Abstract
Fish oil (FO) mediates a number of cardioprotective benefits in patients with cardiovascular disease. In the absence of cardiovascular disease, however, the effects of FO on cardiac structure and function are not clear. In addition, it is not known if an effective dosing strategy for attenuating age-related cardiac dysfunction is also effective at limiting cognitive dysfunction. Therefore, we determined if 4 months of FO supplementation in aged rats would lessen age-related cardiac dysfunction while concomitantly preventing the cognitive decline that is normally observed in this population. The results indicate that FO initiated late in life modifies diastolic function in a small but positive way by attenuating the age-related increases in filling pressure, posterior wall thickness, and interstitial collagen without mitigating age-related deficits in memory or increases in brain inflammation. These data raise the possibility that FO supplementation for purposes of cardiac and brain protection may need to occur earlier in the life span.
Keywords: Cardiac fibrosis, Fish oil, Hippocampus, Microglia, Omega-3 fatty acids
LONG-CHAIN polyunsaturated fatty acids (LC-PUFAs), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are incorporated into neuronal and myocardial membranes and influence membrane fluidity, ion channel function, signal transduction, and gene expression (1–4). LC-PUFAs are primarily obtained from the diet and hepatic synthesis before uptake by other tissues (5,6). Fish oil (FO) is a rich source of omega-3 (n-3) LC-PUFAs, including EPA and DHA. Dietary FO supplementation has been shown to be cardioprotective in primary and secondary heart failure prevention trials (7–9). Likewise, evidence is accumulating that omega-3 PUFAs improve resting hemodynamics and lipid profiles among healthy older individuals (10–15). A remaining question is whether the beneficial impact of FO in the aged cardiovascular (CV) system extends to improving brain health in the same individuals.
In the brain of normal adult animals, diets low in PUFAs result in poor learning, memory, and reduced synaptic plasticity (3). The choice of model used to understand the relationship between PUFAs and brain function is important. Studies employing a generational deficiency model (eg, 16–21), which deplete PUFAs over several generations of breeding, are difficult to translate directly into a normally aging population. Observations from generational depletion models in which PUFA repletion diet is used are likely evaluating the impact of PUFAs on neurobiological development (16–18) and do not extend to studies of aging in which neurobiological development is intact.
Alternatively, replacement models, such as that used in this study, typically compare the effects of diets with high and low levels of omega-3 PUFAs (eg, 22–25). In addition, they reflect a more useful translational model for aging research, in that the experimental designs are more similar to those used in clinical intervention trials, especially if the dietary manipulation occurs in late middle or old age. For example, in normally bred adult mice, 20 g/kg DHA supplementation to the diet for 3 months improved navigational ability in a three-partition maze (26). Four weeks of DHA (300 mg/kg body weight) administration in gerbils increases the number of dendritic spines in the gerbil hippocampus (27). However, there was no assessment of memory in these subjects, so it is not known whether an increase in spine density improves hippocampal function in this setting, or whether the intervention would be effective in the aged brain.
From a therapeutic perspective, it is not known if efficacious dosages of omega-3 PUFAs that improve cardiac function in aging would also be effective for improving brain function. Clinical studies have shown that patients with dementia are likely to have low levels of omega-3 PUFAs (28), but whether omega-3 levels can be restored to normal levels and improve brain and/or cardiac function is not clear (eg, 29). The current study was designed to explore the effects of a dietary intervention on both brain and cardiac function in the same subject using a well-established model of cognitive decline in aging (30–32). This study directly examines the impact of PUFA supplementation in the form of FO on both memory impairment and cardiac dysfunction in aging in rats. CV parameters were assessed by evaluating heart structure and function using noninvasive echocardiography. Furthermore, the anti-inflammatory mechanisms of LC-PUFAs (33) have been proposed to offer protection to the aged brain. This was tested by examining the number of activated microglia in the hippocampus in the same animals used for behavioral and cardiac analyses.
METHODS
Subjects
Subjects included ten 6-month-old and twenty-one 28-month-old Fisher 344 × Brown Norway F1 hybrid (F344 × BNF1) male rats from Harlan Industries (Bethesda, MD). Animals were maintained on a 12-hour light/dark cycle (lights on at 6 AM) with ad libitum access to rat chow (diets described in the following) and water, and housed in pairs. Animal care was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all studies were approved by the Institutional Animal Care and Use Committee of Wake Forest University School of Medicine. The young rats received the purified diet (without FO supplementation) upon arrival at Wake Forest University School of Medicine at 5 months of age. The aged rats began their purified experimental diets (±FO; see Table 1) at 24 months. Before beginning the study, all rats were fed standard laboratory rat chow (Purina) throughout their lifetime. The young rats were on the purified diet for 1 month (age at testing = 6 months) and the aged rats for 4 months (age at testing = 28 months) before cognitive and CV testing (Figure 1). Rats were kept on their respective experimental diets until sacrificed to collect tissues (brain and heart) for analysis. Rats were sacrificed 10–14 days after the completion of water maze testing. Immediately prior to perfusion, blood was obtained transcardially for the analysis of fatty acids in plasma. Blood was collected into tubes containing 38 units of heparin/mL, incubated on ice 1–3 hours, centrifuged at 300g for 15 minutes, and plasma collected and stored in aliquots at −80°C.
Table 1.
Diet Composition
| Ingredient | No Fish Oil |
+Fish Oil |
||
| g/kg Diet | kJ/kg Diet | g/kg Diet | kJ/kg Diet | |
| BiPro whey protein isolate (protein source) | 187.70 | 3,190.9 | 187.70 | 3,190.9 |
| Fish oil beadlets (60% oil by weight) | 0.00 | 15.95 | 368.6 | |
| Soybean oil | 40.00 | 1,515.6 | 30.43 | 1,515.6 |
| L-Cystine | 1.80 | 30.6 | 1.80 | 30.6 |
| L-Alanine | 48.56 | 825.5 | 48.56 | 825.5 |
| Sucrose | 100.00 | 1,680 | 100.00 | 1,680 |
| Cornstarch | 369.43 | 6,206.7 | 363.05 | 6,206.7 |
| Dyetrose | 155.00 | 2,635 | 155.00 | 2,635 |
| t-Butylhydroquinone | 0.01 | 0.01 | ||
| Cellulose | 50.00 | 50.00 | ||
| Mineral mix #210094 | 35.00 | 35.00 | ||
| Vitamin mix # 310025 | 10.00 | 10.00 | ||
| Choline bitartrate | 2.50 | 2.50 | ||
| Total grams | 1,000.00 | 1,000.00 | ||
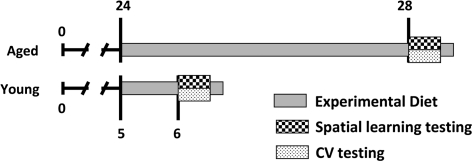
Figure 1.
Time line of experimental diet and testing for aged and young rats. Young and aged rats were fed a purified diet for 1 or 4 months, respectively, indicated by the gray line. The aged rats were further subdivided into two dietary groups (±fish oil). At the ages of 6 or 28 months, the rats underwent cognitive and cardiovascular (CV) testing and were maintained on their respective experimental diets until sacrificed 10–14 days after testing.
Diet Composition
The experimental diets consisted of 4% fat (by weight) and 23% protein (by weight) (whey + amino acids) on the AIN-93M purified base (Dyets Inc., Bethlehem, PA; Table 1). The fat source was soybean oil (alone or fortified with FO). Soybean oil provides medium-chain omega-3 (alpha-linolenic, ∼8%) and omega-6 (linoleic, ∼53%) PUFAs as well as saturated (palmitic, ∼11%; stearic, ∼4%) and monounsaturated (oleic, ∼23%) fatty acids. The FO was microencapsulated and the food was stored light protected at 4°C until use to maintain stability. The FO contributed to 0.96% (by weight) of the total fat (2.2% of calories), with 18% being EPA and 12% DHA. This would afford an estimated 1.75 mg EPA and 1.17 mg DHA per day per animal. Overall, the diet composition was (as percent of calories) 64%–67% carbohydrates, 25% protein, and 9.4% fat (no FO) or 11.2% fat (+FO).
Measurement of Plasma Fatty Acids
Thawed plasma (50 μL in duplicate) was added to tubes containing 25 μg triheptadecanoin (as internal standard; NuChek Prep, Elysian, MN) and subjected to base hydrolysis. Derivatization of free fatty acids was performed in methanolic sodium hydroxide in the presence of boron trifluoride as previously described (34,35). The resultant fatty acid methyl esters were extracted with hexane and 23% sodium chloride, dried under nitrogen gas, and dissolve in iso-octane before analysis by gas chromatography with flame ionization detection by the Department of Pathology Lipid Core Laboratory at Wake Forest University School of Medicine.
Echocardiograms
Echocardiograms were performed in rats that were lightly anesthetized with an isoflurane (1.5%)/O2 mixture via a nose cone during spontaneous ventilation. As previously described (36,37), images were obtained with the animal in a shallow left lateral decubitus position using a 12-MHz phased array transducer and Philips 5500 sector scanner (Philips Medical Systems, Andover, MA). Left ventricular (LV) end-diastolic and end-systolic diameters (LVEDD and LVESD, respectively), LV posterior wall thickness (PWT), and anterior wall thicknesswere measured from midpapillary short-axis images obtained by M-mode echocardiography. The percentage of LV fractional shortening (%FS), an index of contractile function, was calculated as FS (%) = [(LVEDD – LVESD)/LVEDD] × 100. LV systolic function was also determined by heart rate–corrected mean velocity of circumferential fiber shortening, calculated as follows: VCFc = ((LVEDD – LVESD)/(LVEDD × LVET)) × RR interval, whereby LVET represents LV ejection time in seconds from the beginning and end of the aortic valve velocity envelope by continuous-wave Doppler imaging. RR-interval is the time elapsing between two consecutive R waves in the electrocardiogram. LV mass was calculated using a standard cube formula, which assumes a spherical LV geometry according to the following formula: LV mass (LVmass) = 1.04 × [[LVEDD + PWT + AWT]3 – LVEDD], where 1.04 is the specific gravity of muscle and AWT the anterior wall thickness. Relative wall thickness, an index of the geometric pattern of hypertrophy, was calculated as follows: 2 × PWT/LVEDD. Mitral inflow measurements of early filling velocity (Emax), deceleration slope of early filling velocity (Edec slope), and deceleration time of early filling flow velocity (Edec time) were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. Due to relatively high heart rates and fusion of the early and late Doppler profiles, the late transmitral filling velocity, or Amax, was not recorded. The following measurements were made from the septal mitral annular velocity by tissue Doppler imaging: early diastolic (e′) and E/e′, as a measure of filling pressure. All measurements were performed with an off-line analysis system (Xcelera 3.1; Koninklijke Philips Electronics, Amsterdam, The Netherlands) by one observer who was blinded to experimental groups. An average of at least five consecutive cardiac cycles to minimize beat-to-beat variability was used for all measured and calculated systolic and diastolic indices.
Two weeks following the echocardiograms, rats were perfused and the brain and heart extracted and processed for immunohistochemistry and histopathology, respectively.
Cardiac Histopathology
LV collagen (interstitial) volume percentage, at the level below the mitral valve, was measured in picrosirius red (0.1%)–stained, 4-μm cross-sections. Ten optical images (×20) were taken from each left ventricle section for analysis. Adobe PhotoShop was used for collagen, tissue, and background selections. ImageJ (National Institutes of Health) software was then used for image analysis (38). A single investigator, masked to the experimental groups, performed all histological analyses.
Spatial Learning
The water maze is a circular tank (1.83-m diameter and 0.58-m height) with a retractable escape platform and surrounded by black curtains with white patterns that provide spatial cues. During testing, the 25°C–27°C water was clouded by the addition of nontoxic white tempera paint (150 mL) and the top of the escape platform submerged 1 cm below the water surface. Data were analyzed using a video tracking system (Ethovision; Noldus Information Technology, Wageningen, The Netherlands).
Spatial reference memory was assessed during 8 days, in sessions of three trials per day. The rats were trained to locate the escape platform that remains stationary throughout training. During a training trial, the animal was placed in the water at the perimeter of the pool and allowed 90 seconds to locate the platform. If at the end of this interval the rat has failed to escape, he was placed onto the platform and allowed to remain there for 30 seconds. The position of entry for the animal varied at each trial. There was a 60-second intertrial interval. The primary measure of performance during the training trials was the cumulative search—the summation of the distance of the rat from the platform location sampled 10× per second. Lower scores indicate a more accurate search. Every sixth trial consisted of a probe trial that assessed the development of a spatial bias in locating the escape platform. During such trials, the animal swam a total of 30 seconds without the escape platform present; after that time, the platform was raised and made available for escape. The primary measure obtained during the probe trial was a proximity score, which is the sum of the cumulative search during the first 30 seconds of the trial. This measure has been shown to be the most sensitive measure of performance of water maze probe trial performance (39,40).
Spatial reversal learning followed spatial memory assessment. It consisted of 1 day of three training trials, followed by a second day of two training trials and one probe trial. All parameters were identical to the spatial reference memory test described previously.
Cue training occurred on the last day of place training and consists of one session of six trials. During these trials, the visible platform raised 2 cm above the water surface was moved to different locations in the pool to test for sensorimotor and motivational factors that may influence spatial learning. Each rat was given 30 seconds to reach the platform and remained on the platform briefly. Trials were separated by a 30-second intertrial interval.
Immunohistochemistry and Cell Counting
Immunohistochemical staining of the hippocampus was performed on floating 40-μm-thick paraformaldehyde-fixed sections. Every 20th section through the dorsal hippocampus was collected beginning with the appearance of the dorsal blade of the dentate gyrus. While this resulted in counting two sections per animal, an initial subset of tissue was sampled at a 1-in-10 interval (eg, four sections per animal) and demonstrated similar results indicating that this interval is sufficient to reliably calculate density of activated microglia in the dorsal hippocampus in each animal. Sections were washed with tris-buffered saline (pH 7.5) and then quenched with 0.3% H2O2 and blocked in 5% normal horse serum supplemented with 0.1% Triton X-100. Sections were incubated overnight at 4°C with antibodies raised against CD68 (1:500, ED1 clone; AbD Serotec, Raleigh, NC), a marker of activated microglia. Following incubation with biotinylated anti-mouse immunoglobulin G, labeling was revealed with peroxidase-conjugated avidin–biotin complex using nickel-enhanced diaminobenzidine (yields dark purple/black reaction product) as chromogen. All sections were subsequently counterstained with the nuclear binding dye 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich, St. Louis, MO). Sections were mounted on charged glass slides, dehydrated, defatted, and coverslipped. Sections were observed using an Olympus BX51 microscope outfitted with a motorized stage controlled by a PC running Neurolucida software (MicroBrightField Inc., Williston, VT) and a MagnaFire camera (Optronics, Goleta, CA). Boundaries of the hippocampus and its subfields, the dentate gyrus, CA3, and CA1, were defined at low magnification and cells were exhaustively counted using a UPlanFLN ×40 objective lens (N.A. 0.75). As the distribution of activated microglia is not homogenous throughout the extent of the hippocampus, strict stereological procedures were not employed. Rather, measures taken from matched sections have been expressed as a density of immunopositive cells per square millimeter.
Statistical Analysis
Data were analyzed by group (young, aged, aged + FO) using an analysis of variance (ANOVA). Main effects of group were subsequently investigated with a Fisher’s protected least significant difference to fully explore the potential differences between young and aged rats, in addition to the effects of FO supplementation in aged rats compared with both the young and the aged control (unsupplemented) rats. Post hoc Fisher’s protected least significant differences were used for comparison of groups when a significant main effect was observed. Repeated measures ANOVA was used for the water maze training trial data (Group × Training Block), and one-way ANOVAs were used for post hoc comparisons. If homogeneity or normality was not satisfactory (eg, percent cardiac collagen), a nonparametric one-way ANOVA (Kruskal–Wallis) was performed on the ranked measurements followed by Dunn’s multiple-sample comparisons.
RESULTS
Plasma Fatty Acid Levels
Plasma fatty acids levels reflect both dietary intake and metabolism. Ten primary fatty acids were reliably detected in rat plasma (Table 2). There were undetectable levels of the several other known fatty acids including C14:0, C18:3n-6, C18:3n-3, C18:4n-3, C20:2n-6, and C20:3n-6.
Table 2.
Plasma Fatty Acids Profiles in Young and Aged Rats
| Young* | Aged* | Aged + Fish Oil* | |
| Saturated | |||
| C16:0 (palmitic)†,‡ | 21.1 ± 0.5 (38.5 ± 3.4) | 19.4 ± 0.3 (48.25 ± 2.8) | 20.1 ± 0.6 (26.6 ± 2.3) |
| C18:0 (stearic)‡ | 10.4 ± 0.3 (18.6 ± 1.1)§ | 10.3 ± 0.3 (25.8 ± 2.3) | 10.9 ± 0.7 (26.6 ± 2.3) |
| Monounsatured | |||
| C16:1n-7 (palmitoleic) | 2.6 ± 0.4 (5.0 ± 1.1) | 2.9 ± 0.3 (7.3 ± 1.1) | 2.9 ± 0.3 (7.4 ± 1.1) |
| C18:1n-9 (oleic) | 11.0 ± 0.5 (20.1 ± 2.1) | 9.7 ± 0.2‖ (24.2 ± 1.8) | 10.2 ± 0.4 (25.4 ± 2.3) |
| C18:1n-11 (vaccenic) | 3.5 ± 0.2 (6.4 ± 0.7) | 3.1 ± 0.1 (7.7 ± 0.4) | 2.9 ± 0.1‖ (7.3 ± 0.6) |
| Medium-chain PUFA | |||
| C18:2n-6 (linoleic) | 24.4 ± 1.0 (43.4 ± 2.7) | 18.7 ± 0.7¶ (45.9 ± 1.7) | 18.2 ± 0.9¶ (43.9 ± 2.1) |
| Long-chain PUFA | |||
| C20:4n-6 (arachidonic)‡ | 22.4 ± 1.0#,** (39.8 ± 2.4)§ | 31.6 ± 0.6¶,** (78.5 ± 5.3)¶ | 26.3 ± 1.3‖,# (64.1 ± 4.8) |
| C20:5n-3 (eicosapentaenoic)‡ | 1.1 ± 0.4 (2.3 ± 1.0)** | 0.6 ± 0.1 (1.6 ± 0.2)** | 2.4 ± 0.3‖,# (6.0 ± 0.8) |
| C22:5n-3 (docosapentaenoic)‡ | 1.0 ± 0.1 (1.8 ± 0.3)** | 0.9 ± 0.0 (2.3 ± 0.2)** | 1.3 ± 0.1‖,# (3.2 ± 0.3) |
| C22:6n-3 (docosahexaenoic, DHA)‡ | 2.4 ± 0.3 (4.6 ± 0.8)** | 2.8 ± 0.1 (7.1 ± 0.6)** | 4.8 ± 0.3‖,# (12.0 ± 1.0) |
| Total plasma fatty acids (mg/dL) | 180.5 ± 13.7#,** | 248.5 ± 14.8 | 245.0 ± 13.7 |
| Fatty acid ratios†† | |||
| Omega-6/omega-3 | 11.8 ± 1.2 | 11.5 ± 0.4 | 5.7 ± 0.7§,¶ |
| AA/DHA | 9.9 ± 0.8 | 11.3 ± 0.5 | 5.7 ± 0.6‖,# |
Notes: AA = arachidonic acid; DHA = docosahexaenoic acid; PUFA = polyunsaturated fatty acids.
Plasma fatty acid content expressed as percent of total and (mg/dL). Values are the mean ± standard error of the mean for n = 10 (young), 8 (aged), or 11 (aged + fish oil) rats per group.
Fatty acid structure (carbon chain length: number of C–C double bonds, first site from methyl end), and common name and abbreviation.
p < .05, main effect of group.
p < .001 vs aged.
p < .05 vs young.
p < .001 vs young.
p < .05 vs aged.
p < .05 vs aged + fish oil.
Fatty acid ratios are based on mass values (mg/dL).
Age Effects
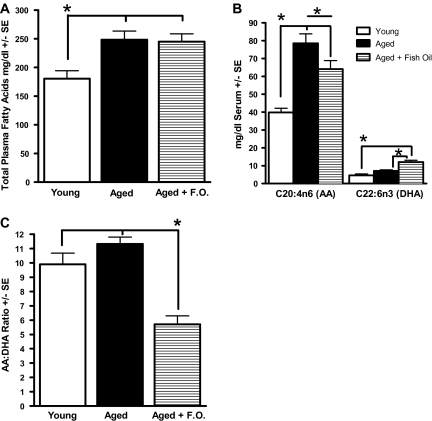
Age altered the total plasma fatty acid content (Figure 2) of rat plasma. Figure 2A shows that the total fatty acid concentration of aged rats was nearly 40% higher than that of young rats. Among the individual fatty acids analyzed, C18:1n-9 (oleic acid) and C18:2n-6 (linoleic acid) showed comparable concentrations across the two age points. However, when these fatty acids are expressed as a percent of total fatty acids, they were significantly lower in the plasma of aged rats compared with the young animals (Table 2). In contrast, the mass quantity of arachidonic acid (AA) was twofold higher in aged rats compared with the young animals and was 41% higher when expressed as a percent of total fatty acids (Table 2). This increase in circulating AA levels occurred even though the two age-groups consumed an identical diet including the content of fatty acids.
Figure 2.
Plasma fatty acids. (A) Total fatty acid mass is lower in the young group compared with the aged rats regardless of diet. (B) Arachidonic acid (AA) is significantly lower in the young group compared with the aged, regardless of diet, and the aged rats on the fish oil (FO) diet have lower AA levels compared with the aged rats on the purified diet. Docosahexaenoic acid (DHA) levels are highest in the aged rats on the FO diet when compared with the aged and young rats on the purified diet. (C) The aged rats that consumed FO had a lower AA/DHA ratio that presumably reflects a less inflammatory diet. Note the lack of a difference between the young and aged rats on the non-FO-supplemented diet. *p < .05.
FO Effects
Aged animals were fed a purified diet for 4 months supplemented with omega-3 PUFA-enriched FO (∼1%; 2.2% of energy). FO supplementation induced an increase in circulating levels of the LC-PUFAs: EPA, DHA, and docosapentaenoic acid (C22:5n-3) when expressed as either mass or percent of total lipids (Figure 2B; Table 2). Additionally, FO supplementation caused a significant reduction in AA levels in aged rats (Figure 2B). Consequently, the AA/DHA ratio, as well as the total omega-6 to total omega-3 ratio, was markedly reduced by almost half when compared with that of the aged rats fed the unsupplemented diet and that of young animals (group, F(2,26) = 20.75, p < .0001; aged + FO < aged control and young, p < .0001; Figure 2C; Table 2). These ratios have long been observed to be a useful indicator of qualitative dietary fatty acid intakes (41).
Due to the perfusion of the brain for an immunohistochemical analysis of inflammation, we were unable to measure the level of fatty acids in the brain.
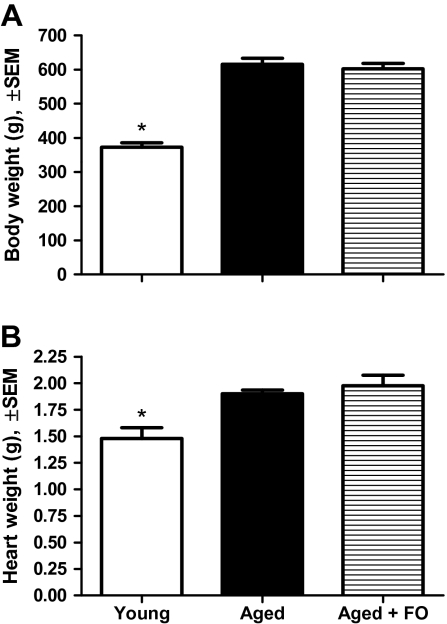
Body and Heart Weights
Body weights were measured after the completion of the water maze testing; heart weights were measured following perfusion and sacrifice. As expected, the aged rats weighed significantly more than the young animals (group, F(2,28) = 43.71, p < .0001; young vs aged, aged + FO, p < .05; Figure 3A) and they had heavier heart weights (group, F(2,28) = 8.905, p < .001; young vs aged, aged + FO, p < .05; Figure 3B). There was no effect of FO supplementation on body weight, heart weight, or heart to body weight ratio, a general index for LV hypertrophy in aged animals. These data reflect the isocaloric diets and suggest that the FO-supplemented diet was as palatable as the control diet.
Figure 3.
(A) Subject weight following water maze testing. A significant effect of group indicated that aged rats were about twice the weight (gram ± standard error of the mean) of young rats. There was no difference between the two aged groups. *p < .05 compared with aged, aged + fish oil (FO). (B) Heart weight after perfusion. Heart weights (gram ± standard error of the mean) significantly increased in aged rats compared with the young rats, regardless of diet. *p < .05 compared with aged, aged + FO.
Cardiac Structure and Function
In vivo analysis of cardiac structure and function was carried out by echocardiography for all groups 1 week after water maze testing. M-mode and Doppler measurements of LV structure and function are summarized in Table 3. Two aged control rats were excluded from cardiac analyses because one had echocardiographic evidence of a large pericardial effusion with associated elevations in filling pressures; E/e′ was more than 2 SDs from the mean of the group (E/e′ = 39), and the other aged animal was in bigeminy at the time of examination. Aged control rats had significantly greater LV chamber dimensions (LVEDD and LVESD) and PWT at end diastole but no alteration in relative wall thickness compared with the young rats (Table 3). This lack of an age effect in relative wall thickness indicates that aging did not impact geometric remodeling. Specifically, aging did not lead to concentric hypertrophy and indicates relatively healthy cardiac integrity in aged F344 × BNF1 rats. FO supplementation significantly reduced the PWT at end diastole compared with the aged control rats, but it had no effect on the relative wall thickness, LVEDD, and LVESD (Table 3). These structural data, when taken together with the significant but slightly thinner posterior walls of the aged + FO rats, indicate that FO supplementation was not harmful to cardiac structure and may be modestly beneficial by reducing the PWT at end diastole. These modest but beneficial results are in contrast to the effects of other dietary supplements such as L-arginine, which has been shown to produce increases in heart weight in aged normotensive and hypertensive rodents and to aggravate cardiac performance (42).
Table 3.
Echocardiographic Indices of Left Ventricular Structure and Function ± Standard Error of the Mean
| Young | Aged | Aged + Fish Oil | |
| LV structure | |||
| HW/BW (mg/g) | 4.0 ± 0.30 | 3.1 ± 0.07 | 3.2 ± 0.16 |
| LVEDD (cm)* | 0.756 ± 0.010† | 0.903 ± 0.035 | 0.905 ± 0.017 |
| LVESD (cm)* | 0.378 ± 0.011† | 0.492 ± 0.033 | 0.512 ± 0.021 |
| PWTed (cm)* | 0.158 ± 0.003† | 0.209 ± 0.005‡ | 0.192 ± 0.004 |
| AWTed (cm) | 0.182 ± 0.006 | 0.203 ± 0.007 | 0.198 ± 0.006 |
| RWT | 0.418 ± 0.009 | 0.469 ± 0.030 | 0.426 ± 0.010 |
| LV mass | 0.589 ± 0.038† | 1.448 ± 0.135 | 1.327 ± 0.061 |
| LV systolic function | |||
| FS (%)* | 50 ± 1‡ | 46 ± 2 | 44 ± 2 |
| VCFc (circ/s) | 0.987 ± 0.023 | 0.994 ± 0.034 | 0.960 ± 0.037 |
| HR (beats/min)* | 348 ± 7† | 291 ± 4 | 304 ± 5 |
| LV diastolic function | |||
| Emax (cm/s) | 72 ± 2 | 77 ± 2 | 74 ± 2 |
| Edec time (s) | 0.050 ± 0.002 | 0.052 ± 0.002 | 0.050 ± 0.002 |
| Edec slope (cm/s) | 14 ± 1 | 14 ± 1 | 15 ± 1 |
| e’ (cm/s)* | 5.0 ± 0.3§ | 3.6 ± 0.3 | 4.1 ± 0.3 |
| E/e’* | 14.9 ± 1.0§ | 21.6 ± 1.2 | 18.6 ± 1.0 |
Notes: AWTed = anterior wall thickness at end diastole; e’ = early mitral annular velocity; E/e’ = transmitral early filling/early mitral annular velocity; Edec = deceleration time of early filling; Emax = transmitral early filling velocity; FS = fractional shortening; HR = heart rate; HW/BW = heart weight/body weight; LV = left ventricular; LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; PWTed = posterior wall thickness at end diastole; RWT = relative wall thickness; VCFc = mean velocity of circumferential fiber shortening corrected for heart rate.
p < .05, main effect of group.
p < .01 vs aged, aged + fish oil.
p < .05 vs aged + fish oil.
p < .05 vs aged.
Systolic function was assessed by calculating FS%) and mean velocity of circumferential fiber shortening corrected for heart rate (Table 3). When compared with the young rats, the FS% was nonsignificantly decreased in aged control rats by 8%, whereas the aged + FO rats had a significant decrease of 12% (group, F(2,26) = 5.14, p < .01; post hoc, young vs aged control, NS; young vs aged + FO, p < .05). There was no difference in FS% between the aged control and aged + FO groups. Also, the mean velocity of circumferential fiber shortening corrected for heart rate was similar among all groups: group, F(2,26) = 0.32, NS. Taken together with the fact that circumferential fiber velocity shortening is a relatively load-independent measure of LV performance, whereas FS% is not (43), these data suggest that systolic function was not overtly influenced by age or FO. As expected, heart rates among aged rats, regardless of diet, were lower when compared with the young rats.
Diastolic function during early LV filling was evaluated using both pulse wave and tissue Doppler. Even though the conventional pulsed Doppler parameters were unaffected by age or diet (Table 3), the load-independent indices of diastolic function, namely e′ and E/e′, revealed the expected age-related declines in diastolic function (36,37). The tissue Doppler–derived measure of myocardial relaxation, e′, was significantly lower in the aged control group when compared with the young rats (group, F(2,26) = 4.37, p < .02, young vs aged control, p < .05). Moreover, LV filling pressure, as depicted by the E/e′ ratio, was significantly higher in the aged control versus young rats (group, F(2,26) = 8.84, p < .001; young vs aged, p < .01). In contrast, the FO modestly attenuated this effect of aging on E/e′ because the aged + FO group was no longer impaired relative to young. Taken together, these results indicate that FO supplementation resulted in a modest attenuation of the effect of age on e′ and E/e′ (Table 3) but the change was not robust enough to result in a significant difference in the numerical mean between the aged control and aged + FO.
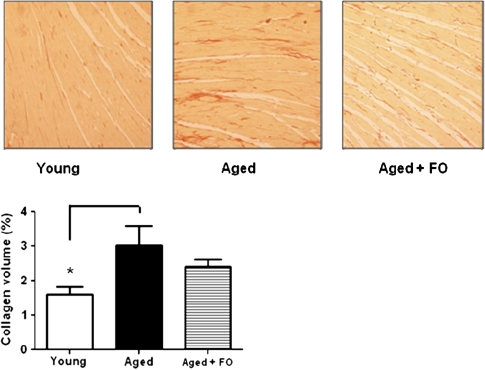
Quantitative histological analysis of cardiac collagen deposition in the left ventricle below the mitral valve from young, aged, and aged + FO groups are presented in Figure 4. This study confirms our previous observation (36) that interstitial collagen is modestly increased in aged control group when compared with the young group (Kruskal–Wallis statistic = 7.201, p < .05; Dunn’s multiple comparisons, young vs aged control, p < .05). Importantly, this age-related collagen deposition was attenuated by FO supplementation (young vs aged + FO: p > .05), which may explain, in part, the subtle mitigation by FO on the age-related elevations in E/e′.
Figure 4.
Cardiac interstitial collagen volume. Representative left ventricular sections (×20) of picrosirius red–stained myocardium from the left ventricle of each group are shown. Aged rats on the control diet had a modest but significant increase in interstitial collagen deposition compared with their younger counterparts on the same diet. This significant increase in collagen deposition in the aged group was reduced with fish oil supplementation. Results are shown as mean ± standard error of the mean. *p < .05 compared with young.
Spatial Memory
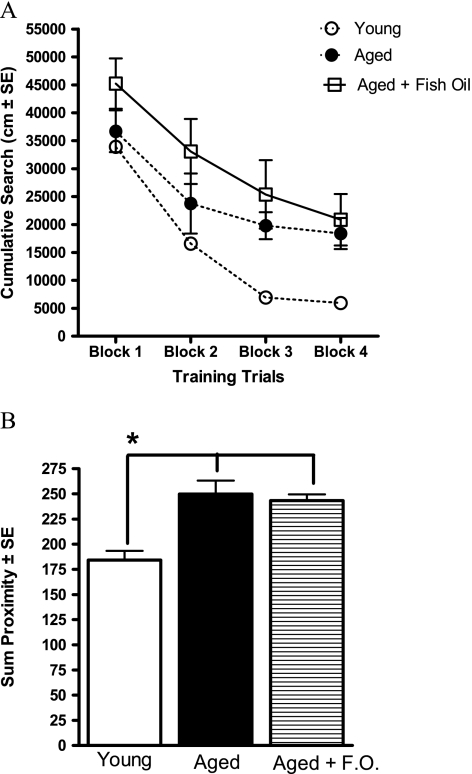
Young rats have better spatial learning ability compared with the aged rats with and without the FO supplement (Figure 5A and B). Training trial data shown in Figure 5A indicate that the young rats were most proficient in acquiring the task compared with the aged control and aged + FO groups (group, F(2,26) = 6.05, p < .01; young vs aged, p = .08; young vs aged + FO, p < .005). Although the statistical analysis with group as a factor showed a trend for a difference between young and aged control rats, if young versus old are compared in the absence of the FO group, there is a significant age effect: F(1,16) = 14.25, p < .005. The young rats had a stronger spatial bias for the escape platform location compared with the aged rats with or without FO supplementation (Figure 5B). Young rats perform significantly better than aged rats with and without FO (group, F(2,26) =15.11, p < .0001; young vs aged, aged + FO, p < .05). Overall, the data demonstrate that spatial learning in aged rats was not affected dietary supplementation of FO.
Figure 5.
(A) The cumulative search during the acquisition of a spatial learning task in the water maze. Each block represents the average of five training trials. Young rats were more proficient in learning the location of the escape platform compared with both the aged control and the aged + fish oil (FO) groups. (B) Proximity to escape platform location during probe trials. Data are the sum of proximity to the escape platform location during probe trials 2–4. Young rats perform significantly better than the aged control and aged + FO groups, and there are no differences between the two aged groups. *p < .05.
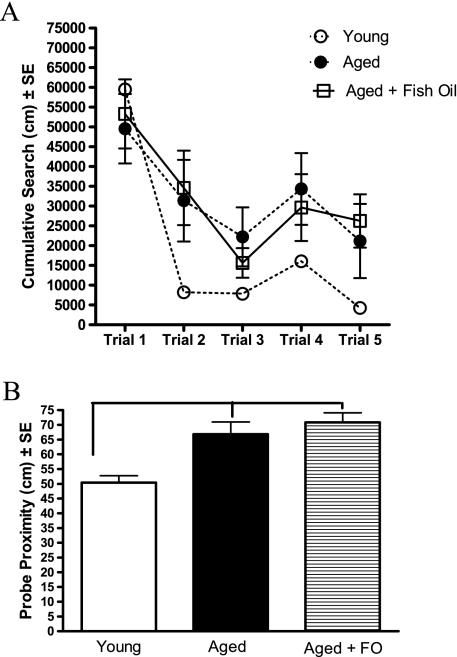
Spatial reversal learning assesses an animal’s ability to inhibit previously learned information (ie, the old platform location) with new information (a new platform location) and is dependent upon the function of the frontal–striatal circuitry (44,45). The reversal acquisition data indicate that the aged rats, regardless of diet, were poorer in learning the new platform location, although the repeated measures ANOVA only approached significance (group, F(2,26) = 2.86, p = .08, and no interaction, p = .18). Fisher’s post hoc indicates that the aged + FO group performed significantly poorer than the young rats (p < .05) and the aged control group only approached significance (p = .06). The rate of learning, indicated by the magnitude of change between trial 1 and trial 2, demonstrates that the young rats were significantly faster at learning the location of the new platform location compared with the aged rats, regardless of the diet (group, F(2,26) = 3.19, p < .056; young vs aged control, aged + FO, p < .05). Finally, a probe trial at the conclusion of reversal learning was used to assess the spatial bias for new platform location (Figure 6B). As a group, the aged rats, regardless of diet, performed more poorly than the young group (group, F(2,26) = 11.73, p < .0005; young vs aged control, p < .005; young vs aged + FO, p < 0.0001; aged vs aged + FO, NS). Overall, the data demonstrate that dietary FO supplementation at a late stage is not effective in the prevention of the effects of age on spatial reversal learning.
Figure 6.
(A) The cumulative distance traveled to reach the escape platform during spatial reversal learning training trials. All groups were equally proficient acquiring the reversal task, although the data approached significance for a main effect of group (see text for explanation). A comparison of the steepness of the learning curve between trials 1 and 2 indicates that the aged control and aged + fish oil (FO) groups were slower to learn the new location of the platform compared with the young group. (B) Reversal learning probe trial performance. Aged rats were significantly impaired compared with their young counterparts regardless of diet composition. *p < .05.
Motor Ability and Vision
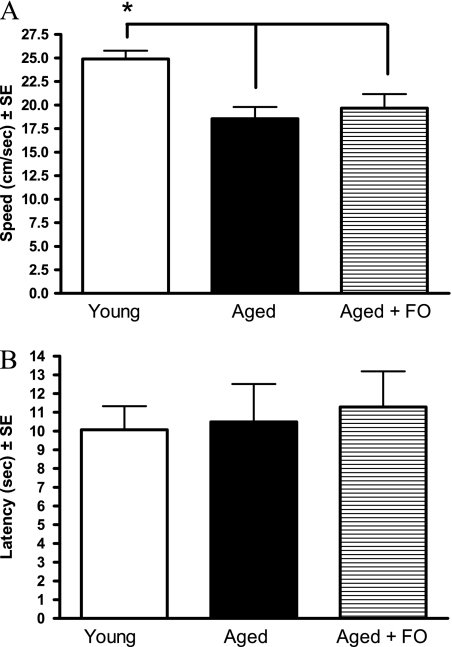
Motor ability was assessed by measuring the swim speed in the water maze during the visible platform trials. Figure 7A shows that all aged rats swam significantly slower than the young group, indicated by a main effect of group: F(2,26) = 9.47, p < .001. There was no effect of FO supplementation on the swim speed of the aged rats.
Figure 7.
(A) Swim speed during water maze cue training. Aged rats swim slower than young rats. Fish oil (FO) supplementation did not improve the swim speed of the aged rats. *p < .05. (B) Latency to reach a visible platform during water maze cue training. There were no effects of age between the aged rats with or without FO supplementation.
Visual acuity was determined by the latency to swim to a visible platform in the water maze and is shown in Figure 7B. There was no effect of age and no effect of FO supplementation. The deficit in latency to reach the visible platform was due to the slower swim speed of the aged rats and not visual deficits.
Effect of Diets on Brain Inflammation in the Hippocampus
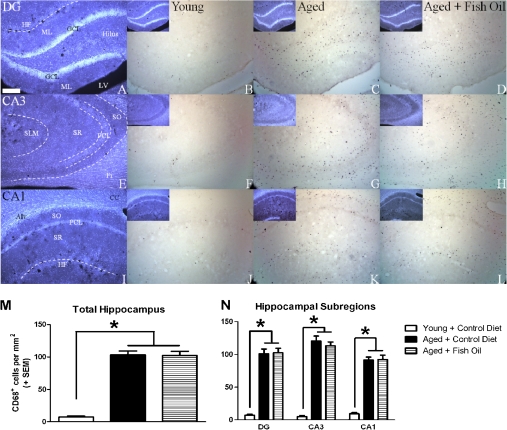
The dorsal hippocampus was specifically selected for an analysis of inflammatory status because this structure is functionally differentiated along its dorsal–ventral axis; the dorsal hippocampus plays a more pronounced role in spatial learning relative to the ventral hippocampus (46). There was a main effect of group, F(2,21) = 92.03, p < .0001, on density of activated microglia, and subsequent post hoc analysis revealed that all aged rats, regardless of diet, had significantly greater density of activated microglia relative to young (p < .0001), but there was no difference observed between the aged control and aged + FO groups (Figure 8). However, the effects of aging are apparent in a subregion-specific pattern, so additional analyses were conducted comparing among discrete hippocampal subregions. Using a repeated measures ANOVA to compare among different groups and subregions, there were main effects of group, F(2,21) = 95.19, p < .0001; subregion, F(2,42) = 33.52, p < .0001; and an interaction between group and subregion, F(4,42) = 14.14, p < .0001. Post hoc tests confirmed the results of the analysis of the whole hippocampus demonstrating that all aged rats, regardless of diet, exhibited higher density of activated microglia relative to young (p < .0001), but aged rats did not differ from each other when comparing control and FO diets. When testing the results of hippocampal subregion on microglial density, a one-way ANOVA did not reveal differences between hippocampal subregions when all animals were included in a single analysis: F(2,69) = 0.66, NS. However, when animals were separated by chronological age, aged rats, but not young rats, did exhibit subregion-specific differences, F(2,48) = 7.10, p < .005, with the CA3 demonstrating a greater density of activated microglia relative to the CA1 (p < .001) and dentate gyrus (p = .03). When aged rats were analyzed with respect to diet, only the rats fed control diet exhibited an effect of subregion, F(2,18) = 5.10, p < .05, whereas only a trend was observed in FO-fed rats, F(2,27) = 2.59, p = .09, NS. However, our sample size may lack the power to detect subregional differences when comparing aged rats on the two diets, and importantly, aged rats maintained on the FO diet did not differ from aged rats fed a control diet when considering whole hippocampus or each subregion in isolation. We have previously observed significantly increased density of activated microglia in the CA3 relative to CA1 in aged rats (47), and recently published reports have suggested that CA3-specific alterations may play a significant role in cognitive aging (48).
Figure 8.
Density of activated microglia is increased in hippocampus of aged rats. 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) staining illustrating anatomical landmarks in the dentate gyrus (A), CA3 (E), and CA1 (I) of young rats. Representative photomicrographs obtained from young (B, F, J) and aged rats (C, G, K), and aged rats fed a fish oil–enriched diet (D, H, L) demonstrating CD68-immunoreactive cells (putative microglia) in the dentate gyrus (B–D), CA3 (F–H), and CA1 (J–L) of the hippocampus. Inset (B–D, F–G, and J–L): DAPI counterstain corresponding to each image shown. CD68+ cells were rarely observed in young rats, but density was significantly increased in aged rats regardless of diet (M and N; *p < .001 vs young control). Among aged rats, the density of CD68+ cells was significantly greater in the CA3 subregion relative to the CA1 (p < .001). Scale bar at bottom left in A is 250 μm. All images were acquired at the same magnification. Alv = alveus; CC = corpus callosum; Fi = fimbria; GCL = granule cell layer; HF = hippocampal fissure; LV = lateral ventricle; ML = molecular layer; PCL = pyramidal cell layer; SLM = stratum lacunosum-moleculare; SO = stratum oriens; SR = stratum radiatum.
DISCUSSION
Earlier studies in rats (49) and humans (50) have suggested that circulating levels of the proinflammatory fatty acid, AA, increase with age. In the current study, aged rats consuming a diet identical to that of young rats had much higher circulating levels of AA compared with the young rats. AA has long been considered to be pro-inflammatory and has been linked to CV disease, elevated blood pressure, and arthritis in humans (51). The mechanism(s) responsible for these age-related differences is not known. It is interesting to note that these differences were observed on a low-fat (4%) diet (relative to the typical Western diet) and raises the important question of whether AA levels would have been even higher had a Western diet (with typical omega-6 fatty acid contents of 5%–10% energy) been utilized.
The addition of about 1% (by weight) FO (providing an estimated 1.75 mg EPA + 1.17 mg DHA per gram diet per day) to the diet of aged rats for 4 months effectively increased the levels of omega-3 PUFAs while decreasing the level of AA in plasma. This is consistent with an anti-inflammatory fatty acid profile. Consequently, this dietary manipulation markedly reduced the omega-6 to omega-3 ratio (Table 2), which would be expected to provide increased omega-3 PUFAs to the tissues, including the heart and brain because omega-6 and omega-3 fatty acids utilize the same uptake and acylation enzymes necessary for incorporation into complex lipids.
Although the fatty acid profile of erythrocyte membranes is thought to be less influenced by dietary fluctuations than that of the plasma in humans (52), the uniform purified diet provided to these animals minimizes the contribution of dietary variation in the current study as shown by the omega-6/omega-3 ratio, which was not different in young and aged rats consuming the unsupplemented diet. Importantly, the impact of FO supplementation in humans on the plasma and red blood cell fatty acid profiles are similar (53).
Whereas increasing omega-3 content late in life did not reverse the senescent cardiac phenotype of the 28-month-old BNF344 rat, the 4-month dietary intervention of FO did attenuate some markers of cardiac aging; specifically, the age-related reductions in e′, and increases in E/e′ and collagen deposition. In contrast to the modest effects on specific cardiac measures, the age-related declines in cognitive function and increases in brain inflammation were not influenced by FO supplementation at the present dosing paradigm (summarized in Table 4).
Table 4.
Summary of Results
| Age-Related Changes | Reversal of Age Effects by Fish Oil Ingestion* | |
| Long-chain PUFA | ||
| C20:4n-6 (arachidonic)* | Increased | Yes |
| C20:5n-3 (eicosapentaenoic)* | No change | Yes |
| C22:5n-3 (docosapentaenoic)* | No change | Yes |
| C22:6n-3 (docosahexaenoic)* | No change (numerical increase, p = .07) | Yes |
| Cardiac measures | ||
| Posterior wall thickness | Increased | Yes |
| E/e’ | Poorer | Yes |
| Fractional shortening | Less | No |
| Spatial learning | ||
| Reference memory | Impaired | No |
| Reversal learning | Impaired | No |
| Brain inflammation | Increased | No |
Notes: PUFA = polyunsaturated fatty acids.
Reversal of the aging phenotype is defined as a value that is significantly different from the aged group. The data may or may not be different from the young group but should change in that direction as it is presumed that the young phenotype is less harmful than the aged phenotype.
Ample evidence has confirmed the altered diastolic compliance of the senescent heart (54,55). Our results build on those data and indicate that aged F344 × BNF1 rats have reduced myocardial relaxation, increased LV filling pressures, and a modest increase in cardiac collagen deposition. We found that FO supplementation to aged F344 × BNF1 rats modestly attenuated age-related lusitropic dysfunction and interstitial fibrosis, indicating a possible contribution of enhanced intracellular calcium regulation and reduced LV remodeling from omega-3 PUFA. It was shown previously that dietary FO supplementation reduced the susceptibility of myocytes to reactive oxygen species–induced injury and the ability to prevent rises in cellular Ca2+ in response to reactive oxygen species (56). Indeed, oxidative stress has been linked to CV aging (57–59) specifically due to a prolonged duration of myocardial relaxation (60). FO-induced alterations in the fatty acid composition of the cell membranes may also contribute to lusitropic function by modifying membrane fluidity and elastic properties of myocardial cells (11,61). Although we did not measure the fatty acid composition of the myocardial membranes among the different groups of rats, our functional findings are supported by results obtained in healthy middle-aged adults (11) and non-human primates (62) showing that dietary FO consumption for 7 weeks to 2 years improved the early phase of LV diastolic filling.
It is possible that the FO-associated benefits on diastolic function could be a consequence of reduced LV remodeling. For instance, FO supplementation increased plasma adiponectin, suppressed inflammation, and prevented cardiac remodeling in a rat model of chronic pressure overload (63) and it reduced LV hypertrophy in hypertensive rats (64). Although cardiac weight was not affected by FO in the present study, the reduced posterior wall thickness and the tendency for lower collagen volume among the aged supplemented rats compared with their unsupplemented cohorts suggest a potential role for omega-3 PUFAs in cardiac antiremodeling, which could, in turn, mitigate the age-related increases in LV filling pressure, or E/e′. Certainly, these cardioprotective effects could be consequences of lower blood pressure and reduced vascular stiffness. Geleijnse and colleagues (65) found that consumption of FO supplements was associated with subtle but significant reductions in blood pressure, particularly among older persons and those with higher blood pressures. Omega-3 PUFA also reduced hypertension and decreased early filling deceleration time, an index of diastolic function, in cyclosporine-treated cardiac transplant recipients (66). Interestingly, Park and Park (67) recently showed that aortic wall thickness was significantly lower in rats supplemented with FO rather than soybean oil or shortening for 4 weeks, suggesting that FO could also have protective effects on vascular remodeling. Unfortunately, we did not obtain blood pressures or examine postmortem aortas from the rats in the present study. Nonetheless, the modest attenuation in age-related diastolic dysfunction in our model fits well with the cardioprotective roles of FO shown in primary and secondary heart failure prevention trials (7–9) and the improved resting hemodynamics and lipid profiles among healthy older individuals (10–15). Indeed, future studies are warranted to determine whether a higher dose and/or earlier commencement of omega-3 PUFA supplementation, for example, at midlife, could reverse or prevent the progression of diastolic dysfunction of aging.
The lack of an effect of FO on spatial learning and on the levels of activated microglia in the hippocampus could indicate that there were no changes in brain PUFA levels as a consequence of the dietary intervention. The limited ability of the brain to synthesis LC-PUFAs compared with the liver render it dependent on dietary- and hepatic-derived LC-PUFAs (5,68). Although we cannot confirm that the levels of PUFAs in the brain were altered since this tissue was processed for immunohistochemistry, it is possible that a higher FO dose may have had an impact of cognitive function parameters. Moreover, Moriguchi and colleagues (69) showed that DHA deficiency in young adult rat brains could be restored to normal levels in 8 weeks with an omega-3 repletion diet (69). This is well within the 16-week supplementation period used in the present study. The Moriguchi studies were done in young rats and may not have similar effects in the aged brain. Indeed, repletion of DHA deficiency is effective at improving performance on a radial arm maze in young adult but not aged rats (16,17), suggesting that improvement of plasma anti-inflammatory properties may not be an effective intervention for brain aging.
Consistent with the hypothesis that improving plasma anti-inflammatory properties in aged animals may not affect brain aging is the apparent lack of an effect of FO on reducing the number of activated microglia in the hippocampus. Microglia are altered in response to inflammation by expressing an “active” phenotype that includes changes in size and ramification, as well as expression of several protein markers, including the one assessed in this study, CD68 (70–72). The quantitative results from this study demonstrate the expected increase in activated microglia in aging, but there was no reduction in this inflammatory marker in aged rats treated with FO. This leaves open the possibilities that dosing parameters were not sufficient or that dietary changes must be started prior to the establishment of a chronic inflammatory state, at least in brain. Indeed, in studies by Riddle and colleagues (73), substantial aging-related increases in activated microglia have been demonstrated in the hippocampus at least as early as 15–18 months of age.
CONCLUSIONS
The results from this study indicate that dietary FO supplementation administered late in life attenuated age-related declines in diastolic function but it did not limit age-related declines in memory or in brain inflammatory status. This study adds to our understanding of the effects of FO in the aged rat due to the comprehensive analysis of the effects of supplementation on memory, brain inflammation, heart, and physical function within the same individuals. Future studies are needed to address the hypothesis that supplementation may need to occur prior to the accumulation of inflammatory damage to be effective in brain, and almost certainly in the heart. If the hypothesis were proven correct, it would indicate that supplementation is beneficial when used preventatively but ineffective at reversing damage if begun late in the life span after chronic inflammation has been established.
FUNDING
This work was supported by the National Institute on Aging at the National Institutes of Health (AG020572 to M.M.N., K08-AG026764 and R01-AG033727 to L.G.), Nestle Nutrition (M.M.N.), and P50-AT002782 (F.H.C.).
Acknowledgments
The authors thank Mona Beardslee, Ashley Donahue, Adam Wilson, Jennifer Souza, Liz Forbes, and Kate Davis for their expert technical assistance.
References
- 1.Farooqui AA, Ong WY, Horrocks LA, Chen P, Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev. 2007;56:443–471. doi: 10.1016/j.brainresrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 3.Assisi A, Banzi R, Buonocore C, et al. Fish oil and mental health: the role of n-3 long-chain polyunsaturated fatty acids in cognitive development and neurological disorders. Int Clin Psychopharmacol. 2006;21:319–336. doi: 10.1097/01.yic.0000224790.98534.11. [DOI] [PubMed] [Google Scholar]
- 4.Harris WS, Sands SA, Windsor SL, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport SI, Igarashi M, Gao F. Quantitative contributions of diet and liver synthesis to docosahexaenoic acid homeostasis. Prostaglandins Leukot Essent Fatty Acids. 2010;82:273–276. doi: 10.1016/j.plefa.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenna JT, Salem N, Jr,, Sinclair AJ, Cunnane SC; International Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Yamagishi K, Iso H, Date C, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr. 2004;79:765–773. doi: 10.1093/ajcn/79.5.765. [DOI] [PubMed] [Google Scholar]
- 11.Grimsgaard S, Bønaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998;68:52–59. doi: 10.1093/ajcn/68.1.52. [DOI] [PubMed] [Google Scholar]
- 12.Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J Nutr. 2007;137:973–978. doi: 10.1093/jn/137.4.973. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 14.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 15.Ryan AS, Keske MA, Hoffman JP, Nelson EB. Clinical overview of algal-docosahexaenoic acid: effects on triglyceride levels and other cardiovascular risk factors. Am J Ther. 2009;16:183–192. doi: 10.1097/MJT.0b013e31817fe2be. [DOI] [PubMed] [Google Scholar]
- 16.Gamoh S, Hashimoto M, Hossain S, Masumura S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol. 2001;28:266–270. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 17.Gamoh S, Hashimoto M, Sugioka K, et al. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93:237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 18.Ikemoto A, Ohishi M, Sato Y, et al. Reversibility of n-3 fatty acid deficiency-induced alterations of learning behavior in the rat: level of n-6 fatty acids as another critical factor. J Lipid Res. 2001;42:1655–1663. [PubMed] [Google Scholar]
- 19.Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- 20.Delion S, Chalon S, Guilloteau D, Lejeune B, Besnard JC, Durand G. Age-related changes in phospholipid fatty acid composition and monoaminergic neurotransmission in the hippocampus of rats fed a balanced or an n-3 polyunsaturated fatty acid-deficient diet. J Lipid Res. 1997;38:680–689. [PubMed] [Google Scholar]
- 21.Delion S, Chalon S, Hérault J, Guilloteau D, Besnard JC, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J Nutr. 1994;124:2466–2476. doi: 10.1093/jn/124.12.466. [DOI] [PubMed] [Google Scholar]
- 22.McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit SC, Clegg DJ. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostaglandins Leukot Essent Fatty Acids. 2006;75:237–257. doi: 10.1016/j.plefa.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Park SJ, Tamura M, Ando S. Effect of the long-term feeding of dietary lipids on the learning ability, fatty acid composition of brain stem phospholipids and synaptic membrane fluidity in adult mice: a comparison of sardine oil diet with palm oil diet. Mech Ageing Dev. 1998;101:119–128. doi: 10.1016/s0047-6374(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 24.Wainwright PE, Xing HC, Ward GR, et al. Water maze performance is unaffected in artificially reared rats fed diets supplemented with arachidonic acid and docosahexaenoic acid. J Nutr. 1999;129:1079–1089. doi: 10.1093/jn/129.5.1079. [DOI] [PubMed] [Google Scholar]
- 25.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim S, Suzuki H. Changes in maze behavior of mice occur after sufficient accumulation of docosahexaenoic acid in brain. J Nutr. 2001;131:319–324. doi: 10.1093/jn/131.2.319. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto T, Cansev M, Wurtman RJ. Oral supplementation with docosahexaenoic acid and uridine-5’-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007;1182:50–59. doi: 10.1016/j.brainres.2007.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherubini A, Andres-Lacueva C, Martin A, et al. Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:1120–1126. doi: 10.1093/gerona/62.10.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bizon JL, Nicolle MM. Rat models of age-related cognitive decline. In: Conn PM, editor. Handbook of Models for Human Aging. London: Elsevier Press; 2006. pp. 379–391. [Google Scholar]
- 31.Burlew BS. Diastolic dysfunction in the elderly—the interstitial issue. Am J Geriatr Cardiol. 2004;13:29–38. doi: 10.1111/j.1076-7460.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 33.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 34.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- 35.Surette ME, Koumenis IL, Edens MB, Tramposch KM, Chilton FH. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin Ther. 2003;25:948–971. doi: 10.1016/s0149-2918(03)80116-9. [DOI] [PubMed] [Google Scholar]
- 36.Groban L, Pailes NA, Bennett CD, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 37.Groban L, Jobe H, Lin M, Houle T, Kitzman DA, Sonntag W. Effects of short-term treadmill exercise training or growth hormone supplementation on diastolic function and exercise tolerance in old rats. J Gerontol A Biol Sci Med Sci. 2008;63:911–920. doi: 10.1093/gerona/63.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasband WS. ImageJ. U.S. National Institutes of Health, Bethesda, MD. http://rsb.info.nih.gov/ij/. Accessed August 23, 2010. [Google Scholar]
- 39.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 40.Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the most sensitive measure of water maze probe test performance? Front Integr Neurosci. 2009;3:4. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 42.Brooks WW, Conrad CH, Robinson KG, Colucci WS, Bing OH. L-arginine fails to prevent ventricular remodeling and heart failure in the spontaneously hypertensive rat. Am J Hypertens. 2009;22:228–234. doi: 10.1038/ajh.2008.334. [DOI] [PubMed] [Google Scholar]
- 43.Paraskos JA, Grossman W, Saltz S, Dalen JE, Dexter L. A noninvasive technique for the determination of velocity of circumferential fiber shortening in man. Circ Res. 1971;29:610–615. doi: 10.1161/01.res.29.6.610. [DOI] [PubMed] [Google Scholar]
- 44.Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Castañé A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 47.McQuail JA, Schindler MK, Riddle DR. The neuroinflammatory response of the adult rat hippocampus to whole brain irradiation is age-dependent and sub-region specific. [Abstract] http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=9beab5e3-b0d1-48e9-a292-2571d3ed1dba&cKey=1d2be109-dc3a-4784-bfc3-8c2369d90bed&mKey=%7bAFEA068D-D012-4520-8E42-10E4D1AF7944%7d. Accessed August 23, 2010. [Google Scholar]
- 48.Haberman RP, Colantuoni C, Stocker AM, Schmidt AC, Pedersen JT, Gallagher M. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging. 2009 Nov 12 doi: 10.1016/j.neurobiolaging.2009.10.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruggiero FM, Cafagna F, Gadaleta MN, Quagliariello E. Effect of aging and acetyl-L-carnitine on the lipid composition of rat plasma and erythrocytes. Biochem Biophys Res Commun. 1990;170:621–626. doi: 10.1016/0006-291x(90)92137-o. [DOI] [PubMed] [Google Scholar]
- 50.High KP, Sinclair J, Easter LH, Case D, Chilton FH. Advanced age, but not anergy, is associated with altered serum polyunsaturated fatty acid levels. J Nutr Health Aging. 2003;7:378–384. [PubMed] [Google Scholar]
- 51.Pepe S. Effect of dietary polyunsaturated fatty acids on age-related changes in cardiac mitochondrial membranes. Exp Gerontol. 2005;40:751–758. doi: 10.1016/j.exger.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem. 2010;43:338–340. doi: 10.1016/j.clinbiochem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007;86:1621–1625. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 54.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 55.LaSarge CL, Nicolle MM. Comparison of different cognitive rat models of human aging. In: Bizon JL, Wood AG, editors. Animal Models of Human Cognitive Aging. New York: Humana Press; 2009. pp. 73–102. [Google Scholar]
- 56.Jahangiri A, Leifert WR, Kind KL, McMurchie EJ. Dietary fish oil alters cardiomyocyte Ca2+ dynamics and antioxidant status. Free Radic Biol Med. 2006;40:1592–1602. doi: 10.1016/j.freeradbiomed.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 57.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 58.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 59.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 60.Li SY, Du M, Dolence EK, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 61.Harris WS. Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol Res. 2007;55:217–223. doi: 10.1016/j.phrs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLennan PL, Barnden LR, Bridle TM, Abeywardena MY, Charnock JS. Dietary fat modulation of left ventricular ejection fraction in the marmoset due to enhanced filling. Cardiovasc Res. 1992;26:871–877. doi: 10.1093/cvr/26.9.871. [DOI] [PubMed] [Google Scholar]
- 63.Duda MK, O’Shea KM, Tintinu A, et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Au D, Brändle M, Rupp H, Jacob R. Influence of a diet rich in fish oil on blood pressure, body weight and cardiac hypertrophy in spontaneously hypertensive rats. Eur J Appl Physiol Occup Physiol. 1988;58:97–99. doi: 10.1007/BF00636610. [DOI] [PubMed] [Google Scholar]
- 65.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Ventura HO, Milani RV, Lavie CJ, et al. Cyclosporine-induced hypertension. Efficacy of omega-3 fatty acids in patients after cardiac transplantation. Circulation. 1993;88(5 Pt 2):II281–II285. [PubMed] [Google Scholar]
- 67.Park S, Park Y. Effects of dietary fish oil and trans fat on rat aorta histopathology and cardiovascular risk markers. Nutr Res Pract. 2009;3:102–107. doi: 10.4162/nrp.2009.3.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rapoport SI, Igarashi M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins Leukot Essent Fatty Acids. 2009;81:119–123. doi: 10.1016/j.plefa.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N., Jr. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res. 2001;42:419–427. [PubMed] [Google Scholar]
- 70.Damoiseaux JG, Döpp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- 72.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 73.Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70:826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]