Abstract
Background
New HIV infections are being observed among men who have sex with men. Understanding the fusion of risky sexual behaviors, stimulant and erectile dysfunction drug use with HIV seroconversion may provide direction for focused intervention.
Methods
During the follow-up period (1998–2008) we identified 57 HIV seroconverters among 1,667 initially HIV-seronegative men. Time to seroconversion was modeled using Cox proportional hazards regression analysis for 7 combinations of sex-drugs (inhaled nitrites or “poppers”, stimulants, and EDDs) used at the current or previous semi-annual visit, adjusting for other risk factors including sexual behavior, alcohol and other drugs used, and depression. Model-based adjusted attributable risks were then calculated.
Results
The risk of seroconversion increased linearly with the number of unprotected receptive anal sex partners (URASP), with hazard ratios (HR) ranging from 1.73 (95% confidence interval [CI]: 0.75, 4.01) for 1 partner, to 4.23 (95% CI: 1.76, 10.17) for 2–4 partners to 14.21 (95% CI: 6.27, 32.20) for 5+ partners, independent of other risk factors. After adjustment, risks for seroconversion increased from 2.99 (95% CI: 1.02, 8.76) for men who reported using stimulants only (1 drug) to 8.45 (95% CI: 2.67, 26.71) for men who reported using all 3 sex-drugs. The use of any of the 7 possible sex-drug combinations accounted for 63% of the nine-year HIV seroincidence in the Multicenter AIDS Cohort Study (MACS). When contributions of increased URASP and combination drug use were analyzed together, the total attributable risk for HIV seroconversion was 74%, with 41% attributable to URASP alone and a residual of 33% due to other direct or indirect effects of sex-drug use.
Conclusions
Use of poppers, stimulants and EDDs increased risk for HIV seroconversion significantly in this cohort. These data reinforce the importance of implementing interventions that target drug-reduction as part of comprehensive and efficacious HIV prevention strategies.
Keywords: Multicenter AIDS cohort study, MSM, stimulants, inhaled nitrites, erectile dysfunction drugs, HIV seroconversion, non-intravenous drug use
INTRODUCTION
With the early recognition of intravenous drug users as being at high risk for AIDS, most of the epidemiologic research on the role of drugs in HIV transmission focused on the risk of sharing needles, syringes and other sources of blood borne infection. However, non-intravenous drug use (NIDU) by gay and bisexual men (hereafter “men who have sex with men” or MSM) may significantly impact HIV infection and progression. Specific NIDU, particularly inhaled nitrites (“poppers”) and methamphetamine, by MSM has been shown to significantly increase the relative risk of infection (defined as seroconversion to HIV-antibody positivity).1–3 More recently, the combined use of methamphetamine and other stimulants (cocaine, crack and ecstasy) with poppers or EDDs has been shown to be behaviorally disinhibiting, especially among MSM at high risk of HIV seroconversion and transmission.4–13
We examined the effects of the combination of stimulant use, poppers and EDDs with risky sexual behavior on HIV seroconversion occurring between 1998 and the present (March, 2008) among MSM who were initially HIV seronegative and followed over time in the Multicenter AIDS Cohort Study (MACS). We predicted that sexually active HIV-seronegative men in the MACS who reported using one or more of these three specific sex-drugs would have significantly higher rates of HIV seroconversion compared to those men who did not report NIDU and that this increased HIV seroconversion risk would be independent of the number of unprotected anal receptive sexual partners (UARSP) and other risk factors. These data allowed us to calculate a measure of the adjusted attributable risk, estimating the contribution of sex-drug use to HIV seroconversion within a large, multi-site cohort of MSM.
METHODS
Population and Study Design
The MACS is an ongoing prospective study of the natural and treated histories of HIV infection among MSM in the United States. A total of 6,972 men were recruited (4,954 in 1984 to 1985, 668 in 1987 to 1,991, and 1350 in 2001 to 2003) at four centers located in Baltimore-Washington, DC; Chicago; Los Angeles; and Pittsburgh. The study design has been described previously14,15 and only methods relevant to the present study are presented here. All MACS questionnaires are available at http://www.statepi.jhsph.edu/macs/forms.html. MACS study protocols were approved by the institutional review boards of each of the participating centers, their community partners, and community advisory boards, and informed consent was obtained from all participants.
MACS participants return every 6 months for detailed interviews, physical examinations, and collection of blood for laboratory testing and storage in a central repository. The interview includes questions about medical conditions, medical treatments, sexual behavior, drug use (e.g., marijuana, poppers, cocaine, crack, heroin, methamphetamine, ecstasy, GHB, ketamine, LSD and other psychedelic drugs, and any erectile dysfunction drug use) and alcohol consumption since the previous visit.
The collection of data on EDD use was started at MACS visit 29 (April 1998–September 1998), obtained from a question concerning “other medications” prescribed. Starting at visit 36 (October 2001–April 2002), a single EDD use question was asked in the medical history review of prescribed medications for diagnosed ED. However, more recently (beginning at visit 43, April 2005–September 2005), a separate set of questions was added to the non-prescribed drug use section about use of EDDs “that were not prescribed by a healthcare provider for diagnosed ED.” All questions concerning sexual and drug use behaviors were assessed using audio computer-assisted self-interviewing (ACASI), a methodology shown to yield more accurate assessments of “sensitive behaviors” than interviewer-administered questionnaires.16
A prospective cohort design was used to examine the effects of EDD and recreational drugs, particularly poppers and stimulants (defined here as methamphetamine and/or crack and/or cocaine) on the risk of HIV seroconversion among initially HIV-negative participants. The cohort included all participants (n=1,667) who were HIV-negative at baseline and had data on EDD and recreational drug use at baseline or follow-up visits. The baseline was defined as the earliest visit that a participant was seen and answered questions on EDD and recreational drugs between visit 29 and 48, i.e., April 1998 to March 2008.
Outcome Variable
ELISAs with confirmatory Western blot tests were performed on all participants initially and every six months thereafter if initially seronegative. Time to HIV seroconversion was the outcome of interest. The date of seroconversion was defined as the midpoint between the dates of the last HIV-seronegative visit and the first HIV-seropositive visit. The person-time contributed was the time from baseline to the date of seroconversion for those who seroconverted or to the date of the last visit seen for persistently seronegative men.
Exposure Variables
The primary exposure of interest was the reported use of EDD and other sex-drugs, specifically poppers and stimulants. Eight different combinations of sex-drug use at the current or previous visits were examined in the analysis, namely (1) None (reference group), (2) EDD alone, (3) poppers alone, (4) EDD + poppers, (5) stimulants alone, (6) stimulants + EDD, (7) stimulants + poppers, and (8) stimulants + EDD + poppers. The use of these drug combinations was defined as “yes” if a participant reported using them at the current visit or the previous visit since HIV infection could have occurred anytime between 1–6 weeks prior to the previous visit up until several weeks prior to the current visit of interest.
Demographics and other behaviors were included in multivariate models to adjust for possible confounding. Age at baseline for these analyses was calculated using self-reported date of birth and was treated as a continuous covariate centered at the approximate median age of 45 years. Race was self-reported at the first MACS study visit (initial baseline) and categorized as White non-Hispanic (reference group), White Hispanic, Black non-Hispanic, Black Hispanic, and “other” (predominantly mixed race). Self-reported highest level of education completed at baseline was categorized as grade 12 or less (reference group), college, and post-college graduate. The number of sexual partners since the prior visit was categorized as none, one, 2–4, and 5 or more. The number of anal sexual partners with whom the participants reported always using a condom was subtracted from the total number of partners for each respective activity (receptive or insertive) to obtain the number of partners with whom the participant engaged in unprotected anal sex and were also categorized as none, one, 2–4, and 5 or more. These partner number categories were derived from preliminary findings that the unadjusted risk of HIV seroconversion increased linearly from 1–5 partners but plateaued after ≥6 partners. Type of alcohol use was classified using frequency of drinking and average number of drinks the participant drank per day since the last visit. Binge drinking was defined as 5 or more drinks per occasion occurring at least monthly. Moderate to heavy drinking was at least weekly drinking of 3–4 drinks or drinking 5 or more drinks less than monthly. The remaining participants who had low to moderate or no drinking comprised the third group of alcohol use in this analysis. Likelihood of clinical depression was indicated by a score of 16 or higher using the Center for Epidemiologic Study of Depression symptom checklist.17
STATISTICAL ANALYSES
Except for demographic data and time of recruitment, all other factors (alcohol use, depression symptom score, URASP and categories of combination sex-drug use) were time varying (i.e., they were updated at each visit). In all analyses, URASP was treated as a confounding variable rather than a mediator between drug use and seroconversion as we did not have data directly linking sexual behavior during or subsequent to specific drug use. Associations between risk co-factors and the time to HIV-seroconversion were estimated using Cox proportional hazards regression models that it can accommodate late entries (technically, left truncated observations - subjects for whom data are available only if they survive to a known observation point).18, 19 This allows models with time dependent covariates to include all subjects at each time point of observation according to their current exposure status (for example, use of a given drug combination or not), which can change over time. Predicted survival curves were calculated by using a nonparametric estimate of the baseline survival function (corresponding to the reference categories for all exposure variables and covariates), and applying the parametric proportionality factors for the hazard estimated by the model using particular choices for the values of the covariates.
Each independent variable was first evaluated in a univariate proportional hazards model. The multivariate model used all 7 combinations of EDD, stimulant, and popper use with all other covariates to test the most recent antecedent exposure to the outcome event. The model included not only the three main effects, but also the three possible two-way interactions and a three-way interaction as well. The reference group was non-use of any drug.
Using the same exposures and covariates as the survival analysis, we computed model-based estimates of the adjusted attributable risk (AR) for use of each of the seven sex-drug combinations, as well as the total AR for any use of these three sex-drugs.20 In addition we calculated the total risk attributable to both URASP (as no additional significant exposure variables were identified in either these or our previous analyses of drug effects on seroconversion in the MACS) and drug use; the difference between this total risk and that attributable to URASP alone was interpreted as the excess risk attributable to sex-drug use after accounting for the disinhibition of risky sexual behavior. For these analyses we used a pooled logistic regression model with a complimentary log-log link corresponding to the discrete time proportional hazards model; standard errors were calculated using bootstrap re-sampling based on 500 samples from the original data.
RESULTS
The baseline characteristics of the study cohort are presented in Table 1. Among the men who were HIV-seronegative in the period of 1998–2008, 57 participants HIV-seroconverted. These men were similar to the persistently seronegative men in terms of age at baseline, race/ethnicity, and attainment of a college education. The seroconverters were predominantly aged 36 to 45 years old, of White, non-Hispanic race/ethnicity, more likely to have participated in the Los Angeles MACS site, and to have used EDDs, poppers and/or stimulants at the current or previous visit. Twenty-one percent of the seroconverters reported 2 or more unprotected receptive anal sex partners in the past six months compared to 5% of the non-seroconverting men. Poppers and EDDs were reportedly used alone or in combination by 33% of seroconverters compared to 23% of non-seroconverting men during the current or previous 6-month visit. 33% of seroconverters reported use of stimulants, alone or in combination with other sex-drugs, which compared to 16% of non-seroconverting men.
TABLE 1.
Baseline* characteristics of MACS HIV seroconverters (SC) and HIV seronegatives (SN) studied from 1998 to 2008.
| Seroconverters | Seronegatives | Overall | |
|---|---|---|---|
| n | 57 | 1610 | 1667 |
| Age (yrs), mean (SD) | 40.3 (10.4) | 45.0 (10.9) | 44.8 (10.9) |
| Age at seroconversion (yrs), mean (SD) | 43.0 (10.5) | ||
| Age categories at enrollment | |||
| 18–25 | 7 (12%) | 107 (7%) | 114 (7%) |
| 26–35 | 8 (14%) | 194 (12%) | 202 (12%) |
| 36–45 | 27 (47%) | 548 (34%) | 575 (34%) |
| 46–55 | 13 (23%) | 514 (32%) | 527 (32%) |
| 56+ | 2 (4%) | 247 (15%) | 249 (15%) |
| Race/ethnicity | |||
| White, non-Hispanic | 36 (63%) | 1064 (66%) | 1100 (66%) |
| White, Hispanic | 3 (5%) | 78 (5%) | 81 (5%) |
| Black (includes non-Hispanic & Hispanic) | 12 (21%) | 396 (25%) | 408 (24%) |
| All Other | 6 (11%) | 72 (4%) | 78 (5%) |
| Educational level | |||
| 12th grade or less | 12 (21%) | 309 (19%) | 321 (19%) |
| Some college or college graduate | 34 (60%) | 764 (48%) | 798 (48%) |
| Some graduate work or graduate degree | 11 (19%) | 519 (33%) | 530 (32%) |
| Cohort enrollment | |||
| 1984–1985 and 1987–1991 (early) | 28 (49%) | 993 (62%) | 1021 (61%) |
| 2001–2003 (late) | 29 (51%) | 617 (38%) | 646 (39%) |
| Center | |||
| Baltimore | 16 (28%) | 392 (24%) | 408 (24%) |
| Chicago | 11 (19%) | 223 (14%) | 234 (14%) |
| Pittsburgh | 10 (18%) | 454 (28%) | 464 (28%) |
| Los Angeles | 20 (35%) | 541 (34%) | 561 (34%) |
| Unprotected receptive anal sex partners | |||
| None | 33(58%) | 1266 (80%) | 1299 (79%) |
| 1 partner | 12 (21%) | 238 (15%) | 250 (15%) |
| 2–4 partners | 5 (9%) | 66 (4%) | 71 (4%) |
| 5+ partners | 7 (12%) | 14 (1%) | 21 (1%) |
| Alcohol Use | |||
| None/Low to Moderate | 34 (60%) | 1083 (68%) | 1117 (67%) |
| Moderate to Heavy | 14 (25%) | 371 (23%) | 385 (23%) |
| Binge | 9 (16%) | 144 (9%) | 153 (9%) |
| Depressive Symptom Score | |||
| ≤ 16 | 41 (79%) | 1149 (76%) | 1190 (76%) |
| > 16 | 11 (21%) | 367 (24%) | 378 (24%) |
|
Used erectile dysfunction drug (EDD) or poppers or stimulant (ST)† |
|||
| None ** | 19 (33%) | 963 (60%) | 982 (60%) |
| Used erectile dysfunction drug (EDD)** | 3 (5%) | 85 (5%) | 88 (5%) |
| Used poppers ** | 15 (26%) | 250 (16%) | 265 (16%) |
| Used EDD + poppers ** | 1 (2%) | 38 (2%) | 39 (2%) |
| Used Stimulant (ST) ** | 8 (14%) | 165 (10%) | 173 (10%) |
| Used ST + EDD ** | 1 (2%) | 18 (1%) | 19 (1%) |
| Used ST + poppers ** | 7 (12%) | 59 (4%) | 66 (4%) |
| Used ST + EDD + poppers ** | 3 (5%) | 14 (1%) | 17 (1%) |
Baseline was defined as the earliest visit that a participant was seen and answered questions on EDD and recreational drugs between visit 29 and 45 (4/1/1998 to 9/30/2008).
Mutually exclusive groups.
Reported during the current or previous 6-month visit.
Table 2 presents univariate and multivariate predictors of HIV seroconversion. As expected, in this cohort of MSM, two or more URASP increased the risk of HIV seroconversion with the risk increasing with greater number of URASP. Protective univariate predictors of HIV seroconversion were older age at baseline and graduate work or graduate degree over high school or less. Being of other race/ethnicity (compared to White, non-Hispanic), late versus early cohort enrollment, and use of EDDs, poppers or stimulants, alone or in combination increased the risk of HIV seroconversion. Moreover any combination of these drugs dramatically raised risk of HIV seroconversion over use of one drug alone.
TABLE 2.
Univariate and multivariate hazard ratios associated with HIV seroconversion using Cox proportional hazard models with time dependent covariates from 1998 to 2008.
| Univariate Hazard Ratio (95% CI) |
Multivariate Hazard Ratio (95% CI)* |
|
|---|---|---|
| Age at enrollment | 0.95 (0.93, 0.97) | 0.99 (0.95,1.02) |
| Race/ethnicity | ||
| White, non-Hispanic | 1 | 1 |
| White, Hispanic | 1.33 (0.41, 4.31) | 1.28 (0.37, 4.41) |
| Black, (includes non-Hispanic and Hispanic) | 1.32 (0.68, 2.53) | 1.27 (0.55, 2.96) |
| All Other | 3.26 (1.37, 7.77) | 1.54 (0.56, 4.20) |
| Educational level | ||
| 12th grade or less | 1 | 1 |
| Some college or college graduate | 0.83 (0.43, 1.61) | 1.07 (0.50, 2.29) |
| Some graduate work or graduate degree | 0.36 (0.16, 0.82) | 0.54 (0.20, 1.48) |
| Cohort enrollment | ||
| 1984–1985 and 1987–1991 (early) | 1 | 1 |
| 2001–2003 (late) | 2.72 (1.57, 4.71) | 1.74 (0.74, 4.06) |
| Alcohol Use | ||
| None/Low to Moderate | 1 | 1 |
| Moderate to Heavy | 1.48 (0.78, 2.79) | 0.89 (0.44, 1.84) |
| Binge | 1.91 (0.85, 4.31) | 0.96 (0.40, 2.29) |
| Depressive Symptom Score | ||
| ≤ 16 | 1 | 1 |
| > 16 | 1.20 (0.63, 2.30) | 0.80 (0.40, 1.59) |
| Unprotected receptive anal sex partners | ||
| None | 1 | 1 |
| 1 partner | 2.02 (0.94, 4.35) | 1.73 (0.75, 4.01) |
| 2–4 partners | 6.90 (3.10, 15.38) | 4.23 (1.76, 10.17) |
| 5+ partners | 31.74 (16.12, 62.48) | 14.21 (6.27, 32.20) |
|
Used erectile dysfunction drug (EDD) or poppers or stimulant (ST)† |
||
| None ** | 1 | 1 |
| Used erectile dysfunction drug (EDD)** | 3.31 (1.13, 9.71) | 3.44 (1.03, 11.53) |
| Used poppers ** | 4.98 (2.21, 11.22) | 3.89 (1.53, 9.87) |
| Used EDD + poppers ** | 4.41 (1.38, 14.11) | 3.28 (0.84, 12.73) |
| Used Stimulant (ST) ** | 5.34 (2.10, 13.58) | 2.99 (1.02, 8.76) |
| Used ST + EDD ** | 6.60 (1.44, 30.11) | 4.91 (1.03, 23.45) |
| Used ST + poppers ** | 12.36 (4.86, 31.44) | 5.36 (1.82, 15.74) |
| Used ST + EDD + poppers ** | 20.15 (7.31, 55.52) | 8.45 (2.67, 26.71) |
Model adjusted for race/ethnicity, age at baseline, cohort, education level, number of unprotected receptive anal sexual partners, and categories of EDD or poppers or stimulant use.
Mutually exclusive groups.
Reported during the current or previous 6-month visit.
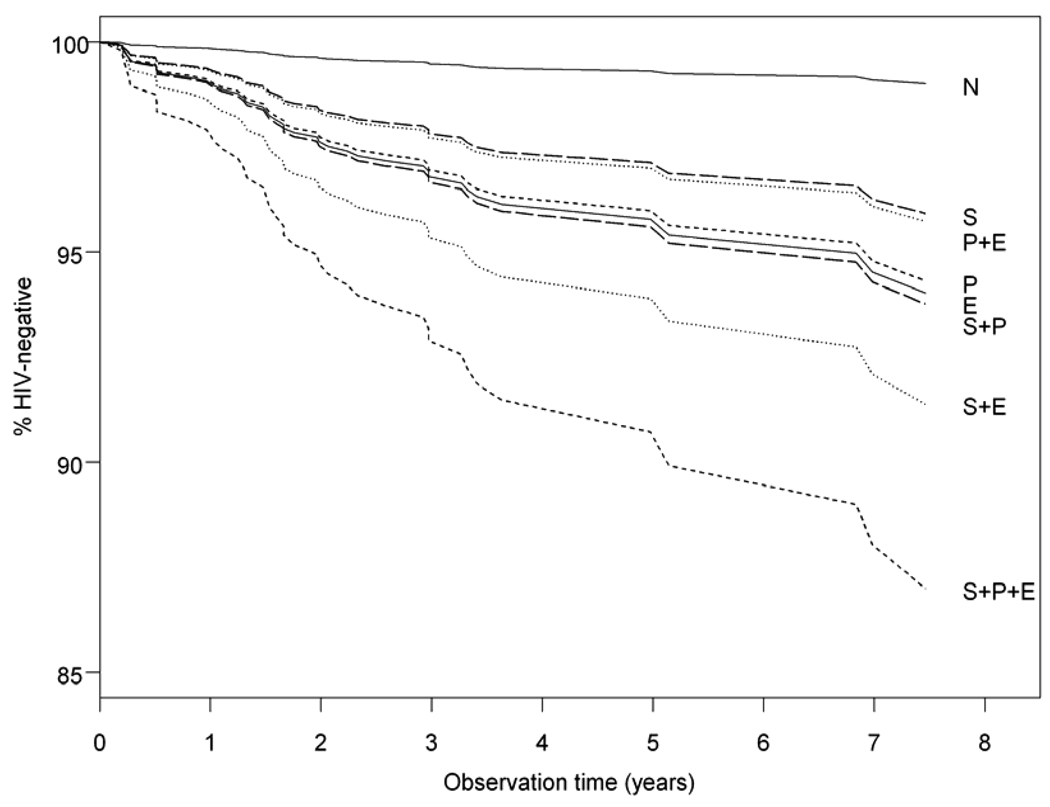
After adjusting for all the covariates, the number of URASP remained an independent predictor of HIV seroconversion. In addition, there was an independent 2.99 [95% CI= 1.02, 8.76] increased hazard rate of HIV seroconversion associated with stimulant use and a 3.89 [95%CI 1.53, 9.87] and 3.44 [95CI 1.03, 11.53] increased risk associated with either popper or EDD use alone, respectively. Reported use of stimulants with any of the other sex-drugs resulted in the highest hazard ratios for HIV seroconversion,. with greatest risk for seroconversion observed in individuals who reported stimulant, poppers and EDD use [8.45, 95%CI:2.67,26.71]. Figure 1 presents the adjusted survival curves for each of the combination sex-drug use subgroups and the referent non-users across the study timeline.
FIGURE 1.
Adjusted time to recent HIV seroconversion by combinations of sex-drug use: none (N), stimulant (S), poppers (P) and EDD (E). All categorical covariates set to the reference value and age was set to the average.
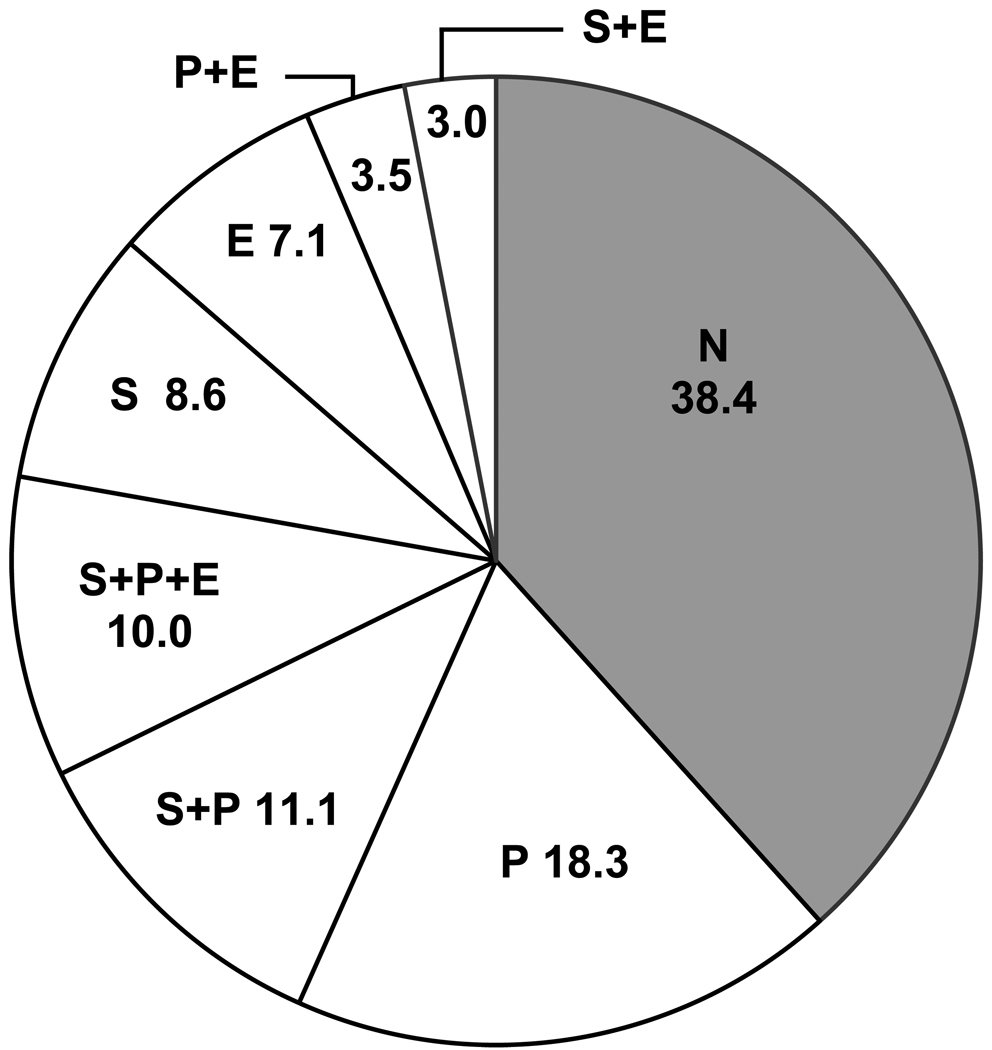
The results of model-based adjusted attributable risk calculations for HIV seroconversion based on this data are summarized in Figure 2. The largest ARs observed are for popper use, either alone or in combination with other drugs. Individual AR values sum to the total risk of seroconversion attributable to sex-drug use. Use of any of the 7 possible sex-drug combinations accounted for 63% of all recent HIV seroincidence in the MACS. Combining the contributions of increased numbers of URASP and sex-drug use in an additive model gave a total AR for HIV seroconversion of 74%. The risk attributable to URASP alone was 41% (22% for 5 or more URASP), resulting in a residual of 33% of recent HIV seroconversions due to the direct or indirect effects of sex-drug use. Additional exposure variables such as alcohol use and elevated depression symptoms were not included in this second calculation but were included as covariates in the regression model, so that all AR values are adjusted for these exposures. The reason for this omission is that, in addition to being non-significant, the relative hazards for both of these exposures were less than one in the multivariate model, indicating that they are somewhat protective of seroconversion risk among this study population. Thus these exposures would make a negative contribution to the attributable risk, and violate analytical model for computing the AR of sex-drug use and behavioral disinhibition. Any difference between attributable risk calculations and the patterns of seroconversion risk seen in Figure 1 are the result of the AR calculations, taking into account the prevalence of the various exposure categories, while the survival curves in Figure 1 do not.
FIGURE 2.
Model-based estimates of the adjusted attributable risk (%) for recent HIV seroconversion by combinations of sex-drug use: none (N), stimulant (S), poppers (P) and EDD (E).
DISCUSSION
We found significant independent associations between stimulant, popper and EDD use after adjusting for other important risk factors such as number of unprotected receptive anal sex partners among the 57 most recent HIV seroconverters in the MACS. Individually, these increased relative hazards for HIV seroconversion were 2.99, 3.89, and 3.44, respectively. However, among men who used all three drugs, the relative hazard escalated to 8 times that of men who reported no use of any of these three drug classes. Most notably, almost two-thirds of these recent seroconversions were associated with the use of these three drugs and approximately half of that AR remained even after taking into account the attributable risk associated with increasing numbers of unprotected receptive anal sex partners and other covariates of combination sex-drug use.
This paper extends the original findings of Plankey, et al.1 by incorporating all new HIV seroconversions through 2008 in the analyses, by examining a broader range of stimulants, by incorporating EDDs in the analyses and by assessing contributions of stimulants, EDDs and poppers, alone and in combination in predicting HIV seroconversion. Although the results from this study support the compounded risks for seroconversion with increasing number of types of sex-drugs found in previous studies 7, 21–23 to the best of our knowledge this is the first published longitudinal study that determined the magnitude of risk of recent HIV seroconversions attributable to the use of these three specific sex-drugs in all possible combinations.
Higher number of sex-drugs used may imply a rudimentary severity marker of behavioral disinhibition that also involves unprotected receptive anal sex with multiple partners. While identical percentages of HIV seronegative men and men who seroconverted (5%) reported use of EDDs, use of EDDs independently increased risk for HIV seroconversion. As the cohort ages, the potential for increased use of EDDs will likely rise among sexually active members of the MACS and with this increase, concomitant risks for continued HIV seroconversion, particularly among those who also report use of poppers and/or cocaine, crack or methamphetamine.
Our findings failed to detect a broad effect of all substance use on HIV seroconversion. In these analyses, severity of alcohol use at the current or previous visit contributed little to HIV seroconversion. Alcohol frequently is used to transact social and sexual connections24–26, but sex-drugs can transcend this function and facilitate extreme forms of sexual behaviors.27, 28
While the use of self-reported behavioral data that measure associations between substance use and high risk sex pose significant methodological challenges, the design used by this study avoids some of the problems. The HIV seroconversion outcome was obtained objectively and precisely, and thus not subjected to misclassification. If the drug use and sexual practices or their concurrence were underreported by participants, the risks reported here are most likely conservative. Participants were not asked about sex-drug use with recent sexual partners (i.e., episodic data), which creates an inability to specify sex-drug use in the context of sexual events. But it remains a major strength of this study that it was performed within a natural history study of exposures ascertained consistently across the cohort and obtained prior to the seroconversion outcome.
Study limitations include a relatively small number of seroconversion events and limited numbers of participants in some of the cells. A consequence of these limitations is that some of the confidence intervals are fairly wide. Thus while some of the estimated hazard ratios could be somewhat smaller, from a public health perspective, we believe that our results are unambiguous.
Important questions remain as to whether and how vasoactive sex-drug use increases the likelihood of HIV infection through unprotected anal sex, even when the behavioral disinhibiting effects of drug use are taken into account. With the findings reported here, it is increasingly clear that combination sex-drug-usage contributes significantly to the spread of HIV infection among vulnerable MSM. From a public health standpoint, interventions that focus on sex-drug reduction strategies in non-treatment settings would appear to sidestep what has otherwise been an impenetrable conundrum of competing hypotheses. Non-injection substance use that commonly accompanies unprotected anal sex with multiple partners remains a potent predictor of HIV seroconversion among MSM and may be increasing in importance as more potent drugs, such as methamphetamine and EDDs, become more popular in high risk sexual settings. The time seems long past to design and evaluate interventions that will disentangle the conjoined epidemics of substance use and high risk sex in this population.
ACKNOWLEDGMENTS
The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (Principal Investigator), Haroutune Armenian, Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, John Hylton, Lisette Johnson, Shenghan Lai, Justin McArthur, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio. Washington, DC: Michael W. Plankey. Chicago: Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: John P. Phair (Principal Investigator), Joan S. Chmiel (Co-Principal Investigator), Sheila Badri, Bruce Cohen, Craig Conover, Maurice O'Gorman, David G Ostrow, Frank Palella, Daina Variakojis, Steven M. Wolinsky. Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (Principal Investigator), Barbara R. Visscher (Co-Principal Investigator), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Thomas Coates, Rita Effros, John Fahey, Beth Jamieson, Otoniel Martínez-Maza, Eric N. Miller, John Oishi, James A. Peck, Paul Satz, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang. Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (Principal Investigator), Lawrence Kingsley (Co-Principal Investigator), James T. Becker, Robert L. Cook, Robert W. Evans, John Mellors, Sharon Riddler, Anthony Silvestre, Ron Stall. Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (Principal Investigator), Alvaro Muñoz (Co-Principal Investigator), Haitao Chu, Stephen R. Cole, Christopher Cox, Stephen J. Gange, Janet Schollenberger, Eric C. Seaberg, Sol Su. NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez; National Heart, Lung and Blood Institute: Cheryl McDonald. Website located at http://www.statepi.jhsph.edu/macs/macs.html. The authors wish to thank Michael Costa for his technical assistance in the preparation of this manuscript.
Sources of support:
The Multicenter AIDS Cohort Study is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute; and the National Heart, Lung, and Blood Institute: UO1-AI-35042, 5-M01-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, and UO1-AI-35041. Additional support was provided by the National Institute of Drug Research through 1R01DA022936, "Long Term Health Effects of Methamphetamine Use in the MACS", Ronald Stall, PhD., PI.
REFERENCES
- 1.Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45(1):85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchacz K, McFarland W, Kellogg TA, et al. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19(13):1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- 3.Chesney MA, Barrett DC, Stall R. Histories of substance use and risk behavior: precursors to HIV seroconversion in homosexual men. Am J Public Health. 1998;88(1):113–116. doi: 10.2105/ajph.88.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colfax GN, Mansergh G, Guzman R, et al. Drug use and sexual risk behavior among gay and bisexual men who attend circuit parties: a venue-based comparison. J Acquir Immune Defic Syndr. 2001;28(4):373–379. doi: 10.1097/00126334-200112010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Crosby R, Metty A. A descriptive analysis of HIV risk behavior among men having sex with men attending a large sex resort. J Acquir Immune Defic Syndr. 2004;37(4):1496–1499. doi: 10.1097/01.qai.0000127065.61454.b4. [DOI] [PubMed] [Google Scholar]
- 6.Hirshfield S, Remien RH, Humberstone M, Walavalkar I, Chiasson MA. Substance use and high-risk sex among men who have sex with men: a national online study in the USA. AIDS Care. 2004;16(8):1036–1047. doi: 10.1080/09540120412331292525. [DOI] [PubMed] [Google Scholar]
- 7.Mansergh G, Shouse RL, Marks G, et al. Methamphetamine and sildenafil (Viagra) use are linked to unprotected receptive and insertive anal sex, respectively, in a sample of men who have sex with men. Sex Transm Infect. 2006;82(2):131–134. doi: 10.1136/sti.2005.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez TH, Gallagher KM. Factors associated with recent sildenafil (Viagra) use among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2006;42(1):95–100. doi: 10.1097/01.qai.0000218361.36335.77. [DOI] [PubMed] [Google Scholar]
- 9.Halkitis PN, Palamar JJ, Mukherjee PP. Poly-club-drug use among gay and bisexual men: a longitudinal analysis. Drug Alcohol Depend. 2007;89(2–3):153–160. doi: 10.1016/j.drugalcdep.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarcz S, Scheer S, McFarland W, et al. Prevalence of HIV infection and predictors of high-transmission sexual risk behaviors among men who have sex with men. Am J Public Health. 2007;97(6):1067–1075. doi: 10.2105/AJPH.2005.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple S, Zians J, Strathdee S, Patterson TL. Sexual marathons and methamphetamine use among HIV-positive men who have sex with men. Arch Sex Behav. 2007 doi: 10.1007/s10508-007-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spindler HH, Scheer S, Chen SY, et al. Viagra, Methamphetamine, and HIV Risk: Results From a Probability Sample of MSM, San Francisco. Sex Transm Dis. 2007 doi: 10.1097/01.olq.0000258339.17325.93. [DOI] [PubMed] [Google Scholar]
- 13.Drumright LN, Strathdee SA, Little SJ, et al. Unprotected Anal Intercourse and Substance Use Before and After HIV Diagnosis Among Recently HIV-Infected Men Who Have Sex With Men. Sex Transm Dis. 2007;34(6):401–407. doi: 10.1097/01.olq.0000245959.18612.a1. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 15.Dudley J, Jin S, Hoover D, Metz S, et al. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142(3):323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 16.Gribble JN, Miller HG, Cooley PC, et al. The impact of T-ACASI interviewing on reported drug use among men who have sex with men. Subst Use Misuse. 2000;35(6–8):869–890. doi: 10.3109/10826080009148425. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol. 1979;88(2):174–181. doi: 10.1037//0021-843x.88.2.174. [DOI] [PubMed] [Google Scholar]
- 18.Cox DR, Oakes R. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- 19.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. 2nd. ed. New York: Springer-Verlag; 2003. [Google Scholar]
- 20.Cox C. Model-based estimation of the attributable risk in case-control and cohort studies. Stat Med. 2006;15:611–625. doi: 10.1177/0962280206071930. [DOI] [PubMed] [Google Scholar]
- 21.McNall M, Remafedi G. Relationship of amphetamine and other substance use to unprotected intercourse among young men who have sex with men. Arch Pediatr Adolesc Med. 1999;153(11):1130–1135. doi: 10.1001/archpedi.153.11.1130. [DOI] [PubMed] [Google Scholar]
- 22.Patterson TL, Semple SJ, Zians JK, Strathdee SA. Methamphetamine-using HIV-positive men who have sex with men: correlates of polydrug use. J Urban Health. 2005;82(1) Suppl 1:i120–i126. doi: 10.1093/jurban/jti031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey JW, Mejia R, Bingham T, et al. Drug Use, High-Risk Sex Behaviors, and Increased Risk for Recent HIV Infection among Men who Have Sex with Men in Chicago and Los Angeles. AIDS Behav. 2008 doi: 10.1007/s10461-008-9403-3. [DOI] [PubMed] [Google Scholar]
- 24.Elam G, Macdonald N, Hickson FC, et al. Risky sexual behaviour in context: qualitative results from an investigation into risk factors for seroconversion among gay men who test for HIV. Sex Transm Infect. 2008;84(6):473–477. doi: 10.1136/sti.2008.031468. [DOI] [PubMed] [Google Scholar]
- 25.Stall R, Paul JP, Greenwood G, et al. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men's Health Study. Addiction. 2001;96(11):1589–1601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- 26.Vanable PA, McKirnan DJ, Buchbinder SP, et al. Alcohol use and high-risk sexual behavior among men who have sex with men: the effects of consumption level and partner type. Health Psychol. 2004;23(5):525–532. doi: 10.1037/0278-6133.23.5.525. [DOI] [PubMed] [Google Scholar]
- 27.Koblin BA, Murrill C, Camacho M, et al. Amphetamine use and sexual risk among men who have sex with men: results from the National HIV Behavioral Surveillance study--New York City. Subst Use Misuse. 2007;42(10):1613–1628. doi: 10.1080/10826080701212519. [DOI] [PubMed] [Google Scholar]
- 28.Reback CJ, Larkins S, Shoptaw S. Changes in the meaning of sexual risk behaviors among gay and bisexual male methamphetamine abusers before and after drug treatment. AIDS Behav. 2004;8(1):87–98. doi: 10.1023/b:aibe.0000017528.39338.75. [DOI] [PubMed] [Google Scholar]