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson DH, Jones GR, Rice CL. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Can J Public Health. 2007;98(suppl 2):S69–S108. [PubMed] [Google Scholar]
- 11.Thomas EE, De Vito G, Macaluso A. Speed training with body weight unloading improves walking energy cost and maximal speed in 75- to 85-year-old healthy women. J Appl Physiol. 2007;103:1598–1603. doi: 10.1152/japplphysiol.00399.2007. [DOI] [PubMed] [Google Scholar]
- 12.Hortobágyi T, Solnik S, Gruber A, et al. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture. 2009;29:558–564. doi: 10.1016/j.gaitpost.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Hortobágyi T, DeVita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev. 2006;34:29–35. doi: 10.1097/00003677-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 15.Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture. 2010;31:355–359. doi: 10.1016/j.gaitpost.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Larsen AH, Puggaard L, Hamalainen U, Aagaard P. Comparison of ground reaction forces and antagonist muscle coactivation during stair walking with ageing. J Electromyogr Kinesiol. 2008;18:568–580. doi: 10.1016/j.jelekin.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Kang HG, Dingwell JB. Dynamics and stability of muscle activations during walking in healthy young and older adults. J Biomech. 2009;42:2231–2237. doi: 10.1016/j.jbiomech.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokuno CD, Cresswell AG, Thorstensson A, Carpenter MG. Age-related changes in postural responses revealed by support-surface translations with a long acceleration-deceleration interval. Clin Neurophysiol. 2010;121:109–117. doi: 10.1016/j.clinph.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Bigland-Ritchie B, Woods JJ. Integrated electromyogram and oxygen uptake during positive and negative work. J Physiol. 1976;260:267–277. doi: 10.1113/jphysiol.1976.sp011515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hortobágyi T, DeVita P. Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J Electromyogr Kinesiol. 2000;10:117–126. doi: 10.1016/s1050-6411(99)00026-7. [DOI] [PubMed] [Google Scholar]
- 21.Hortobágyi T, DeVita P. Altered movement strategy increases lower extremity stiffness during stepping down in the aged. J Gerontol A Biol Sci. 1999;54:B63–B70. doi: 10.1093/gerona/54.2.b63. [DOI] [PubMed] [Google Scholar]
- 22.Mian OS, Thom JM, Narici MV, Baltzopoulos V. Kinematics of stair descent in young and older adults and the impact of exercise training. Gait Posture. 2007;25:9–17. doi: 10.1016/j.gaitpost.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Häkkinen K, Kallinen M, Izquierdo M, et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol. 1998;84:1341–1349. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- 24.Hsu MJ, Wei SH, Yu YH, Chang YJ. Leg stiffness and electromyography of knee extensors/flexors: Comparison between older and younger adults during stair descent. J Rehabil Res Dev. 2007;44:429–436. doi: 10.1682/jrrd.2006.04.0033. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hortobágyi T, Mosciki B, Rider P, et al. Effects of age and gait tasks on muscle to muscle coherence. 2010. pp. 16S1–S14. Parkinsonism Rel Disord. [Google Scholar]
- 27.Brockway JM. Derivation of formulae used to calculate energy expenditure in man. Hum Nutr Clin Nutr. 1987;41:463–471. [PubMed] [Google Scholar]
- 28.Umberger BR. Stance and swing phase costs in human walking. J R Soc Interface. 2010;7:1329–1340. doi: 10.1098/rsif.2010.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solnik S, Rider P, Steinweg K, Devita P, Hortobagyi T. Teager-Kaiser energy operator signal conditioning improves EMG onset detection. Eur J Appl Physiol. 2010;110:489–498. doi: 10.1007/s00421-010-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCann DJ, Adams WC. A dimensional paradigm for identifying the size-independent cost of walking. Med Sci Sports Exerc. 2002;34:1009–1017. doi: 10.1097/00005768-200206000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003;177:69–78. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 33.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 34.VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C, Studenski SA. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol A Biol Sci Med Sci. 2009;64:1190–1198. doi: 10.1093/gerona/glp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Hortobágyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol Med Sci. 2003;58A:M453–M460. doi: 10.1093/gerona/58.5.m453. [DOI] [PubMed] [Google Scholar]
- 37.Bieryla KA, Anderson DE, Madigan ML. Estimations of relative effort during sit-to-stand increase when accounting for variations in maximum voluntary torque with joint angle and angular velocity. J Electromyogr Kinesiol. 2009;19:139–144. doi: 10.1016/j.jelekin.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Hahn ME, Lee HJ, Chou LS. Increased muscular challenge in older adults during obstructed gait. Gait Posture. 2005;22:356–361. doi: 10.1016/j.gaitpost.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Billot M, Simoneau EM, Van Hoecke J, Martin A. Age-related relative increases in electromyography activity and torque according to the maximal capacity during upright standing. Eur J Appl Physiol. 2010;109:669–680. doi: 10.1007/s00421-010-1397-7. [DOI] [PubMed] [Google Scholar]
- 40.Hortobágyi T, Del Olmo MF, Rothwell JC. Age reduces cortical reciprocal inhibition in humans. Exp Brain Res. 2006;171:322–329. doi: 10.1007/s00221-005-0274-9. [DOI] [PubMed] [Google Scholar]
- 41.Mattay VS, Fera F, Tessitore A, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- 42.Sailer A, Dichgans J, Gerloff C. The influence of normal aging on the cortical processing of a simple motor task. Neurology. 2000;55:979–985. doi: 10.1212/wnl.55.7.979. [DOI] [PubMed] [Google Scholar]
- 43.Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage. 2008;40:1772–1781. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186:59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- 46.Hortobágyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The effects of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol Biol Sci. 1995;50A:B399–B406. doi: 10.1093/gerona/50a.6.b399. [DOI] [PubMed] [Google Scholar]
- 47.Startzell JK, Owens DA, Mulfinger LM, Cavanagh PR. Stair negotiation in older people: a review. J Am Geriatr Soc. 2000;48:567–580. doi: 10.1111/j.1532-5415.2000.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 48.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 49.Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Dietz V. Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull. 2009;78:I–VI. doi: 10.1016/S0361-9230(08)00410-3. [DOI] [PubMed] [Google Scholar]
- 52.Harkema SJ. Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]