Abstract
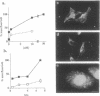
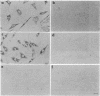
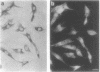
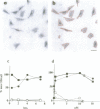
Cells internalized synthetic oligonucleotides (oligos) in culture. The hybridization of these molecules to target RNA in the living cell was subsequently detected and characterized after fixation of the cells, with or without previous detergent extraction. Hybridized oligo was distinguished from free oligo in the cell using an in situ reverse transcription technique. This assay exploited the ability of the hybridized oligo to prime synthesis of a specific cDNA strand; unhybridized oligo present in the cell could not act as a primer for reverse transcription. Phosphorothioate and fluorochrome-labeled phosphodiester oligo dT were found to enter cells rapidly and hybridize to poly (A) RNA within 30 min. Hybrids containing phosphorothioate oligo dT were detectable in cells after up to 4 h of efflux time. Phosphodiester bonded oligo dT containing covalently-linked fluorochromes appeared more stable in the cell than unmodified phosphodiester oligo dT; hybrids containing these oligos could be detected in cells as long as 18h after efflux began. The in situ transcription assay was also sensitive enough to detect hybridization of anti-actin oligos to actin mRNA in the cell. It is probable, therefore, that this assay can be used to help assess the efficacy of antisense oligos by their hybridization to a target mRNA in cells or tissues; hybridized oligos are more likely to induce a specific antisense effect. Additionally, this assay will help to identify probes that would be useful as stable hybridization tags to follow RNA movement in living cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassell G. J., Powers C. M., Taneja K. L., Singer R. H. Single mRNAs visualized by ultrastructural in situ hybridization are principally localized at actin filament intersections in fibroblasts. J Cell Biol. 1994 Aug;126(4):863–876. doi: 10.1083/jcb.126.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T., Burri M., Thoma G., Bopp D., Richstein S., Frigerio G., Noll M., Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988 Jun;7(6):1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberwine J., Spencer C., Miyashiro K., Mackler S., Finnell R. Complementary DNA synthesis in situ: methods and applications. Methods Enzymol. 1992;216:80–100. doi: 10.1016/0076-6879(92)16011-8. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Akin D. Role of nuclear trafficking in regulating cellular activity. Int Rev Cytol. 1994;151:183–228. doi: 10.1016/s0074-7696(08)62633-9. [DOI] [PubMed] [Google Scholar]
- Fisher T. L., Terhorst T., Cao X., Wagner R. W. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993 Aug 11;21(16):3857–3865. doi: 10.1093/nar/21.16.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992 Oct 16;71(2):301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- Hughes J. A., Avrutskaya A. V., Juliano R. L. Influence of base composition on membrane binding and cellular uptake of 10-mer phosphorothioate oligonucleotides in Chinese hamster ovary (CHRC5) cells. Antisense Res Dev. 1994 Fall;4(3):211–215. doi: 10.1089/ard.1994.4.211. [DOI] [PubMed] [Google Scholar]
- Iversen P. L., Zhu S., Meyer A., Zon G. Cellular uptake and subcellular distribution of phosphorothioate oligonucleotides into cultured cells. Antisense Res Dev. 1992 Fall;2(3):211–222. doi: 10.1089/ard.1992.2.211. [DOI] [PubMed] [Google Scholar]
- Kislauskis E. H., Li Z., Singer R. H., Taneja K. L. Isoform-specific 3'-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J Cell Biol. 1993 Oct;123(1):165–172. doi: 10.1083/jcb.123.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis E. H., Zhu X., Singer R. H. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol. 1994 Oct;127(2):441–451. doi: 10.1083/jcb.127.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M., Etkin L. D. Delocalization of Vg1 mRNA from the vegetal cortex in Xenopus oocytes after destruction of Xlsirt RNA. Science. 1994 Aug 19;265(5175):1101–1103. doi: 10.1126/science.7520603. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Taneja K., Singer R. H. Temporal resolution and sequential expression of muscle-specific genes revealed by in situ hybridization. Dev Biol. 1989 May;133(1):235–246. doi: 10.1016/0012-1606(89)90314-x. [DOI] [PubMed] [Google Scholar]
- Leonetti J. P., Mechti N., Degols G., Gagnor C., Lebleu B. Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2702–2706. doi: 10.1073/pnas.88.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J., Kølvraa S., Hindkjaer J., Petersen S., Koch J., Nygård M., Jensen T., Gregersen N., Junker S., Bolund L. Nonradioactive, sequence-specific detection of RNA in situ by primed in situ labeling (PRINS). Exp Cell Res. 1991 Sep;196(1):92–98. doi: 10.1016/0014-4827(91)90459-8. [DOI] [PubMed] [Google Scholar]
- Morishita R., Gibbons G. H., Kaneda Y., Ogihara T., Dzau V. J. Pharmacokinetics of antisense oligodeoxyribonucleotides (cyclin B1 and CDC 2 kinase) in the vessel wall in vivo: enhanced therapeutic utility for restenosis by HVJ-liposome delivery. Gene. 1994 Nov 4;149(1):13–19. doi: 10.1016/0378-1119(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Sachs A. The role of poly(A) in the translation and stability of mRNA. Curr Opin Cell Biol. 1990 Dec;2(6):1092–1098. doi: 10.1016/0955-0674(90)90161-7. [DOI] [PubMed] [Google Scholar]
- Scherczinger C. A., Knecht D. A. Co-suppression of Dictyostelium discoideum myosin II heavy-chain gene expression by a sense orientation transcript. Antisense Res Dev. 1993 Summer;3(2):207–217. doi: 10.1089/ard.1993.3.207. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Singer R. H. RNA zipcodes for cytoplasmic addresses. Curr Biol. 1993 Oct 1;3(10):719–721. doi: 10.1016/0960-9822(93)90079-4. [DOI] [PubMed] [Google Scholar]
- Sixou S., Szoka F. C., Jr, Green G. A., Giusti B., Zon G., Chin D. J. Intracellular oligonucleotide hybridization detected by fluorescence resonance energy transfer (FRET). Nucleic Acids Res. 1994 Feb 25;22(4):662–668. doi: 10.1093/nar/22.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Krieg A. M. Problems in interpretation of data derived from in vitro and in vivo use of antisense oligodeoxynucleotides. Antisense Res Dev. 1994 Summer;4(2):67–69. doi: 10.1089/ard.1994.4.67. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja K. L., Lifshitz L. M., Fay F. S., Singer R. H. Poly(A) RNA codistribution with microfilaments: evaluation by in situ hybridization and quantitative digital imaging microscopy. J Cell Biol. 1992 Dec;119(5):1245–1260. doi: 10.1083/jcb.119.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkinson J. L., Stein C. A. Patterns of intracellular compartmentalization, trafficking and acidification of 5'-fluorescein labeled phosphodiester and phosphorothioate oligodeoxynucleotides in HL60 cells. Nucleic Acids Res. 1994 Oct 11;22(20):4268–4275. doi: 10.1093/nar/22.20.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov V. V., Balakireva L. A., Yakubov L. A. Transport of oligonucleotides across natural and model membranes. Biochim Biophys Acta. 1994 Jun 29;1197(2):95–108. doi: 10.1016/0304-4157(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Wagner R. W. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994 Nov 24;372(6504):333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- Wintersberger U. Ribonucleases H of retroviral and cellular origin. Pharmacol Ther. 1990;48(2):259–280. doi: 10.1016/0163-7258(90)90083-e. [DOI] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P., Aghajanian J., Zamecnik M., Goodchild J., Witman G. Electron micrographic studies of transport of oligodeoxynucleotides across eukaryotic cell membranes. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3156–3160. doi: 10.1073/pnas.91.8.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Matson S., Herrera C. J., Fisher E., Yu H., Krieg A. M. Comparison of cellular binding and uptake of antisense phosphodiester, phosphorothioate, and mixed phosphorothioate and methylphosphonate oligonucleotides. Antisense Res Dev. 1993 Spring;3(1):53–66. doi: 10.1089/ard.1993.3.53. [DOI] [PubMed] [Google Scholar]